ABSTRACT
Hepatocellular Carcinoma (HCC) is the most common type of liver cancer and can lead to multiple complications if left untreated. Disease incidence has increased and is expected to continue doing so in the coming years. However, treatment is expensive, which limits its application in poorer communities. Consequently, this study aimed to evaluate the cost-effectiveness of the combination of atezolizumab and bevacizumab versus sorafenib in the treatment of HCC. We performed a systematic review of published economic evaluation studies investigating the cost-effectiveness of atezolizumab and bevacizumab versus sorafenib using the following databases: PubMed, NHS Economic Evaluation, Cochran, and Scopus. This study was conducted according to the PRISMA reporting guidelines. Three independent reviewers screened all relevant articles published before March 2022. The Cheers checklist was used to assess the quality of the selected studies, and all relevant outcomes were assessed. Six studies met the inclusion criteria. QALY ranged from 0.811 to 2.7 in the combination group, and from 0.84 to 1.021 in the sorafenib group. The incremental cost-effectiveness ratio varied from $104,242/LY to $250,907/LY and from $61,613/QALY to $322,500/QALY. We concluded that atezolizumab plus bevacizumab was no more cost-effective than sorafenib, with the major contributing factor being the price of medication; thus, a reduction in price may improve cost-effectiveness. Further studies are needed that consider a greater variety of middle-to-low income populations in both clinical trial and practice.
INTRODUCTION
Hepatocellular Carcinoma (HCC) is the most common active type of liver cancer worldwide and is likely to be fatal if left untreated. Thus, early diagnosis is crucial for patient survival. The incidence of the disease is high in various countries, especially among individuals with untreated liver diseases.1 The disease has a high prevalence in Asia and Europe, and rates of HCC have drastically increased in both males and females—from 2.7 per 100,000 males in 1997 to 8.8 per 100,000 in 2016 and from 0.8 per 100,000 females in 1997 to 2.2 per 100,000 in 2016, respectively.2 Currently, the number of cases is 905,683 worldwide and already constitutes a considerable financial burden on patients. However, this number is expected to surpass 1 million in the next three to four years.2 Given the economic burden of HCC management, many patients are unable to afford the high cost of treatment, leading to further deterioration of liver function and possibly death. Thus, the identification of high risk individuals could be vital in preserving their financial security and overall survival.4 Among the risk factors is the presence of scar tissue caused by infection with hepatitis B or C. Viral load plays a critical role in the development of liver carcinoma. Although vaccines are important in protecting against infection, vaccinated individuals can still be infected.5 Early diagnosis (with immunoassays and biopsies) and treatment of hepatitis B or C infections could prevent HCC. Otherwise, the primary prevention measures for HCC are screening and diagnosis, which help in preventing the many complications caused by liver cirrhosis.5,6
Tumor resection is the most effective treatment option. In conditions where surgery is dangerous or ineffective, radiation, ablation, or transplants, may be considered. Medications play a key role in the treatment of unresectable tissue.7 Sorafenib was the first approved medication to treat HCC and the only systemic agent available. Sorafenib—classified as a tyrosine kinase, angiogenesis, immune checkpoint, and vascular endothelial growth factor inhibitor—works by blocking the growth of blood vessels that support cancer cells, preventing metastasis of liver cancer cells, and decreasing the functioning of cancer cells.1 However, the cost of this medication is high. Lenvatinib—a multiple kinase receptor inhibitor—is an alternative treatment,8 but most clinicians use it only as a second-line treatment because it is inferior to sorafenib. Recently, a new systemic therapy comprising atezolizumab and bevacizumab has emerged. It was approved by the Food and Drug Administration (FDA) on May 29, 2020, and was added to treatment guidelines worldwide. These biological agents have a dual mechanism of action. Atezolizumab prevents PD-L1 from interacting with B7-1 receptors, thereby increasing T cell activity,10 bevacizumab targets vascular endothelial growth factor 1, leading to inhibition of angiogenesis and tumor growth.8 Their combined use has shown better outcomes than standard therapy, making it a potential agent to replace sorafenib. However, this combined treatment is extremely expensive and lacks a comprehensive cost-effectiveness analysis.11
Various biological therapies that target PD-L1 have been developed and have shown promising results in the treatment of multiple cancers. However, their high cost limits their clinical application.11 Moreover, little is known of the long-term cost-effectiveness of these medications. Consequently, this systematic review aimed to determine the cost-effectiveness (i.e., economic costs versus clinical outcomes) of the combination of atezolizumab and bevacizumab versus sorafenib for patients with HCC. Our findings could aid clinical institutions in prioritizing resources and reducing expenses while providing patients with cost- and clinically effective treatments.
METHODOLOGY
Study design and data sources
This systematic review collates the existing literature concerning the cost-effectiveness of atezolizumab and bevacizumab combination therapy versus sorafenib in the treatment of HCC. The study design was based on the PRISMA guidelines. We used four databases (PubMed, Google Scholar, Cochrane Library, and Scopus) to identify all publications published prior to March 2022 that are associated with the following keywords: cost-effectiveness analysis, cost-utility analysis, cost-benefit analysis, economic evaluation, atezolizumab plus bevacizumab, atezolizumab, bevacizumab, hepatocellular carcinoma, hepatic carcinoma, and liver cancer). We also conducted additional searches of the gray literature, such as government reports, policy statements, and conference-published abstracts.
Study selection
Only studies published in English were considered. The search results from the databases were merged and duplicates were removed. The reviewers (B.B., A.A., and F.A.) screened the title and abstract of each article independently according to the specific inclusion and exclusion criteria of this review. All studies directly related to the economic evaluation of atezolizumab and bevacizumab treatments in patients with HCC were included. No restrictions were placed on sex, age, or nationality. We excluded all studies reporting only outcomes, partial economic evaluations (cost analysis, cost minimization), budget impact and cost-of-illness studies, and non-original articles (editorials, commentary, reviews, conference proceedings). If a study could not be excluded based on the title and abstract, the full text was reviewed to determine eligibility. Any inconsistencies between reviewers were resolved by discussion to minimize the exclusion of relevant studies.
Data extraction and quality management
The purpose of data synthesis in the review was to examine the outcomes of economic evaluation studies. Two reviewers (A.A. and F.A.) independently extracted the data from each study into an Excel sheet, while another (B.B.) reviewed the dataset for discrepancies. Data extracted from each included the title, study design, population, setting, perspective, intervention, outcomes, cost threshold, time horizon, and type of cost. Owing to the nature of economic evaluation and heterogeneity among studies, a quantitative meta-analysis was not performed, as there are methodological complications in conducting meta-analyses of economic evaluations. Accordingly, we did not pool the incremental effects.
Quality of reporting assessment
Quality assessment of the selected economic evaluations was performed using the ISPOR CHEERS checklist (Consolidated Health Economic Evaluation Reporting Standards) (Figure 1).
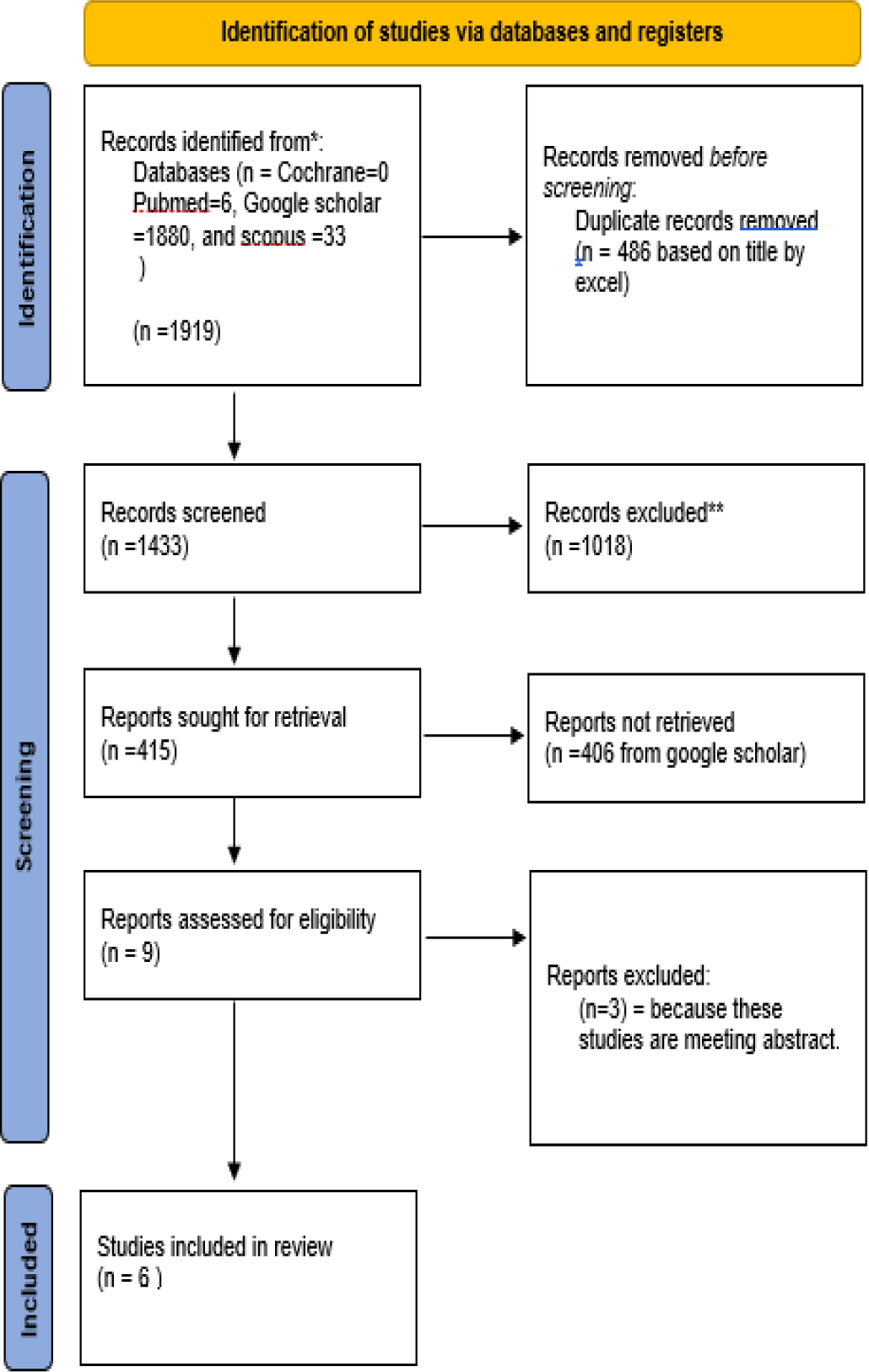
Figure 1.
PRISMA flowchart for article selection process.
RESULTS
Study characteristics
The literature search identified 1,919 studies, of which six studies were eligible (Figure 1). The characteristics of the eligible studies are shown in Table 1, which includes study setting, intervention and comparator, perspective, and cost analysis, with a discount rate of 3%. The study populations were based on the IMbrave150 trial, and the total number of participants was 501; 336 were assigned to the atezolizumab plus bevacizumab group, and 165 were assigned to the sorafenib group. Large variations between studies were observed in terms of population, setting, and intervention; all studies were conducted in China, except one study conducted in both the USA and China.
| Study | Population | Setting | Perspective | Comparator | Outcomes | Intervention | Time Horizon | Model Type | Cost Threshold |
|---|---|---|---|---|---|---|---|---|---|
| (Li, et al., 2021)12 | Simulate IMbrave150 trial | China | Payer Perspective | Sorafenib 400 mg twice daily | Life-years QALY | Atezolizumab 1200 mg/ kg every 3 weeks Bevacizumab 15mg /kg every 3 weeks | NA | Markov Model | $150,000/QALY |
| (Wen, et al., 2021)10 | Simulate IMbrave150 trial | USA and China | Payer Perspective | Sorafenib 400 mg twice daily | QALY | Atezolizumab 1200 mg/ kg every 3 weeks Bevacizumab 15mg /kg every 3 weeks | 10 years | Markov Model | $28,527.00/QALY in the China. $150,000.00/QALY in the USA. |
| (Hou & Wu, 2020)5 | Simulate IMbrave150 trial | China | Chinese health sector Perspective | Sorafenib 400 mg twice daily | Life-years QALY | Atezolizumab 1200 mg/ kg every 3 weeks Bevacizumab 15mg /kg every 3 weeks | NA | Survival model | $30,828/QALY |
| (Chiang, Chan, Lee, & Choi, 2021)1 | Simulate IMbrave150 trial | China | Payer Perspective | Sorafenib 400 mg twice daily | QALY | Atezolizumab 1200 mg/ kg every 3 weeks Bevacizumab 15mg /kg every 3 weeks | 5 years | Markov model | 150,000/QALY and 100,000/QALY |
| (Su, Wu, & Shi, 2021)13 | Simulate IMbrave150 trial | China | Payer Perspective | Sorafenib 400 mg twice daily | Life-years QALY | Atezolizumab 1200 mg/ kg every 3 weeks Bevacizumab 15mg /kg every 3 weeks | 10 months | Decision tree combining with survival model | 150,000/QALY |
| (Zhang, et al., 2021)14 | Hypothetical number 424 (266 were on Atezolizumab + bevacizumab and 158) | China | Payer Perspective | Sorafenib 400 mg twice daily | QALY | Atezolizumab 1200 mg/ kg every 3 weeks Bevacizumab 15mg /kg every 3 weeks | 6 years | survival model | 150,000/QALY and 100,000/QALY |
All of the studies considered direct medical costs incurred on the payer. (Direct nonmedical or indirect costs were not reported in these studies). The intervention arm was a combination of atezolizumab and bevacizumab and the comparator was sorafenib. The outcomes varied between the studies, three QALY and life years (LY), and three used QALY only. Three studies employed the Markov model, whereas the other three used the survival model (Table 2). The cost-effectiveness threshold varied from $28.527/QALY,10 to $150,000/QALY,10,12,13 (Table 2).
| Study | QALY | Life Years | ICER |
|---|---|---|---|
| (Li, et al., 2021)12 | For Combination = 2.7 QALYFor sorafenib = 1.86 QALY | For Combination = 3.46 LYFor sorafenib = 2.29 LYIncremental = 1.17 LY | ICER = $288,663.04/QALYIf the price of atezolizumab were reduced by 75%, the probability of atezolizumab being cost-effective was over 50% at threshold ($150,000 per QALY)Also bevacizumab if reduce the price to 50% that will make the regimen more cost-effective |
| (Wen, et al., 2021)10 | For combinationIn China:PD state = 0.76 QALYSD state = 0.64 QALYTotal = 1.4 QALYIn USA:PD state = 0.64 QALYSD state =0 .76 QALYTotal = 1.40 QALYfor SorafenibIn China =PD state = 0.37 QALYSD state = 0.50 QALYTotal = 0.87 QALYIn USA =PD state = 0.37 QALYSD state = 0.50QALY Total = 0.87 QALY | For CombinationPD state = 1.11 LYSD state = 0.84 LYTotal = 1.96 LYFor sorafenibPD state = 0.74 LYSD state = 0.49 LYTotal = 1.22 | Cost/LY = 104,242.42$ Cost /QALY = 145,546.21$ in China.Cost/Years = 120,345.96$Cost/QALY = $168,030.21 in the USA.A reduction in price to make this combination cost-effective.if the price of atezolizumab was 30% of the primary price, the total cost for both groups was the same. |
| (Hou & Wu, 2020)5 | For Combination = 1.984 QALYSorafenib = 1.173 QALY | For Combination = 3.033 LYSorafenib = 1.736 LY | ICER = 61,613$/QALYIf the cost of Atezolizumab and bevacizumab is reduced by 50% then it will be cost-effective |
| (Chiang, Chan, Lee, & Choi, 2021)1 | For Combination1.426 QALY on base case group1.227 QALY on pessimistic group1.825 QALY on Optimistic groupFor sorafenib = 0.987 QALY | For Combination2.02 LY on base case group1.78 LY on Pessimistic Survival group2.48 LY on Optimistic survival groupFor sorafenib = 1.51 LY | Cost/LY = 155,047$Cost/QALY = 179,729$Price reduction would recommended to being cost-effective. |
| (Su, Wu, & Shi, 2021)13 | For Combination = 1.551 QALYFor sorafenib = 1.021 QALY | For Combination0.938 LY on Progression-free groupOverall = 3.033 LYFor sorafenib0.548 LY on Progression-free groupOverall = 1.736 LY | NA |
| (Zhang, et al., 2021)14 | For Combination = 1.412 QALYFor sorafenib = 0.928 QALY | For Combination = 1.840 LYFor sorafenib = 1.218 LY | Cost/LY = 250,907$Cost/QALY = 322,500$Price reduction would recommended to being cost-effective. |
The cost of each medication varied from $3690.13 to $240,137 for atezolizumab and bevacizumab and from $2328.05 to $109,355 for sorafenib (Table 3). The incremental cost varied between China and the USA as well as according to the use of a drug as the first- or second-line treatment.
| Study | Atzolizumab Price ($USD) | Bevacizumab Price ($USD) | Sorafenib Price ($USD) | Total Cost ($USD) | Study References in Price |
|---|---|---|---|---|---|
| (Li, et al., 2021)12 | Cost Per Cycle: 9192 | Cost Per Cycle: 367.87 | Cost Per Cycle: 10656.64 | Combination cost: 48,3673.34Sorafenib cost: 241,225.94Incremental: 242447.40 | The drug prices were obtained from the Red Book. According to NCCN guideline recommendations. |
| (Wen, et al., 2021)10 | (In CHINA)Unit Price = 4638.01Per Cycle = 4638.01Monthly cost = 6184.01(In USA)Unit Price = 10,816.50Per Cycle = 10,816.50Monthly cost = 12,500.00Note: All costs were converted into US dollars, with an exchange rate of $1 =¥7.07 | (In CHINA)Unit Price = 212.10Per cycle = 1908.90Monthly cost = 2545.25(In USA)Unit Price = 841.51Per Cycle =7573.79Monthly cost =10,098.12Note: All costs were converted into US dollars, with an exchange rate of $1 =¥7.07 | Cost in CHINA= 2328.05Cost in theUSA=18,847.00Note: All costs were converted into US dollars, with an exchange rate of $1 = ¥7.07 | In ChinaCombination = 95,972.83Sorafenib = 18,833.34Incremental cost = 77,139.49In the USA Combination =283,304.15Sorafenib = 194,248.14Incremental cost 89,056.01 | Prices in China were from the Chinese national pricePrices in the USA were sourced from the Red book. |
| (Hou & Wu, 2020)5 | For Combination, not each medication alone = 65,172 | For Combination, not each medication alone = 65,172$ | 15,178 | NA | From Database: Mahipal A, Tella SH, Kommalapati A, Lim A, Kim R. Immunotherapy in Hepatocellular Carcinoma: Is There a Light at the End of the Tunnel? Cancers (Basel). 1078 2019;11(8). |
| (Chiang, Chan, Lee, & Choi, 2021)1 | Atezolizumab (1200 mg) (fixed) (every 3 weeks): 9419.16 cost of post-therapy per cycle: 4612.66 | Bevacizumab (15 mg/kg) (every 3 weeks)=117.60cost of post-therapy per cycle: 4612.66 | Sorafenib (every 3 weeks)14,609.28cost of post-therapy per cycle:4825.93 | NA | Academy of Managed Care Pharmacy.U.S. Department of Veterans Affairs |
| (Su, Wu, & Shi, 2021)13 | They mention the cost of the combination only:As First Line = $14 ,210$ As Second= $78,570 | They mention the cost of the combination only:As First Line = 214 ,210$ As Second or other = 78,570$ | As First Line = 109,355As Second or other = 93 619 | Total cost of combination = 292,780Sorafenib = 202,973 | The prices were collected from public databases in USA:Centers for Medicare & Medicaid Services. 2019 ASP drug pricing files Red Book |
| (Zhang, et al., 2021)14 | They mention the cost of the combination only:As drug = 240,137As non-drug = 73,056 | They mention the cost of the combination only:As drug = 240,137As non- drug=73,056 | As drug = 94,920As non-drug= 62,064 | Total cost of combination = 313,193$Total cost of sorafenib = 156,984Total Incermental change = 156,210 | Atezolizumab and bevacizumab costs were obtained from the Centers for Medicare and Medicaid Services.Sorafenib cost was obtained from Micromedex Redbook. |
The included articles reported the QALYs, LY, and ICER.5 The outcome values varied between studies. The QALY ranged from 0.811 to 2.7 in the combination group and from 0.84 to 1.021 in the sorafenib group. QALY also varied between subgroups of progressive disease state versus stable disease state and pessimistic group versus optimistic group (Table 2).
The ICER was estimated for some studies in two ways per LY or per QALY, ranging from $104,242.42/LY to $250,907/LY and from $61,613/QALY to $322,500/QALY. Some studies calculated the ICER using only the QALY.12
Quality assessment results
The majority of economic evaluation studies were of good quality. The quality scores ranging from 24 to 26 out of 28 (Table 4). One study was of low quality, with a score of 16 out of 28. All of the studies clearly described the population characteristics and competing alternatives while also having a clear objective.
| ITEM | Guidance of the report | (Li, et al., 2021)12 | (Wen, et al., 2021)13 | (Hou & Wu, 2020) 14 | (Chiang, Chan, Lee, & Choi, 2021) 15 | (Su, Wu, & Shi, 2021) 16 | (Zhang, et al., 2021)17 | |
|---|---|---|---|---|---|---|---|---|
| TITLE | ||||||||
| Title | 1 | Identify the study as an economic evaluation and specify the interventions being compared. | √ | √ | √ | √ | √ | √ |
| ABSTRACT | ||||||||
| Abstract | 2 | Provide a structured summary that highlights context, key methods, results and alternative analyses. | √ | √ | × | √ | √ | √ |
| INTRODUCTION | ||||||||
| Background and Objective | 3 | Give the context for the study, the study question and its practical relevance for decision making in policy or practice. | √ | √ | × | √ | √ | √ |
| METHOD | ||||||||
| Health economic analysis plan | 4 | Indicate whether a health economic analysis plan was developed and where available. | √ | √ | √ | √ | √ | √ |
| Study population | 5 | Describe characteristics of the study population (such as age range, demographics, socioeconomic, or clinical characteristics). | √ | √ | √ | √ | √ | √ |
| Setting and location | 6 | Provide relevant contextual information that may influence findings. | √ | √ | √ | √ | √ | √ |
| Comparators | 7 | Describe the interventions or strategies being compared and why chosen. | √ | √ | √ | √ | √ | √ |
| Perspective | 8 | State the perspective(s) adopted by the study and why chosen. | √ | √ | √ | √ | √ | √ |
| Time horizon | 9 | State the time horizon for the study and why appropriate. | × | √ | × | √ | √ | √ |
| Discount rate | 10 | Report the discount rate(s) and reason chosen. | √ | √ | × | √ | √ | √ |
| Selection of outcome | 11 | Describe what outcomes were used as the measure(s) of benefit(s) and harm(s). | √ | √ | √ | √ | √ | √ |
| Measurement of outcome | 12 | Describe how outcomes used to capture benefit(s) and harm(s) were measured. | √ | √ | √ | √ | √ | √ |
| Valuation of outcome | 13 | Describe the population and methods used to measure and value outcomes. | √ | √ | √ | √ | √ | √ |
| Measurement and valuation of resources and costs | 14 | Describe how costs were valued. | √ | √ | √ | √ | √ | √ |
| Currency, price date, and conversion | 15 | Report the dates of the estimated resource quantities and unit costs, plus the currency and year of conversion. | √ | √ | × | √ | √ | × |
| Rationale and description of model | 16 | If modelling is used, describe in detail and why used. Report if the model is publicly available and where it can be accessed. | √ | √ | × | √ | √ | √ |
| Analytics and assumptions | 17 | Describe any methods for analysing or statistically transforming data, any extrapolation methods, and approaches for validating any model used. | √ | √ | × | √ | √ | √ |
| Characterizing heterogeneity | 18 | Describe any methods used for estimating how the results of the study vary for sub-groups. | × | × | √ | √ | √ | √ |
| Characterizing distributional effects | 19 | Describe how impacts are distributed across different individuals or adjustments made to reflect priority populations. | √ | √ | √ | √ | √ | √ |
| Characterizing uncertainty | 20 | Describe methods to characterize any sources of uncertainty in the analysis. | × | √ | × | √ | √ | √ |
| Approach to engagement with patients and others affected by the study | 21 | Describe any approaches to engage patients or service recipients, the general public, communities, or stakeholders (e.g., clinicians or payers) in the design of the study. | × | × | × | × | × | × |
| RESULT | ||||||||
| Study parameters | 22 | Report all analytic inputs (e.g., values, ranges, references) including uncertainty or distributional assumptions. | √ | √ | × | √ | √ | √ |
| Summary of main result | 23 | Report the mean values for the main categories of costs and outcomes of interest and summarise them in the most appropriate overall measure. | √ | √ | √ | √ | √ | √ |
| Effect of uncertainty | 24 | Describe how uncertainty about analytic judgments, inputs, or projections affect findings. Report the effect of choice of discount rate and time horizon, if applicable. | × | √ | × | √ | √ | × |
| Effect of engagement with patients and others affected by the study | 25 | Report on any difference patient/service recipient, general public, community, or stakeholder involvement made to the approach or findings of the study (LIKE 21) | × | × | × | × | × | × |
| DISCUSSION | ||||||||
| Study findings, limitations, generalizability, and current knowledge | 26 | Report key findings, limitations, ethical or equity considerations not captured, and how these could impact patients, policy, or practice. | √ | √ | √ | √ | √ | √ |
| OTHER RELEVANT INFORMATION | ||||||||
| Source of funding | 27 | Describe how the study was funded and any role of the funder in the identification, design, conduct, and reporting of the analysis | √ | √ | √ | √ | √ | √ |
| Conflicts of interest | 28 | Report authors conflicts of interest according to journal or International Committee of Medical Journal Editors requirements. | √ | √ | √ | √ | √ | √ |
| SCORE out of 28 | 22/28 | 25/28 | 16/28 | 26/28 | 26/28 | 24/28 | ||
DISCUSSION
We systematically reviewed studies on the cost-effectiveness of atezolizumab and bevacizumab versus sorafenib in the treatment of HCC. We identified few eligible studies and observed considerable variability in outcomes between studies. Atezolizumab and bevacizumab treatment is clinically more effective and offers better outcomes in terms of overall survival and progression-free survival than sorafenib alone,15,16 however, their combined use is particularly expensive in terms of short-and long-term treatment, which led to an ICER of above the willingness-to-pay threshold. We estimate that a 75% reduction in the price of atezolizumab and 50% reduction in the price of bevacizumab is required to make their use more cost effective than sorafenib. Given that the number of HCC cases is only estimated to increase within the next few years, patient survival and quality of life could be improved by adjusting the price of these medications.
The combination of atezolizumab and bevacizumab has shown promising patient outcomes. In updated studies (with a median follow-up of 15.6 months), the median overall survival with the combination therapy was about 19.2 months—the longest overall survival rate for any phase 3 advanced-HCC trial—and was associated by an overall response rate of 30%, more than double that of sorafenib.9 More recently, data from the COSMIC-312 trial demonstrated that the atezolizumab plus cabozantinib consider a a potential treatment option for first-line HCC treatment.17
Studies that included only direct medical costs and indirect costs, such as loss of productivity, morbidity, and mortality attributed to hepatic cancer from a societal perspective, were not included in our study; thus the full cost may not be captured. Indirect costs account for over 50% of the total economic burden of most cancers. A previous study showed that the mean indirect costs from morbidity were $574; and those from mortality were $30,837.18 The cost of side effects varied between studies; three studies reported that either atezolizumab or bevacizumab had comparable side effects to sorafenib,1 one study showed that a combination was more costly in the treatment of side effect,14 and another showed that the two treatments were equal for the Chinese population and sorafenib was more costly for the USA population.10 The side effects that can incur costs and reduce treatment success include hypertension, aspartate aminotransferase increase, diarrhea, alanine aminotransferase increase, blood bilirubin increase, platelet count decrease, rash, palmar–plantar erythrodysesthesia syndrome, and hand-foot syndrome. Thus, there is a need for more studies assessing the side effect costs of medications for HCC.
We acknowledge some inherent limitations to our systematic review that limit interpretation of the results. Research on the cost-effectiveness of atezolizumab plus bevacizumab is currently limited to China and the USA. The generalizability of the results of these cost-effectiveness analyses may be limited to the population being studied because efficacy and safety data are mainly restricted to patients who met the inclusion criteria for the biologic agent trials. The parameters in the reported studies are difficult to compare because of heterogeneity in study design, definitions of costs, willingness-to-pay thresholds, treatment/follow-up periods (from months to years), variation in demand and supply, and differences in patient risk and treatment sequences. Although they were not considered in the eligible studies, indirect costs (e.g., loss in productivity) are an important part of the overall cost and may impact the cost-effectiveness analysis result. Moreover, eligible studies were limited to the English language; therefore, relevant studies in other languages could have been excluded.
Multiple studies were based on efficacy data from randomized controlled trial (RCT), which may not reflect the actual effectiveness of real world evidence in daily practice. There are important differences between clinical trials and real-world practice that could produce different results in cost-effectiveness analyses. For example, clinical practice may produce different effectiveness data because of patient characteristics, underlying chronic diseases, and adherence issues; thus, an update of cost-effectiveness is warranted based on data from clinical practice. Furthermore, the actual price of the medication after discounting may result in a significant reduction in medication costs.
Given the potential clinical benefits offered by combinatorial therapies with atezolizumab and bevacizumab but also the limitations of the available studies, we emphasize that further cost-effectiveness analyses using larger robust datasets from middle- and low-income populations and both clinical trials and practice are urgently needed. In practice, cost-effectiveness needs to be balanced with several other considerations to inform the formulation of medical resource budgets.
CONCLUSION
Atezolizumab plus bevacizumab is a promising treatment for HCC; however, their combined use is less cost-effective than treatment with sorafenib because of their high price, regardless of the positive clinical outcomes. Although there is evidence of cost-effectiveness, larger robust studies are needed to verify/update our conclusions; further studies are needed to investigate the difference in cost-effectiveness between this and other treatment options in geographically and economically varied populations. A reduction in price would promote cost-effectiveness and could potentially save a considerable number of lives.
References
- Chiang CL, Chan SK, Lee SF, Choi HCW. First-line atezolizumab plus bevacizumab versus sorafenib in hepatocellular carcinoma: A cost-effectiveness analysis. Cancers. 2021;13(5):931 [PubMed] | [CrossRef] | [Google Scholar]
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4-13. [PubMed] | [CrossRef] | [Google Scholar]
- Aly A, Ronnebaum S, Patel D, Doleh Y, Benavente F. Epidemiologic, humanistic and economic burden of hepatocellular carcinoma in the USA: A systematic literature review. Hepat Oncol. 2020;7(3):HEP27 [PubMed] | [CrossRef] | [Google Scholar]
- Yuen SC, Amaefule AQ, Kim HH, Owoo BV, Gorman EF, Mattingly TJ, et al. A systematic review of cost-effectiveness analyses for hepatocellular carcinoma treatment. Pharmacoecon Open. 2022;6(1):9-19. [PubMed] | [CrossRef] | [Google Scholar]
- Hou Y, Wu B. Atezolizumab plus bevacizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma: A cost-effectiveness analysis. Cancer Commun (Lond). 2020;40(12):743-5. [PubMed] | [CrossRef] | [Google Scholar]
- Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(Suppl 4):14-22. [PubMed] | [CrossRef] | [Google Scholar]
- Dubois de Gennes C, Mazaleyrat B, Sanchez Alvarez J, Lueza B. POSB49 cost effectiveness model of atezolizumab plus bevacizumab in untreated locally advanced or metastatic hepatocellular carcinoma (HCC) in French setting. Value Health. 2022;25(Array) [CrossRef] | [Google Scholar]
- Drugs list. Saudi Food and Drug Authority [cited Sep 20, 2022]. Available from: https://www.sfda.gov.sa/en/drugs-list [CrossRef] | [Google Scholar]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-905. [PubMed] | [CrossRef] | [Google Scholar]
- Wen F, Zheng H, Zhang P, Liao W, Zhou K, Li Q, et al. Atezolizumab and bevacizumab combination compared with sorafenib as the first-line systemic treatment for patients with unresectable hepatocellular carcinoma: A cost-effectiveness analysis in China and the United States. Liver Int. 2021;41(5):1097-104. [PubMed] | [CrossRef] | [Google Scholar]
- Patel K, Stein S, Luther J, Huntington SF. Cost-effectiveness of atezolizumab and bevacizumab in advanced hepatocellular carcinoma. J Clin Oncol. 2021;39(15_ suppl):e18829 [CrossRef] | [Google Scholar]
- Li M, Lin P, Luo S, Huang X, Huang X, Nian D, et al. A cost-effectiveness analysis of atezolizumab and bevacizumab verses sorafenib in patients with advanced hepatocellular carcinoma; 2021 [unpublished manuscript]. [CrossRef] | [Google Scholar]
- Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. 2021;4(2):e210037 [PubMed] | [CrossRef] | [Google Scholar]
- Zhang X, Wang J, Shi J, Jia X, Dang S, Wang W, et al. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib for patients with unresectable or metastatic hepatocellular carcinoma. JAMA Netw Open. 2021;4(4):e214846 [PubMed] | [CrossRef] | [Google Scholar]
- Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862-73. [PubMed] | [CrossRef] | [Google Scholar]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(3_suppl):267 [CrossRef] | [Google Scholar]
- Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995-1008. [PubMed] | [CrossRef] | [Google Scholar]
- Huang SY, Chen HM, Liao KH, Ko BS, Hsiao FY. Economic burden of cancers in Taiwan: A direct and indirect cost estimate for 2007-2017. BMJ Open. 2020;10(10):e036341 [PubMed] | [CrossRef] | [Google Scholar]
