ABSTRACT
Aim and Background: The coronavirus disease 2019 (Covid-19) virus pandemic is still ravaging the world with its ongoing resurgence and the continuous mutation, suggesting the need for continuous research on safe and effective novel vaccines. Presently several types of vaccines have been developed and emerged in the global market to control COVID-19 virus. Consequently, the knowledge and information on COVID-19 have been expanding at a high level. Researchers need to gain relevant knowledge regarding the different vaccines; however scattered information makes this process time-consuming and laborious. The present study aimed to evaluate the characteristics and trends in global COVID-19 vaccine high-cited literature using bibliometric and visualizations methods and offer some directions and suggestions for future research. Methodology: Studies published between December 2019 and 22 Nov 2022 on COVID-19 vaccines were retrieved from the Scopus database. From the 16026 studies retrieved, 406 were identified as high-cited papers (HCPs) having received 100 or more citations. From the 406 HCPs, information about publications outputs, countries, institutions, journals, keywords, and citation counts was identified. Data analysis and visualization were conducted using Microsoft Excel, VOSviewer and Bibliometrix R software. Results: The 406 global HCPs on COVID-19 vaccines research were identified in Scopus database since Dec 2019 till 30 Nov 2022 using a search strategy, which received 123614 citations, averaging 304.17 citations per publication (CPP). An external funding was received by 53.20% (216 publications), which were cited 76107 times (with an average of 352.35 CPP). The 7086 authors from 694 organizations affiliated to 76 countries and publishing in 121 journals were involved in global COVID-19 vaccine research. The most productive countries were USA (n=213), U.K (n=91), China (n=36) and Germany (n=35). The most impactful countries in terms of citations per paper (CPP) and relative citation index (RCI) were South Africa (794.68 and 2.61), Germany (507.11 and 1.67), U.K. (396.59 and 1.30) and Spain (367.5 and 1.121). The most productive organizations were University of Oxford, U.K., Imperial College London, U.K. (n=25 each), Center for Disease Control and Prevention (CDC), USA and Tel Aviv University (n=19 each) and the most impactful organizations were University of Cambridge, U.K (783.4 and 2.57), Emory University, USA (780.1 and 2.56), John Hopkins Bloomberg School of Public Health, USA (702.67 and 2.31) and National Institute of Allergy and Infectious Diseases. USA (676.41 and 2.22). The most productive authors were A.J. Pollard (n=16) and T. Lambe (n=14) (of University of Oxford), O. Tureci and P.R. Dormitzer (n=12 each) (of BioNTechSE, Germany) and the most impactful were D. Cooper (1239.22 and 4.07), K.J. Janseu (1228.11 and 4.03) (BioNTechSE, Germany, K.A. Swanson (987.0 and 3.24) (University of Oxford, U.K.) and P.R. Dormitzer (983 and 3.23) (BioNTechSE, Germany). The most productive journals were New England Journal of Medicine (n=53), The Lancet (n=28), Nature (n=22) and JAMA (N=17). The most impactful journals (as per citations per paper) were New England Journal of Medicine (613.15), Lancet (496.39), Human Vaccines and Immunotherapeutics (369.67) and Nature (360.64). Among population age groups, the major focus was on adults (51.48%) and Middle Aged (39.16%). Among publication types, the major focus was Clinical Studies (26.85%), Epidemiology (22.66%) and Genetics (21.92%). The most significant keywords by frequency of appearances were “Covid-19” (n=388), “Covid-19 Vaccines” (n=357), “Vaccination” (n=221), “Prevention and Control” (n=181) and “Vaccine Immunogenicity” (n=133). Conclusion: The HCPs in COVID-19 vaccine research was done mainly by the authors and institutions of high-income Countries (HIC) and was published in high-impact medical journals. Our research has identified the leading countries, institutions, journals, hotspots and development trend in the field that could provide the foundation for further investigations. The bibliometric analysis will help the clinicians to rapidly identify the potential collaborative partners, identify significant studies, and research topics within their domains of COVID-19 vaccines.
INTRODUCTION
The world has witnessed severe outbreaks of coronavirus diseases, including COVID-19 pandemic, which was first reported in China in December 2019. WHO declared a public health emergency of international concern (PHEIC) on 30 January 2020, shortly after the etiological agent causing the new respiratory disease later called COVID-19 had been isolated and the first genomic sequence had been completed.1
The coronavirus disease 2019 (COVID-19) pandemic was caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 752,517,552 confirmed global cases and 6,804,491 deaths in more than 200 countries, as reported by WHO on 23 Jan 2023.2
COVID-19, the disease caused by SARS-CoV-2, is asymptomatic for some, for others it can cause illness ranging from mild flu-like symptoms with the most serious cases manifesting with acute respiratory distress syndrome (ARDS), pneumonia and death. This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems.3 Despite the implementation of stringent public health measures, there have been devasting effects in various sectors contributing to our economy
Owing to the absence of definitive treatment, the global burden of the pandemic requires an efficacious vaccine that elicits a lasting protective immune response against SARS-CoV-2. This will be an essential armament for the prevention and mitigation of the downstream morbidity and mortality caused by SARS-CoV-2 infection.4 The vaccination against COVID-19 emerged as an effective strategy against the spread of the pandemic. Acceptance of the COVID-19 vaccine has advanced considerably, and there has been a rapid expansion in global vaccine research focusing on exploiting the novel discoveries on the pathophysiology, genomics, and molecular biology of the severe acute respiratory syndrome coronavirus 2 or COVID-19 infections over the last three years.4
The demand for the COVID-19 vaccines arose since the World Health Organization (WHO) declared the COVID-19 outbreak a pandemic. Scientific/academic institutions and manufacturers started to work on the development of COVID-19 vaccines only after the declaration of the PHEIC. Now, given the urgent need for COVID-19 vaccines, unprecedented financial investments and scientific collaborations were put in place for the development of vaccines.5
As a result, since the beginning of 2021 saw numerous vaccines given emergency approval and begins their roll out in countries across the world. These vaccines are designed mainly based on three approaches. Their differences lie in whether they use a whole virus or bacterium; just the parts of the germ that triggers the immune system; or just the genetic material that provides the instructions for making specific proteins and not the whole virus.6
At present different kinds of COVID-19 vaccine candidates are under development. The WHO COVID-19 vaccine tracker and landscape has been regularly compiling detailed information of each COVID-19 vaccine candidate in development by closely monitoring their progress through the pipeline.7 As on 24 January 2023, the number of vaccines in clinical development was 176 and their number in pre-clinical development were 199. The classification of 172 vaccine candidates in clinical phase were: (i) Protein Subunit (PS)(57, 33%), Viral Vector (non-replicating) (VVnr)(23, 13%), DNA (16, 9%), Inactivated Virus (IV)(22, 13%), RNA (41, 23%), Viral Vector (replicating)(4, 2%), Virus Like Particle (VLP)(7, 4%), VVr + Antigen Presenting Cell (VVr + APC)(1. 1%), Live Attenuated Virus (LAV) (2. 1%), VVnr + Antigen Presenting Cell (VVnr + APC) (2, 1%) and Bacterial antigen-spore expression vector (BacAg-SpV) (1, 1%). On classifying them by route of administration, (i) Oral (5, 3%), (Injectable (159, 90%): (i) Sub-cutaneous (SC)(5, 3%), (ii) Intradermal (ID)(9, 5%), (iii) Intra muscular (IM)(145, 82%), (iv)Intra nasal (IN)(14, 8%), Aerosol (AE)(1, 1%), (vi) Inhaled ) (IH)(2. 1%) and (iii) No Data (12., 7%).4 As of 23 January 2023, a total of 13,156,047,747 vaccine doses have been administered by different countries as indicated by WHO.5
Worldwide research on this topic has led to an explosion of publications on COVID-19 and COVID-19 vaccines, by increasing at exponential pace to understand different aspects of the disease and vaccine development since December 2019.7
A bibliometric study enables the mapping and expansion of knowledge in a research area, evidencing connections between the main publications, authors, institutions, themes, and other characteristics of the field under study8 For instance, a bibliometric analysis can be used to analyze trends in an area of research, provide evidence for the impact of the research area, find new and emerging areas of research, identify potential research collaborators or identify suitable sources in which to publish.9
Articles with high-citation frequency can provide important information about the current research situation in a certain field.10 Papers with a high number of citations are considered central in research. Therefore, the high- cited papers provide evidence and information about research trends and scientific progress in a specific field.11 High-cited papers provide interesting information about the contributors, articles and topics which are influential in the research community during a certain period and have a greater chance of visibility, thus attracting greater attention among researchers.12 Evaluating the top cited publications content is therefore very useful for obtaining information about the trends of specific fields in the perspective of research progress.13
Although many bibliometric studies have been performed in relation to COVID-19 in general,14–22 but the specific subject of the COVID-19 vaccine has so far attracted comparatively less attention from researchers. A few bibliometric studies have been published on assessment of global COVID-19 vaccines literature, which make the quantitative and qualitative assessment by way of analyzing various features of research output and citations.7–9,23–30 With the rapid growth COVID-19 vaccines literature, it was difficult to go through such large number of publications appearing in various databases. It is observed that there has been a non-availability of any bibliometric study on HCPs papers on COVID-19 vaccine and any not study providing a quick, comprehensive synthesis of relevant information on the related scientific orientations, gaps, collaborations, themes, or dynamisms on COVID-19 vaccines. Considering significance of COVID-19 vaccine research and the importance of high-cited articles, we qualitatively and quantitatively analyzed the top 406 HCPs in the field of COVID-19 vaccines, to evaluate their overall global research output and trends with an aim to identify the major players (countries, organizations, authors and journals), major themes being pursued using significant keywords, besides analyzing and constructing visualization network of co-authorship in countries, organizations and authors, co-occurrence of keywords and co-citation of sources and to provide a reference for future research in this field.
METHODOLOGY
On Nov 30, 2022, a comprehensive search string was developed and used in Scopus database for identification, retrieval and downloading of relevant papers published between December 2019 till 30 Nov 2022 on COVID-19 vaccine. The keywords related to COVID-19 and Vaccine was used in Title search tag, as shown below. The search resulted in 16027 documents, which were rearranged in the decreasing order of citations, to obtain top 406 documents having received 100 or more citations. These 406 retrieved records were further refined and sorted out by broad subject area, document type, source type, organizations, author, journal and keywords. The data was used to manage the extracted data and perform statistical analysis and develop bibliometric indicators. The 406 records were exported as a CSV file and imported by VOSviewer software, which provides a network visualization of the publications, including bibliographic coupling, co-authorship, co-citation, and co-occurrence analysis of publications, countries, organizations, authors, journals and keywords. Labels represent items in the network visualization using circles. The weight of an item determines the size of the label and the circle around it. The label of some items may not be visible to prevent possible overlapping labels. An item’s color is determined by the cluster to which it belongs. The lines between items represent links.7
TITLE ( “covid 19” OR “2019 novel coronavirus” OR “coronavirus 2019” OR “coronavirus disease 2019” OR “2019-novel CoV” OR “2019 nov” OR covid 2019 OR corvidae OR “corona virus 2019” OR ncov-2019 OR ncov2019 OR “nov 2019” OR 2019-ncov OR covid-19 OR “Severe acute respiratory syndrome coronavirus 2” OR “SARS-CoV-2” ) OR KEY ( “covid 19” OR “2019 novel coronavirus” OR “coronavirus 2019” OR “coronavirus disease 2019” OR “2019-novel CoV” OR “2019 nov” OR covid 2019 OR corvidae OR “corona virus 2019” OR ncov-2019 OR ncov2019 OR “nov 2019” OR 2019-ncov OR covid-19 OR “Severe acute respiratory syndrome coronavirus 2” OR “SARS-CoV-2” ) AND TITLE ( vaccine* )
ANALYSIS AND RESULTS
Overall View
From the 16027 papers indexed in Scopus database till 30.11.2022 on COVID-19 Vaccine research, only 406 (2.53%) received 100 to 6423 citations. These 406 papers (2020=129, 2021=258 and 2022=19) are assumed here as high-cited papers (HCPs) and they together received 12,36,14 citations, averaging 304.17 citations per paper. The 406 HCPs show uneven distribution of citations: The 1, 11 and 41 papers fall in citation range 5000-6423, 1000-4999 and 500-999 as against 136 and 217 papers fall in citation range 200-499 and 100-199.
Of the 406 HCPs, 68.28% (277) constitute articles, 19.21% (78) reviews, 7.14% (29) letters, 4.19% (17) notes, 0.74% (3) short surveys and 0.49% (2) editorials.
Of the 406 HCPs, 216 (53.20%) received external funding support from more than 150 funding agencies and together received 76107 citations, averaging 352.35 citations per paper. The major external funding agencies supporting research (along with their output) in this area were National Institute of Health, USA (70 papers), National Institute of Allergy and Infectious Diseases, USA (51 papers), Pfizer, USA (29 papers), National Institute of Health Research (25 papers), Bill and Melinda Gates Foundation, USA (21 papers), U.K. Research and Innovation (20 papers), The Welcome Trust (19 papers), Merck (17 papers), Medical Research Council (14 papers), National Institute of Advanced Translational Research (13 papers), Centers for Disease Control and Protection, USA (11 papers), National Key Research and Development Program of China (10 papers), National Natural Science Foundation of China and GlaxoSmithKline (8 papers each), National Cancer Institute and U.S. Department of Health and Human Service (7 papers each), European Commission (6 papers), etc.
Of the 406 HCPs, 209 (51.48%) were focused on “Adults”, followed by “Middle Aged” (159 papers, 39.16%), “Aged” (137 papers; 33.74%), “Adolescents” (92 papers; 22.66%) and “Children” (16 papers; 3.94%). There is overlapping of literature under various population age groups.
Among types of research, Clinical Studies accounts for the largest number and share (109, 26.85%) of total HCPs, followed by Epidemiology (92 papers, 22.66%), genetics (89 papers, 21.92%), Adverse Events (83 papers, 20.44%), Treatment (43 papers, 10.59%), Risk Factors (21 papers, 5.17%), Pathophysiology (13 papers, 3.20%), and Complications (9 papers, 2.22%).
Among another publication type, Controlled Studies account for the largest number and share (173 papers), 42.61%), followed by Clinical Trials (77 papers, 18.97%), Randomized Clinical Trials (59 papers, 14.53%), Cross-Sectional Studies (34 papers, 8.37%), Prospective Studies (23 papers, 5.67%), Observational Studies (17 papers, 4.19%), Retrospective Studies and Controlled Clinical Trials (10 papers, 2.46% each), Meta-Analysis (6 papers, 1.48%) and Case Reports (5 papers, 1.23%)
Most Productive and Impactful Countries
In all 76 countries participated in global COVID-19 vaccine research, of which 57 countries contributed 1-5 papers each, 6 countries contributed 6-10 papers each, 12 countries contributed 11-100 papers each and 1 country contributed 213 papers.
The top 23 countries contributed 5 to 213 papers and these together contributed 605 papers which received 205806 citations, accounting more than 100.0% share each in global publications and citations. Further it was observed that only 6 countries contributed more than the average publication productivity (26.30) of all top 23 countries: USA (213 papers and 52.46% share) and U.K. (91 papers and 22.41% share), China (36 papers and 8.87% share), Germany (35 papers and 8.62% share), Israel (28 papers and 8.62% share) and Canada (27 papers and 6.65% share). Only 5 countries registered average citation per paper (CPP) and relative citation index (RCI) above the group average (340.18 and 1.12) of top 23 countries: South Africa (794.68 and 2.61), Germany (507.11 and 1.67), U.K (396.59 and 1.30), Spain (367.5 and 1.21) and Russia Federation (361.67 and 1.19). The share of international collaborative papers of top 23 countries varied from 20.00% to 100.0%, with an average of 62.31% [Table 1].
The total link strength of top 23 countries varied from 2 to 183, with highest collaboration strength and intensity depicted by USA (183 linkages), U.K. (123 linkages), Germany (62 linkages), South Africa (56 linkages), Australia (44 linkages), Netherlands (42 linkages), etc. The country to country collaborative linkages among top 23 countries varied from 1 to 42, with highest collaborative linkages and intensity (42), depicted by country pairs “USA-U.K”, followed by country pairs such as “USA-Germany” (21 linkages), “USA-Netherland” (17 linkages), “U.K.-Germany” (14 linkages), “USA-South Africa” (13 linkages), “U.K and South Africa” (11 linkages), “USA-Australia” (10 linkages), “USA-Brazil” (9 linkages), “USA-Canada” and “USA-Belgium” (8 linkages), “USA-Israel” and “Germany-South Africa” (7 linkages each), etc. Among top 23 countries, USA became the center of collaboration attraction, followed by U.K. and Germany.
The country co-authorship network of HCPs in COVID-19 Vaccine, built by the VOSviewer, is presented in Figure 1. By network analysis, the country co-authorship data has been presented in five clusters: Cluster 1 includes 7 countries namely Australia, Belgium, Brazil etc. Cluster 2 includes 6 countries, namely Canada, China, India, etc. Cluster 3 includes 5 countries, namely France, Qatar, UK, etc. Cluster 4 includes four countries, namely Israel, Italy, Russia, and Spain and cluster 5 includes one country, i.e., Germany. The 23 countries, institutions, and authors with the most publications are listed in Table 1.
Most Productive and Impactful Organizations
In all, 694 organizations participated in global COVID-19 vaccine research. The top 32 organizations contributed 5 to 34 papers and these together contributed 409 papers which received 158390 citations, accounting more than 100.0% share each in global publications and citations.
On further analysis, it was observed that 14 organizations contributed more than the average publication productivity (12.78) of all 30 organizations: University of Oxford, U.K. (34 papers, 8.37% share), Imperial College, London, U.K. ( 25 papers, 6.16% share), The Center for Disease Control and Prevention, USA, Tel Aviv University, Israel, London School of Hygiene and Tropical Medicine, U.K and University College London, U.K. (19 papers and 4.68% share each), Harvard Medical School, USA and University of Washington, USA (18 papers and 4.43% share), etc. Only 16 organizations registered average citation per paper (CPP) and relative citation index (RCI) above the group average (387.26 and 1.27) of top 32 organizations: University of Cambridge, U.K. (783.4 and 2.57), Emory University, USA (780.1 and 2.56), John Hopkins Bloomberg School of Public Health, USA (702.67 and 2.31), National Institute of Allergy and Infectious Diseases. USA (676.41 and 2.22), Brigham and Women’s Hospital, USA (643.91 and 2.11), Baylor College of Medicine, USA (531.08 and 1.74), London School of Hygiene and Tropical Medicine, U.K. (512.47 and 1.68), Chinese Academy of Sciences and Peking Union Medical College, China (448.2 and 1.47), et al. Table 2 presents the bibliometric profile of top 8 most productive and 8 most impactful organizations.
The total link strength of top 32 organizations varying from 1 to 49, with highest collaboration strength and intensity (49 linkages) depicted by National Institute of Allergy and Infectious Diseases, USA, followed by University of Washington (40 linkages), Emory University (40 linkages), University of North Carolina at Chapel Hill, USA (37 linkages), National Institute of Health, USA (36 linkages), Vanderbilt University Medical Center, USA (35 linkages), University College, London (33), University of Oxford and Imperial College London (31 each), Brigham and Women’s Hospital, USA (29), London School of Hygiene and Tropical Medicine, U.K. (25), etc.
| Sl. No | Country | TP | TC | CPP | RCI | ICP | %ICP | TLS | %TP |
|---|---|---|---|---|---|---|---|---|---|
| 1 | United States | 213 | 72164 | 338.80 | 1.11 | 123 | 57.75 | 183 | 52.46 |
| 2 | United Kingdom | 91 | 36090 | 396.59 | 1.30 | 62 | 68.13 | 123 | 22.41 |
| 3 | China | 36 | 11488 | 319.11 | 1.05 | 15 | 41.67 | 17 | 8.87 |
| 4 | Germany | 35 | 17749 | 507.11 | 1.67 | 31 | 88.57 | 62 | 8.62 |
| 5 | Israel | 28 | 8337 | 297.75 | 0.98 | 9 | 32.14 | 22 | 6.90 |
| 6 | Canada | 27 | 4986 | 184.67 | 0.61 | 17 | 62.96 | 34 | 6.65 |
| 7 | Netherlands | 20 | 5588 | 279.40 | 0.92 | 20 | 100.00 | 42 | 4.93 |
| 8 | South Africa | 19 | 15099 | 794.68 | 2.61 | 14 | 73.68 | 56 | 4.68 |
| 9 | Australia | 18 | 4470 | 248.33 | 0.82 | 15 | 83.33 | 44 | 4.43 |
| 10 | Italy | 17 | 3904 | 229.65 | 0.75 | 10 | 58.82 | 23 | 4.19 |
| 11 | Brazil | 12 | 3783 | 315.25 | 1.04 | 12 | 100.00 | 37 | 2.96 |
| 12 | India | 12 | 2659 | 221.58 | 0.73 | 8 | 66.67 | 16 | 2.96 |
| 13 | Belgium | 11 | 3490 | 317.27 | 1.04 | 9 | 81.82 | 31 | 2.71 |
| 14 | France | 9 | 1997 | 221.89 | 0.73 | 4 | 44.44 | 8 | 2.22 |
| 15 | Switzerland | 9 | 1591 | 176.78 | 0.58 | 6 | 66.67 | 29 | 2.22 |
| 16 | Thailand | 9 | 1369 | 152.11 | 0.50 | 6 | 66.67 | 18 | 2.22 |
| 17 | Qatar | 7 | 1639 | 234.14 | 0.77 | 5 | 71.43 | 11 | 1.72 |
| 18 | Spain | 6 | 2205 | 367.50 | 1.21 | 3 | 50.00 | 10 | 1.48 |
| 19 | Russian Federation | 6 | 2170 | 361.67 | 1.19 | 2 | 33.33 | 11 | 1.48 |
| 20 | Hong Kong | 5 | 1531 | 306.20 | 1.01 | 2 | 40.00 | 2 | 1.23 |
| 21 | South Korea | 5 | 1464 | 292.80 | 0.96 | 1 | 20.00 | 6 | 1.23 |
| 22 | Singapore | 5 | 1195 | 239.00 | 0.78 | 2 | 40.00 | 9 | 1.23 |
| 23 | Saudi Arabia | 5 | 838 | 167.60 | 0.55 | 1 | 20.00 | 5 | 1.23 |
| 605 | 205806 | 340.18 | 1.12 | 377 | 62.31 | ||||
| 406 | 123614 | 304.47 |
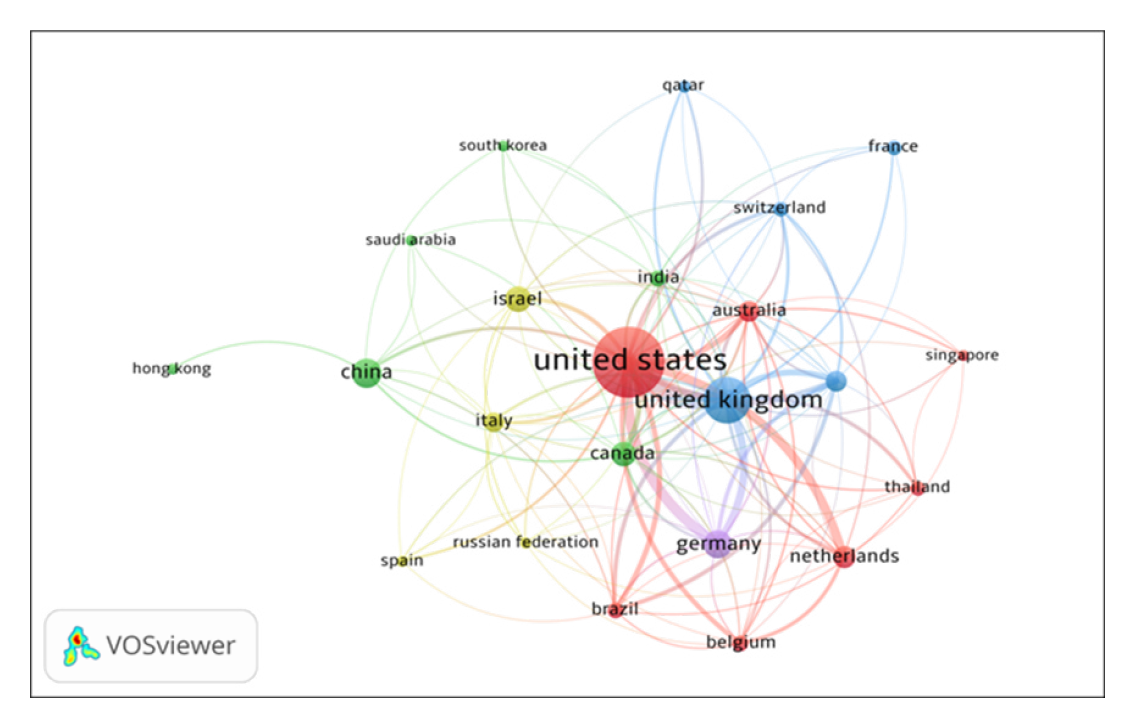
Figure 1.
Countries Collaborations Network Map of top 23 Countries.
The organization-to-organization collaborative linkages among top 32 organizations varied from 1 to 13, with highest collaborative linkages (13) depicted by institutional pair “National Institute of Allergy and Infectious Diseases, USA and National Institute of Health, USA”, followed by institutional pairs, such as “University of Oxford, U.K and Imperial College London, U.K.” (12 linkages), “Tel Aviv University, Israel and Chaimsheba Medical Center, Israel” (11 linkages), “University of Oxford, U.K and London School of Hygiene and Tropical Medicine, U.K.” (10 linkages), “University of Oxford, U.K and University College, London, U.K”., “MIT, USA and Massachusetts General Hospital, USA” and “Harvard Medical School, USA and Massachusetts General Hospital, USA” (9 linkages each), “Center for Disease Control and Prevention, USA and Stanford University School of Medicine, USA”, “Massachusetts General Hospital, USA and Brigham and Women’s Hospital, USA”, “University of Washington, USA and Stanford University School of Medicine, USA” and “Imperial College, London, U.K and University College London, U.K.” (7 linkages each), “John Hopkins University, USA and John Hopkins Bloomberg School of Public Health, USA” (6 linkages), etc.
| Sl. No | Name of the Organization | TP | TC | CPP | RCI | TLS | %TP |
|---|---|---|---|---|---|---|---|
| Top 8 Most Productive Organizations | |||||||
| 1 | University of Oxford, U.K. | 34 | 13994 | 411.59 | 1.35 | 31 | 8.37 |
| 2 | Imperial College, London | 25 | 10567 | 422.68 | 1.39 | 31 | 6.16 |
| 3 | Center for Disease Control and Prevention, USA | 19 | 3799 | 199.95 | 0.66 | 17 | 4.68 |
| 4 | Tel Aviv University, Israel | 19 | 4955 | 260.79 | 0.86 | 1 | 4.68 |
| 5 | London School of Hygiene and Tropical Medicine, U.K. | 19 | 9737 | 512.47 | 1.68 | 25 | 4.68 |
| 6 | University College London, U.K. | 19 | 8030 | 422.63 | 1.39 | 33 | 4.68 |
| 7 | Harvard Medical School, USA | 18 | 5696 | 316.44 | 1.04 | 19 | 4.43 |
| 8 | University of Washington, USA | 18 | 3889 | 216.06 | 0.71 | 47 | 4.43 |
| Top 8 Most Impactful Organizations | |||||||
| 1 | University of Cambridge, U.K. | 5 | 3917 | 783.4 | 2.57 | 21 | 1.23 |
| 2 | Emory University, USA | 10 | 7801 | 780.1 | 2.56 | 40 | 2.46 |
| 3 | John Hopkins Bloomberg School of Public Health, USA | 12 | 8432 | 702.67 | 2.31 | 9 | 2.96 |
| 4 | National Institute of Allergy and Infectious Diseases. USA | 17 | 11499 | 676.41 | 2.22 | 49 | 4.19 |
| 5 | Brigham and Women’s Hospital, USA | 11 | 7083 | 643.91 | 2.11 | 29 | 2.71 |
| 6 | Baylor College of Medicine, USA | 13 | 6904 | 531.08 | 1.74 | 21 | 3.2 |
| 7 | London School of Hygiene and Tropical Medicine, U.K. | 19 | 9737 | 512.47 | 1.68 | 25 | 4.68 |
| 8 | Chinese Academy of Sciences and Peking Union Medical College, China | 5 | 2241 | 448.2 | 1.47 | 6 | 1.23 |
The institutional collaboration network of top 32 organizations, using biblioshiny, is presented in Figure 2. By network analysis, the institutional co-authorship data has been presented in five clusters represented by different colors: (i) Cluster 1 (Green with 17 institutes), includes organizations such as University of Oxford, University of Washington, Imperial College London, University College London, University of Cambridge, Tel Aviv University, King’s College London, London School of Hygiene and Tropical Medicine and others; (ii) Cluster 2 (lavender with 11 institutions), includes organizations such as University of Pennsylvania, Harvard medical school, National Institute of Allergy and Infectious Diseases, Emory university, Johns Hopkins university, Massachusetts general hospital, and others; (iii) Cluster 3 (Red with 4 institutions), includes organizations such as University of Michigan, Ann Arbor, USA, INSERM, France, Chinese Academy of Sciences and Peking Union Medical College, China and Organisation Mondale de la Sante. All the 32 institutes have 145 links and 339 total link strengths.
Most Productive and Impactful Authors
In all 7086 authors participated in global COVID-19 vaccine research, of which 6808 contributed 1-5 papers each, 264 authors 6-10 papers each, 12 authors 11-15 papers each and 1 author 16 papers. The top 50 authors contributed 4 to 16 papers and these together contributed 383 papers which received 260590 citations, accounting for 94.33% and more than 100.0% share each in global publications and citations.
On further analysis, it was observed that 18 authors contributed more than the average group productivity (7.66) of all 50 authors: A.J. Pollard (16 and 3.94% share), T. Lambe (14 papers and 3.45% share), O. Tureci, P.R. Dormitzer and K.A.Swanson (12 papers and 2.96% share each), B. Hallis and S. Bibi (11 papers and 2.71% share each), D.H. Barouch, S.Belij-Ramnerstorfer, S.J. Dunachie, D. Cooper, K.J. Janseu, K.J. Janseu, P.K. Alley and E. Barnes (9 papers and 2.22% share each). Twenty eight authors have registered citations per paper and relative citation index above the group average (680.39 and 2.23) of all 50 authors: D. Cooper (1239.22 and 4.07), K.J. Janseu (1228.11 and 4.03), K.A. Swanson (987.0 and 3.24), P.R. Dormitzer (983.25 and 3.23), S.C. Gilbert and P. Cicconi (967.67 and 3.18 each), O. Tureci (965.08 and 3.17), S. Rhead (901.29 and 2.96), L. Corey (884.71 and 2.6), A.V.S. Hill , P.M. Folegatti, A.D. Douglas, D.Jenin, D. Jenin, S. Kerridge, (74 C. Dold , B.Angus, E.A. Clutterbuck and K.R.W. Emary, (851.14 and 1.72 each), E. Plested, H. Robinson, R. Song and K.J. Ever (836.76 and 1.72), M. Voysey (828.75 and 2.72), S. Bittaye (819.0 and 2.69), S.Belij – Ramnerstorfer and E. Barnes (759.22 and 2.49 each), S.J. Dunachie (712.33 and 2.34) and P.K. Alley (710.11 and 2.22). Table 3 presents the bibliometric profile of top 8 most productive and 8 most impactful authors.

Figure 2a.
Institutional Collaboration Network Map.
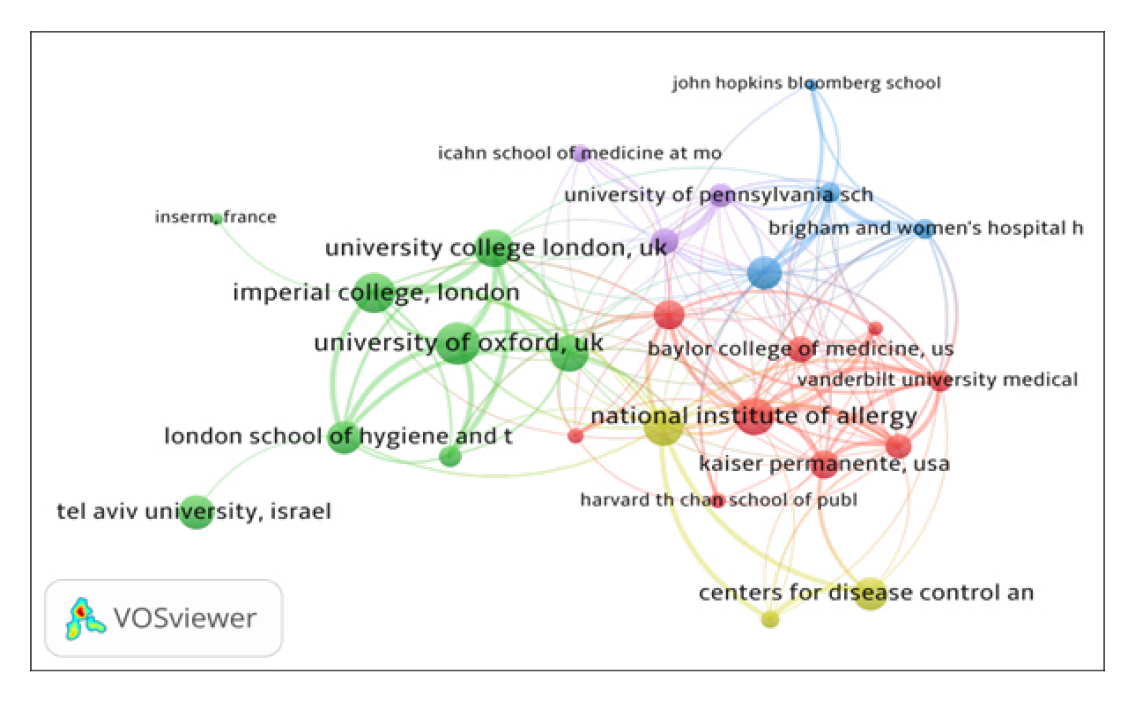
Figure 2b.
Institutional Collaboration Network Map.
The total link strength of top 50 authors varied from 1 to 200, with highest strength and intensity (200 links) depicted by S. Bibi, followed by A.J. Pollard (198 linkages), B. Hallis (197 linkages), C. Dold (194 linkages), P.K. Alley, H. Robinson and E.A. Cutterbuck (181 linkages each), E. Plested (176 linkages), E. Bames and S. Belij – Rammerstorfer (174 linkages each), M. Bittaye and S.J. Dunachie (172 linkages ach), M. Voysey (171 linkages), A.D. Douglas, K.J. Ewer, P.M. Follegatti, A.V.S. Hill, D. Jennkin, S. Kerridgen and R. Song (168 linkages each), etc.
The author to author collaborative linkages among top 50 authors varied from 1 to 11, with highest collaborative linkages and intensity (13 each) depicted by author pairs such as “A.J. Pollard and T. Lambe” and “A. Pollard and S. Bibi” and “T. Lambe and S.Bibi”, followed by “P.R. Dormitzer and K.A. Swanson”, “P.R. Dormitzer and O. Tureci” and “K.A. Swanson and O. Tureci (11 linkages each), “P.R. Dormitzer and K.U. Jensen”, “S. Bibi and C. Dold”, “P.R. Dormitzer and C. Cooper” and “S. Bibi and B. Hallis” (10 linkages each), etc.
The co-authorship network of top 50 authors, using VOSviewer, is presented in Figure 3. By network analysis, the co-authorship data has been presented in five clusters represented by different colors: (i) Cluster 1 (Red with 26 authors), includes P.K. Aley, B. Angus, E. Barnes, S. Belij – Rammerstorfer, S. Bibi, C. Dold, A.D. Douglas, S.J. Dunachie and others; (ii) Cluster 2 (Green with 11 authors) includes Bittaye M., Bell B.P., Gaglani M., Gee J. and others; (iii) Cluster 3 (Blue with 5 authors), includes Alter G., Barouch D.H., Corey L. and others; (iv) Cluster 4 (Lavender with 5 authors), includes Cooper D., Dormitzer P.R., Jansen K.U. and others); and (v) Cluster 5 (Yellow with 3 authors) includes Andrews N., Ramsay M. and, Stowe J. All the authors have 385 links and 2499 total link strengths.
Most Productive and Impactful Journals
The 406 HCPs were published in 121 journals, of which 106 journals published 1-5 papers each, 5 journals 6-10 papers each, 8 journals 11-20 papers each and 1 journal 53 papers. The top 30 most productive journals contributed 3 to 53 papers and together contributed 285 papers and 98795 citations, accounting for 70.2% and 79.29% share respectively of global COVID-19 papers and citations. The top 6 most productive journals were: New England Journal of Medicine (n=53), The Lancet (n=28), Nature (n=22), JAMA and Nature Medicine (n=17 each). The top 6 journals in terms of citations per papers were: New England Journal of Medicine (613.15), The Lancet (496.33), Human Vaccines and Immuno-therapeutic (369.67), Nature (360.37), European Journal of Epidemiology (341.8) and Cell (332.45). The top 6 journals by IF were The Lancet (29 papers) (IF=202.73), New England Journal of Medicine (53 papers) (IF=176.079), The BMJ (6 papers) (IF=96.216), The Lancet Infectious Diseases (10 papers) (71.421), Nature (22 papers) (69.504), etc. Table 4 presents the bibliometric profile of top 8 most productive and 8 most impactful journals.
Journals co-citation analysis is not only an effective way to study the structure and characteristics of research area, but also reveals the construction and characteristics of articles. VOSviewer software used to map the journals co-citation network of publications. Figure 4 shows the co-citation network of journals with 168 nodes and 969 total link strengths. The size of a node shows the activity of the journal and the number of articles published. The distance between two nodes also matters a lot. The smaller the distance between two nodes, the higher the citation frequency. All 30 journals were distributed in 5 clusters represented by various colors. (i) The Cluster 1 (Red color) includes journals such as Vaccine, Vaccines, European journal of Epidemiology, eClinical Medicine, PLOS One and so on. Here only specific medical journals are included; (ii) The Cluster 2 (Green color), includes journals such as JAMA – Journal of the American Medical Association, New England Journal of Medicine and Lancet, etc. Here multidisciplinary science and medical journals are included; (iii) The Cluster 3 (Blue color) includes journals such as ASC Nano, Cell, Nature communications, Science and others. This cluster represents science and technology journals; (iv) The Cluster 4 (Yellow) includes information journals; and (v) The Cluster 5 (Lavender) includes immunology journals.
| Sl. No | Author | Affiliation of the author | TP | TC | CPP | RCI | %TP |
|---|---|---|---|---|---|---|---|
| Top 8 Most Productive Authors | |||||||
| 1 | A.J. Pollard | University of Oxford, U.K. | 16 | 8899 | 556.19 | 1.83 | 3.94 |
| 2 | T. Lambe | University of Oxford, U.K. | 14 | 8671 | 619.36 | 2.03 | 3.45 |
| 3 | O. Tureci | BioNTechSE, Germany | 12 | 11581 | 965.08 | 3.17 | 2.96 |
| 4 | P.R. Dormitzer | BioNTechSE, Germany | 12 | 11799 | 983.25 | 3.23 | 2.96 |
| 5 | K.A. Swanson | University of Oxford, U.K. | 12 | 11844 | 987.00 | 3.24 | 2.96 |
| 6 | B. Hallis | University of Oxford, U.K. | 11 | 7214 | 655.82 | 2.15 | 2.71 |
| 7 | S. Bibi | University of Oxford, U.K. | 11 | 7379 | 670.82 | 2.20 | 2.71 |
| 8 | D.H. Barouch | Beth Israel Deaconess Medical Center, USA | 9 | 3063 | 340.33 | 1.12 | 2.22 |
| Top 8 Most Impactful Authors | |||||||
| 1 | D.Cooper | BioNTechSE, Germany | 9 | 11153 | 1239.22 | 4.07 | 2.22 |
| 2 | K.J. Janseu | BioNTechSE, Germany | 9 | 11053 | 1228.11 | 4.03 | 2.22 |
| 3 | K.A.Swanson | University of Oxford, U.K. | 12 | 11844 | 987.00 | 3.24 | 2.96 |
| 4 | P.R. Dormitzer | BioNTechSE, Germany | 12 | 11799 | 983.25 | 3.23 | 2.96 |
| 5 | S.C. Gilbert | University of Oxford, U.K. | 6 | 5806 | 967.67 | 3.18 | 1.48 |
| 6 | P.Cicconi | University of Oxford, U.K. | 6 | 5806 | 967.67 | 3.18 | 1.48 |
| 7 | O. Tureci | BioNTechSE, Germany | 12 | 11581 | 965.08 | 3.17 | 2.96 |
| 8 | S. Rhead | University of Oxford, U.K. | 7 | 6309 | 901.29 | 2.96 | 1.72 |
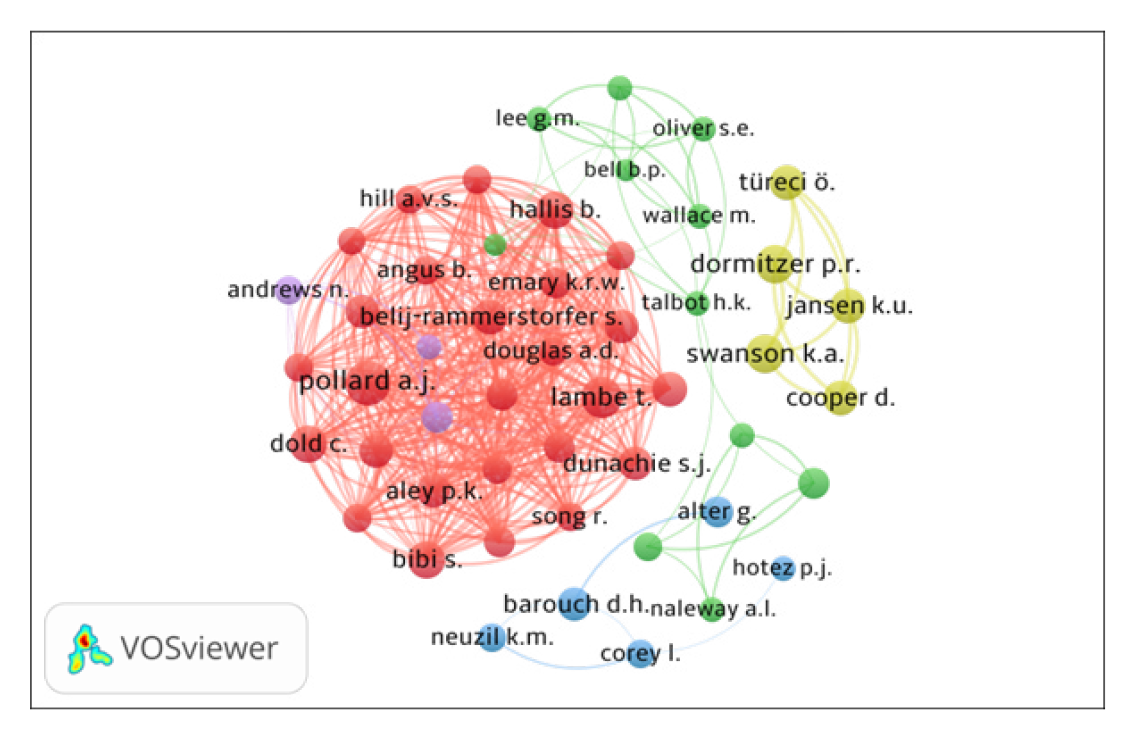
Figure 3.
Top 50 Authors Collaboration Network Map.
Significant Keywords
A total of 3302 author keywords were identified from the 406 HCPs on COVID-19 Vaccine, of which 1857 keywords occurred 1 time, 899 keywords 2-5 times, 231 keywords 6-10 times, 286 keywords 11-100 times and 29 keywords 101-388 times. The frequency of occurrence of keywords indicates their importance in this area. The 46 significant keywords selected with a frequency of 9 or more are presented in Table 5. Here keywords are arranged by number of occurrences. The leading significant keywords were: COVID-19 (388), COVID-19 Vaccines (357), Vaccination (221), Prevention and Control (181), Vaccine Immunogenicity (133), Drug Efficacy (121), etc.
The 46 significant keywords with a frequency equal to 9 or more were selected for co-occurrence network analysis and mapping using VOSviewer (Figure 5). The co-occurrence keyword analysis examined the frequency of two co-occurred keywords. The higher the frequency of co-occurrence of two words, the closer the relationship between them, indicated by the position of the two words. The size of the node indicates how often the keyword appeared with other keywords. Figure 5 presents divides the top 46 keywords in 3 clusters. All the keywords together have 936 links and 17423 total link strengths. Figure 6 presents the world cloud of 46 significant keywords.
Cluster 1 (Red with 16 keywords) includes keywords such as Neutralizing Antibody, Coronavirus Spike Glycoprotein, Virus Vaccine, Viral Vaccines, Genetics, Immune Response, RNA Vaccine, Virus Neutralization, Recombinant Vaccine, Synthetic Vaccines, MRNA Vaccine, RNA Messenger, Angiotensin Converting Enzyme, Virus Replication and Lipid Nanoparticle;
| Sl. No | Source | TP | %TP | TC | CPP | IF (2022) | TLS |
|---|---|---|---|---|---|---|---|
| Top 8 Most Productive Journals | |||||||
| 1 | New England Journal of Medicine | 53 | 13.054 | 32497 | 613.151 | 176.079 | 390 |
| 2 | The Lancet | 28 | 6.897 | 13899 | 496.393 | 202.731 | 264 |
| 3 | Nature | 22 | 5.419 | 7934 | 360.636 | 69.504 | 165 |
| 4 | Jama – Journal of the American Medical Association | 17 | 4.187 | 3758 | 221.059 | 69.504 | 96 |
| 5 | Nature Medicine | 17 | 4.187 | 4424 | 260.235 | 35.09 | 95 |
| 6 | Science | 14 | 3.448 | 4334 | 309.571 | 41.84 | 132 |
| 7 | Vaccines | 14 | 3.448 | 2960 | 211.429 | 4.82 | 100 |
| 8 | Morbidity and Mortality Weekly Report | 13 | 3.202 | 2572 | 197.846 | 17.586 | 21 |
| Top 8 Most Impactful Journals | |||||||
| 1 | New England Journal of Medicine | 53 | 13.054 | 32497 | 613.151 | 176.079 | 390 |
| 2 | The Lancet | 28 | 6.897 | 13899 | 496.393 | 202.731 | 264 |
| 3 | Human Vaccines and Immuno-therapeutics | 3 | 0.739 | 1109 | 369.667 | 4.526 | 6 |
| 4 | Nature | 22 | 5.419 | 7934 | 360.636 | 69.504 | 165 |
| 5 | European Journal of Epidemiology | 5 | 1.232 | 1709 | 341.8 | 8.082 | 20 |
| 6 | Cell | 11 | 2.709 | 3657 | 332.455 | 66.85 | 90 |
| 7 | The Lancet Infectious Diseases | 10 | 2.463 | 3269 | 326.9 | 71.421 | 84 |
| 8 | Viruses | 3 | 0.739 | 953 | 317.667 | 5.71 | 7 |
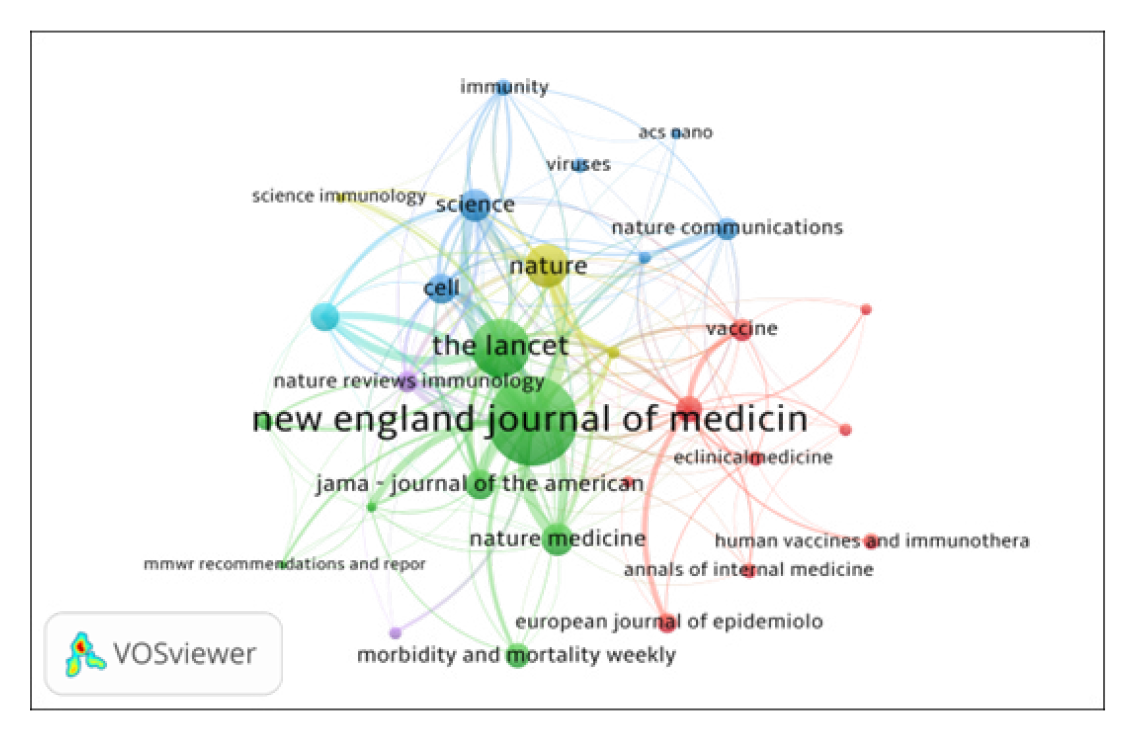
Figure 4.
Top 30 Journals Co-citation Network Map.
Cluster 2 (Green with 16 keywords) includes keywords such as COVID-19; COVID-19 Vaccines. Vaccination, Prevention and Control, Drug Efficacy, BNT162 Vaccine, Epidemiology, Tozinameran, Vaccine Hesitancy, Comirnaty, Elasomeran, Drug Therapy, Incidence, Vaxzevria, AD26.COV.S Vaccine and social media
Cluster 3 (Blue with 14 keywords) includes keywords such as Vaccine Immunogenicity, Virus Antibody, Drug Safety, Adverse Event, Antibody Response, Antibody Titer, Humoral Immunity, Immunization, Immunoglobulin G, Myalgia, Inactivated Vaccine, Arthralgia, Seroconversion and Immunosuppressive Treatment
SUMMARY
Bibliometric analysis is a powerful tool to identify, highlight, and identify quantitative information on research trends and relevance, including geographical location, author co-authorship, country, research institutions, research category, and citations. The present study utilized a bibliometric methods approach to retrieve and analyze the most important articles on COVID-19 vaccines and describe the trends in global COVID-19 vaccine research. We hope that this analysis will provide useful insight into the citation frequency of high-cited cited articles published in global scholars in this field to help recognize the quality of the works, discoveries, and the trends steering the study on COVID-19 vaccines. Vaccines are assumed to play an essential role in increasing population immunity, preventing severe disease, and reducing the ongoing health crisis. Because of this, pharmaceutical and vaccine-related research has experienced an unprecedented surge. This surge has also led to the proliferation of research articles on related subjects.
| SI. No | Keyword | Occurrences | Cluster | TLS | SNo | Keyword | Occurrences | Cluster | TLS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Covid-19 | 388 | Green | 3112 | 24 | Tozinameran | 55 | Green | 540 |
| 2 | Covid-19 Vaccines | 357 | Green | 2991 | 25 | Vaccines, Synthetic | 52 | Red | 587 |
| 3 | Vaccination | 221 | Green | 1921 | 26 | Immunization | 51 | Blue | 534 |
| 4 | Prevention And Control | 181 | Green | 1618 | 27 | Immunoglobulin G | 51 | Blue | 671 |
| 5 | Vaccine Immunogenicity | 133 | Blue | 1498 | 28 | MRNA Vaccine | 51 | Red | 575 |
| 6 | Drug Efficacy | 121 | Green | 1180 | 29 | Vaccine Hesitancy | 48 | Green | 228 |
| 7 | Neutralizing Antibody | 120 | Red | 1395 | 30 | Myalgia | 44 | Blue | 561 |
| 8 | Virus Antibody | 114 | Blue | 1354 | 31 | Messenger RNA | 38 | Red | 455 |
| 9 | Coronavirus Spike Glycoprotein | 107 | Red | 1179 | 32 | RNA Messenger | 38 | Red | 429 |
| 10 | Drug Safety | 98 | Blue | 1033 | 33 | Angiotensin Converting Enzyme | 35 | Red | 357 |
| 11 | Virus Vaccine | 94 | Red | 786 | 34 | Comirnaty | 30 | Green | 331 |
| 12 | BNT162 Vaccine | 85 | Green | 833 | 35 | Inactivated Vaccine | 30 | Blue | 327 |
| 13 | Viral Vaccines | 84 | Red | 730 | 36 | Elasomeran | 29 | Green | 310 |
| 14 | Genetics | 83 | Red | 863 | 37 | Arthralgia | 27 | Blue | 358 |
| 15 | Adverse Event | 80 | Blue | 777 | 38 | Drug Therapy | 23 | Green | 210 |
| 16 | Antibody Response | 79 | Blue | 937 | 39 | Incidence | 22 | Green | 215 |
| 17 | Epidemiology | 77 | Green | 598 | 40 | Seroconversion | 20 | Blue | 277 |
| 18 | Immune Response | 73 | Red | 775 | 41 | Virus Replication | 20 | Red | 189 |
| 19 | Antibody Titer | 66 | Blue | 788 | 42 | Vaxzevria | 19 | Green | 191 |
| 20 | RNA Vaccine | 60 | Red | 656 | 43 | AD26.COV.S Vaccine | 18 | Green | 181 |
| 21 | Virus Neutralization | 59 | Red | 689 | 44 | Immunosuppressive Treatment | 12 | Blue | 141 |
| 22 | Humoral Immunity | 58 | Blue | 661 | 45 | Lipid Nanoparticle | 10 | Red | 106 |
| 23 | Recombinant Vaccine | 58 | Red | 646 | 46 | Social Media | 9 | Green | 53 |
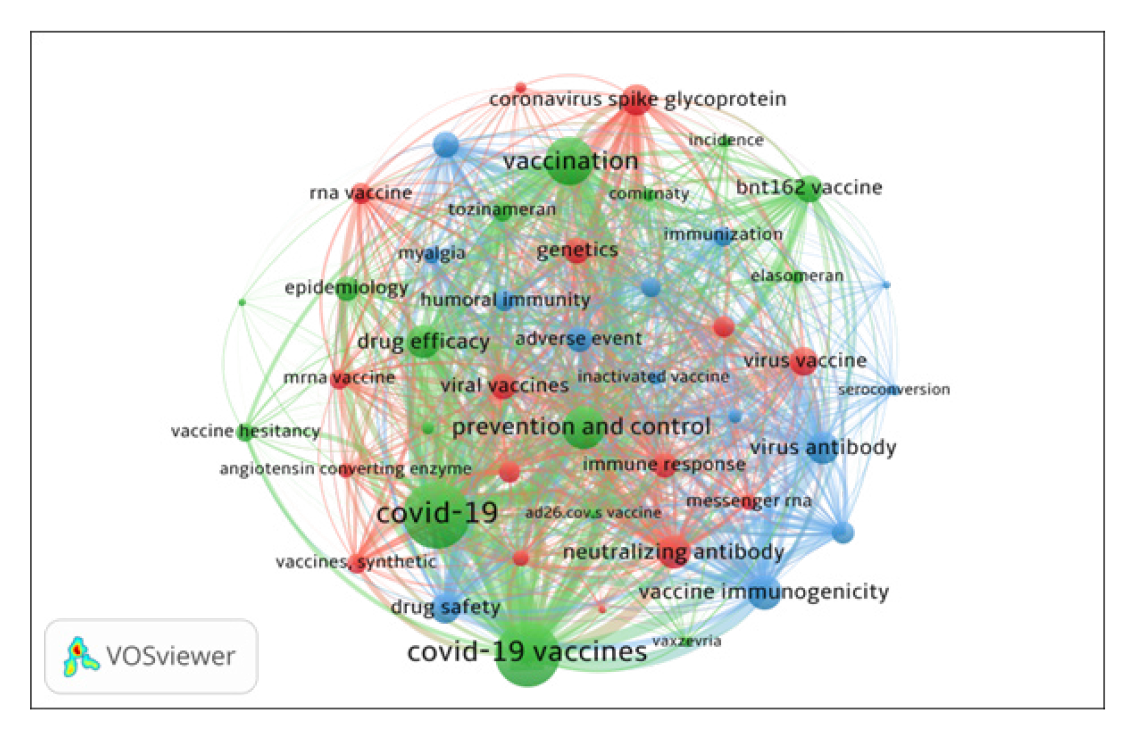
Figure 5.
Top 46 Selected Keywords Co-Occurrence Network.

Figure 6.
Word Cloud Map of 46 Significant Keywords.
The aggregate research publication was extracted from the Scopus database from 1 December, 2019 to 30 Nov 2022, a total of 406 high-cited papers (HCPs) were published in the database. The 406 HCPs were contributed by 7086 authors from 694 organizations affiliated to 76 countries and published in 121 journals. Majority of the retrieved high-cited articles consisted of articles (n = 277, 68.28%). Around 53% of 406 HCPs received external funding support from more than 100 funding agencies and registered 352.35 CPP. The largest funding support came from United States funding agencies, with higher funding support coming from National Institute of Health, USA (70 papers), National Institute of Allergy and Infectious Diseases, USA (51 papers), Pfizer, USA (29 papers), National Institute of Health Research (25 papers), Bill and Melinda Gates Foundation, USA (21 papers), etc. At country level, the active and productive countries with most papers were USA (n=213), U.K (n=91), China (n=36) and Germany (n=35), in contrast to most impactful countries with largest CPP and RCI were South Africa (968.56 and 3.53), Germany (609.62 and 2.22) and U.K. (381.47 and 1.39). At the organization level, the most prolific organizations were University of Oxford (34 papers), Imperial College, London (25 papers), Center for Disease Control and Prevention, USA, Tel Aviv University, Israel, London School of Hygiene and Tropical Medicine, U.K and University College London, U.K. and Harvard Medical School, USA (19 papers each) and the most impactful organizations in terms of CPP and RCI were University of Cambridge, U.K. (783.4 and 2.57), Emory University, USA (780.1 and 2.56), John Hopkins Bloomberg School of Public Health, USA (702.67 and 2.31) and National Institute of Allergy and Infectious Diseases. USA (676.41 and 2.22). At the author level, the most prolific authors were A.J. Pollard (n=16), T. Lambe (n=14), O. Tureci, P.R. Dormitzer and K.A.Swanson (n=12) and the most impactful authors in terms of CPP and RCI were D. Cooper (1239.22 and 4.07), K.J. Janseu (1228.11 and 4.03), K.A. Swanson (987.0 and 3.24) and P.R. Dormitzer (983.25 and 3.23). The leading journals in COVID-19 vaccine were New England Journal of Medicine (n=53), The Lancet (n=28), Nature (n=22), JAMA and Nature Medicine (n=17 papers each) and most impactful journals were New England Journal of Medicine (613.15), The Lancet (496.33), Human Vaccines and Immuno-therapeutic (369.67), Nature (360.37), European Journal of Epidemiology (341.8) and Cell (332.45). The most frequently used keywords, measured by the frequency of occurrences were Covid-19 (n=388), Covid-19 Vaccines (n=357), Vaccination (n=221), Prevention and Control (n=181), Vaccine Immunogenicity (n=133) and Drug Efficacy (n=121). Specific themes were identified in the conceptual mapping of the 46 significant keywords through co-occurrence analysis, which were distributed across 3 major clusters representing the sub-themes in COVID-19 vaccine research.
The interaction among productive countries, organizations, authors, and keywords were analyzed. In terms of Total Link Strength (TLS), the countries with most collaborative linkages were USA (183), U.K. (123), Germany (62) and South Africa (56). Among them, USA was the center of star attraction in collaborative linkages with other countries: USA-U.K. (42 linkages), USA-Germany (21 linkages), USA-Netherland (17 linkages), U.K.-Germany (14 linkages), USA-South Africa (13 linkages), U.K and South Africa (11 linkages), etc.
Among participating organizations, USA and U.K. top organizations depicted the largest number of collaboration linkages and also co-author linkages. Also their co-authorship analysis indicates their presence in five clusters with strong collaborative linkages among organizations in each cluster.
Our analysis highlights the characteristics of the most influential articles on COVID-19 vaccine research. The findings provided valuable insight into the scientific research progress in this domain and suggest scaling-up research and development, increased collaboration and improved information dissemination on COVID-19 vaccine research.
Finally, it is assumed that the findings of this study provide global status, research hotspots and potential trends in the field of COVID-19 vaccines, which may be helpful for researchers and policymakers in mastering the knowledge structure, and evaluating and guiding the future development trends of global COVID-19 vaccine research. The study may also be helpful to rapidly identify the potential partners, landmark studies and research topics within their domains of interest. It is also helpful for funding agencies to assess ongoing research and future research trends in COVID-19 vaccines. Effective vaccine development and treatment therapy is still a hot zone for future research directions.
Limitations
This study has several limitations. First of all, different researchers may use different words to express the same thing, and thereby some publications with varied expressions may be excluded while collecting publications from Scopus database. Furthermore, Scopus is used as database for amassing publications in this study, and some publications only included in other databases (e.g., Scopus, PubMed and Google Scholar) may be excluded. We, however, believe that the use of the Scopus database for analysis provides a robust insight for understanding research performance focusing on COVID-19 Vaccine, as it covers all PubMed/Medline publications. Furthermore, mixing the information and data derived from the other sources would have been heterogeneous making the scientometric analysis complex and inaccurate.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- WHO. [Accessed on 2.2.23];COVID-19 vaccines.
https://www.who.int/teams/regulation-prequalification/eul/covid-19 - WHO Coronavirus (COVID-19) Dashboard. 2023
https://covid19.who.int/ - Yap C, Ali A, Prabhakar A, Prabhakar A, Pal A, Lim YY, Kakodkar P, et al. Comprehensive literature review on Covid-19 vaccines and role of SARS-CoV-2 variants in the pandemic. Therapeutic Advances in Vaccines and Immunotherapy, Nov 2021. 2021 [CrossRef] | [Google Scholar]
- WHO. The COVID-19 vaccine tracker and landscape. 2023
https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines - WHO. 2021
https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained
The different types of COVID-19 vaccines. - Sarirete A. A bibliometric Analysis of COVID-19 vaccines and sentiment analysis. Procedia Computer Science. 2021;194:280-7. [CrossRef] | [Google Scholar]
- Srivastava PR. Intellectual structure and publication pattern in Journal of Global Information Management: A bibliometric analysis during 2002-2020. Journal of Global Information Management. 2021;29(4):1-31. [CrossRef] | [Google Scholar]
- Tchuente Dieudonne, Nyawa Serge, Wamba Samuel Fosso. COVID-19 vaccine global information management through bibliometrics. Journal of Global Information Management. 2021;29(6):22 [CrossRef] | [Google Scholar]
- Yi K, Xu JG, Yang KL, Zhang X, Ma L, You T, et al. The top-100 most cited articles of biomarkers in congenital heart disease: A bibliometric analysis. Ann Palliat Med. 2021;11:1700-13. [CrossRef] | [Google Scholar]
- Shah SM, Ahmad T, Chen S, Yuting G, Liu X, Yuan YA, et al. bibliometric analysis of the one hundred most cited studies in psychosomatic research. Psychother Psychosom. 2021;90:425-30. [CrossRef] | [Google Scholar]
- Wohlin C. An Analysis of the most cited articles in software engineering journals – 1999. Inf Softw Technolog. 2005;47(15):957-64. [CrossRef] | [Google Scholar]
- Fardi A. Top-cited articles in endodontic journals. J Endo. 2011;37(9):1183-90. [CrossRef] | [Google Scholar]
- Gupta BM, Dhawan SM, Ahmed KK Mueen, Mamdapur Ghouse Modin. Global research on Covid-19 disease: A scientific assessment of publications during 2020-21. International Journal of Medicine and Public Health. 2021;11(2):76-84. [CrossRef] | [Google Scholar]
- Sa’ed HZ, Al-Jabi SW. Mapping the situation of research on coronavirus disease-19 (COVID-19): A preliminary bibliometric analysis during the early stage of the outbreak. BMC Infect Dis. 2020;20:561 [CrossRef] | [Google Scholar]
- Chahrour M, Assi S, Bejjani M, Nasrallah AA, Salhab H, Fares M, et al. A bibliometric analysis of COVID-19 research activity: A call for increased output. Cureus. 2020;12(3):e7357 [CrossRef] | [Google Scholar]
- Yu Y, Li Y, Zhang Z, Gu Z, Zhong H, Zha Q, Yang L, Zhu C, Chen EA, et al. bibliometric analysis using VOSviewer of publications on COVID-19. Ann Transl Med. 2020;8(13):816 [CrossRef] | [Google Scholar]
- Lou J, Tian SJ, Niu SM, Kang XQ, Lian HX, Zhang LX, et al. Coronavirus disease 2019: A bibliometric analysis and review. Eur Rev Med Pharmacol Sci. 2020;4(6):3411-21. [CrossRef] | [Google Scholar]
- Mao X, Guo L, Fu P, Xiang C. The status and trends of coronavirus research: A global bibliometric and visualized analysis. Medicine. 2020;99(22):e20137 [CrossRef] | [Google Scholar]
- Tao Z, Zhou S, Yao R, Wen K, Da W, Meng Y, et al. COVID-19 will stimulate a new coronavirus research breakthrough: A 20-year bibliometric analysis. Ann Transl Med. 2020;8(8):528
https://atm.amegroups.com/article/view/40214/html
[CrossRef] | [Google Scholar] - Zhai F, Zhai Y, Cong C, Song T, Xiang R, Feng T, et al. Research progress of coronavirus based on bibliometric analysis. Int J Environ Res Public Health. 2020;17(11):3766 [CrossRef] | [Google Scholar]
- Zhou Y, Chen L. Twenty-year span of global coronavirus research trends: A bibliometric analysis. Int J Environ Res Public Health. 2020;17(9):3082 [CrossRef] | [Google Scholar]
- Ahmad T, Murad MA, Baig M, Hui J. Research trends in COVID-19 vaccine: A bibliometric analysis. Hum Vaccin Immunother. 2021;17(8):2367-72. [CrossRef] | [Google Scholar]
- Gan P, Pan X, Huang S, Xia H, Zhou X, Tang X, et al. Current status of coronavirus disease 2019 vaccine research based on bibliometric analysis. Hum Vaccin Immunother. 2022;18(6):2119766 [CrossRef] | [Google Scholar]
- Wei W-T, Wei C-K, Wu C-C. Trends in research about COVID-19 vaccine documented through bibliometric and visualization analysis. Healthcare. 2022;10:1942 [CrossRef] | [Google Scholar]
- Chen Y, Cheng L, Lian R, Song Z, Tian J. COVID-19 vaccine research focuses on safety, efficacy, immunoinformatics, and vaccine production and delivery: A bibliometric analysis based on VOSviewer. Biosci Trends. 2021;15:64-73. [CrossRef] | [Google Scholar]
- Xu Z, Qu H, Ren Y, Gong Z, Ri HJ, Zhang F, Chen X, Zhu W, Shao S, Chen X, et al. Update on the COVID-19 vaccine research trends: A bibliometric analysis. Infect Drug Resist. 2021;14:4237-47. [CrossRef] | [Google Scholar]
- Noruzi A, Gholampour B, Gholampour S, Jafari S, Farshid R, Stanek A, Saboury AA, et al. Current and future perspectives on the COVID-19 vaccine: A scientometric Review. J Clin Med. 2022;11:750 [CrossRef] | [Google Scholar]
- Alkan-ęeviker S, Öntürk H, Alıravcı ID, Sıddıkoğlu D. Trends of Covid 19 vaccines: International collaboration and visualized analysis. Infect Dis Clin Microbiol. 2021;3:129-36. [CrossRef] | [Google Scholar]
- Gupta BM, Mueen Ahmed KK. Covid 2019 vaccine: A scientometric assessment of global publications during 2020. Journal of Young Pharmacists, Journal of Young Pharmacists. 2022;14(1):37-45. [CrossRef] | [Google Scholar]
- Zeng D, Wang J, Xiao B, Zhang H, Ma X. A Bibliometric Visualization Analysis on Vaccine Development of Coronavirus Disease 2019 (COVID-19). Vaccines. 2023;11:295 [CrossRef] | [Google Scholar]
