ABSTRACT
Plasmalogens, glycerophospholipids, can alleviate Parkinson’s and Alzheimer’s disease. It helps in neural communication, such as vesicular or membrane fusion and ion transport. It counteracts oxidative stress by acting on Reactive Oxygen Species (ROS). Plasmalogens may improve memory in Alzheimer’s patients as observed using Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). Plasmalogens are usually isolated from Ascidians and Scallops. In the current review, we discuss the role of plasmalogen in neurodegenerative disorders.
INTRODUCTION
Plasmalogens are the class of glycerophospholipids that are present in bacteria, mammals, and invertebrates. It is only present in anaerobic bacteria and not found in plants and aerobic bacteria.1 It makes up 15-20% of the cell membranes. 90% of the Ethanolamine Plasmalogen (PlsEtn) is present in different brain regions.2 Phospholipid may be sub-divided into distinct sub-classes based on the nature of the polar headgroup at the sn-3 position of the glycerol backbone in eukaryotes.2 The grey and white matter of the brain constitutes 60 – 80 % of Plasmalogen Ethanolamine.3 It can either have the choline group or the ethanolamine group attached. A decrease in plasmalogens has been associated with a decline in cognitive function.4 Due to its presence in a high amount of lipid membranes in the brain, it is not surprising to observe the decrease of plasmalogens in neurological disorders such as Zellweger Syndrome, Alzheimer’s Disease, Parkinson’s Disorder, and Down’s syndrome.5,6 Plasminogens mediate the activity of peroxisomes, which can serve as a biomarker for neurological disorders and aging.7 Its structure contains a vinyl ether bond in the sn-1 position. It links the fatty aldehyde to the glycerol moiety. On the 1st and the 2nd position, a fatty acyl moiety is attached. The ester bond of a glycerol backbone is present at sn-2. The fatty acids present on sn-1 are as follows:
C16:0 Palmitic Acid
C 18:0 Stearic Acid
C 18:1 Oleic Acid
The head group contains either the ethanolamine group or the choline group (Figures 1, 2).8
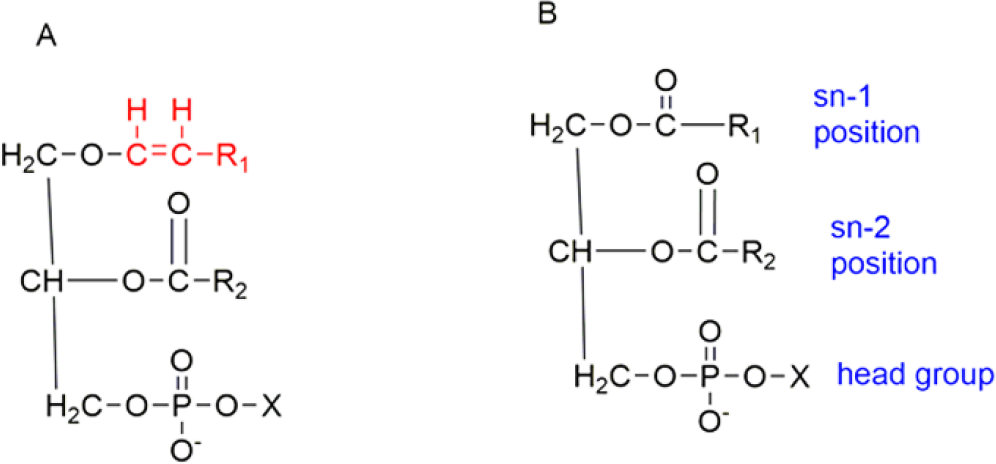
Figure 1.
Example of Plasmalogens showing vinyl ether bond at sn-1 position and an ester linkage at sn-2 position.
Figure 2.
Basic structure of plasmalogen.
It has been hypothesized that plasmalogens evolved as respiration evolved in animal cells with increased oxygen concentrations in the air. It led to the development of plasmalogen function in mammals and other species.9 Plasmalogens are predominantly expressed in the nervous system.8 They are affected by various neurodegenerative disorders like Alzheimer’s and Parkinson’s. Deficiencies in plasmalogens demonstrated in Alzheimer’s disease have been linked that PlsEtn.10 The role of plasmalogens in Parkinson’s disease is not well defined. However, the experimental evidence suggests that plasmalogens can be used as a biomarker in Parkinson’s disorder. Zellweger’s syndrome is a disorder phenotypically similar to Down’s syndrome caused by a mutation in one of the 13 PEX genes. It is characterized by the dysfunction of peroxisomes which ultimately leads to the deficiency of plasmalogens.11 In this disease, the total phospholipid content decreases by about 20%. A decrease in phosphatidylcholine and phosphatidylethanolamine is responsible for the change.12 In this review, we discuss the involvement of plasmalogens in various neurological disorders and their potential as therapeutic agents.
PHYSIOLOGICAL ROLES OF PLASMALOGEN
Plasmalogens are associated with neurological disorders by the following mechanisms.
Membrane fusion
Loss of ether lipids can affect the fusion of synaptic vesicles at the axon terminal. This process affects the release of neurotransmitters involved in mediating inter-neuronal communication.13 Alzheimer’s disease is associated with reduced ethyl plasmalogens contributing to vesicular fusion in neurons.14 Synaptic vesicular fusion at the axon terminal leads to the release of neurotransmitters. Lack of ether lipids can reduce neurotransmission.13 Thus, alterations in these lipids may contribute to diseases of the Central Nervous System (CNS).
Oxidative Stress
Oxidative stress in the brain is the imbalance between the ROS and antioxidant defenses. Oxidative stress can further lead to injuries to the tissues, neurons, or other parts of the brain, leading to neurodegenerative diseases like Alzheimer’s.15 Plasmalogens are effective as endogenous antioxidants. Structurally plasmalogens are sensitive to oxidative reaction due to the presence of enol ether in its structure.16 This, in turn, protects the necessary lipids and lipoproteins from oxidative damage. It acts as a scavenger to protect against free radical attack. The increase in plasmalogen content increases resistance against ROS and ROS generators.17 In Alzheimer’s brain, a significant reduction of plasmalogens has been linked to oxidative stress-mediated damage to the neuronal tissues and altered membrane properties.8 Kuczynski et al. proposed that the Plasmalogen Ethanolamine (PlsEtn) elevation can protect the brain from oxidative stress, which concluded by highlighting the role of PlsEtn in scavenging free radicals.18
Ion Transport
Ca2+ channels and plasmalogens are present in the skeletal muscle sarcoplasmic reticulum. The acyl side-chain orientation of plasmalogens helps provide a particular lipid environment where the anionic glycerophospholipids serve as boundary lipids and help regulate the Na+-Ca2+ exchanges. It enhances the Na+-Ca+2 exchange by altering the dynamics of the anionic phospholipid membrane,19 and the sarcolemmal Na+-Ca2+ exchange activity depends on the membrane lipid environment.20
IMPROVEMENT IN THE CASES OF DEMENTIA
Clinical trials using plasmalogens to restore cognitive deficits in dementia patients show promising results. Alzheimer’s disease is a neurodegenerative disorder where dementia is prevalent. Plasmalogens can prevent cognitive decline in mild to moderate Alzheimer’s patients. Plasmalogen deficiency also contributes to the deposition of Aβ plaques and is associated with APOE, a genetic risk factor for Alzheimer’s disease.21 In a study, Yamashita et al., infused Ascidian Viscera ethanolamine glycerophospholipid (EtnGpl), rich in plasmalogen ethanolamine, in ß-amyloid infused rats, improving the reference and working memory-related learning ability. In ß-amyloid infused rats, increased oxidative stress and chronic inflammation lead to the activation of PLA2 followed by degradation of brain plasmalogen ethanolamine. It can also protect neurons against oxidative injuries caused by iron.22 Plasmalogens may decrease glial activation and accumulation of Aß proteins in the murine brain, induced by chronic LPS injection. In a previous study by Hossain et al.23 Pls prevented neuronal cell death by activating Protein kinase B (AKT)24 and inhibited caspase 3, which suggested that Pls is an essential lipid capable of improving neuronal survival. Wood et al. conducted cognition tests in mild to moderate Alzheimer’s patients using Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), a memory assessment scale. Plasmalogens have been reported to reduce by up to 75% in Alzheimer’s patients. On treatment with plasmalogens, the results demonstrated improvement in ADAS-Cog score in AD patients, which correlated with the increased circulating plasmalogen levels.25
Fujino et al. conducted a Multicentred, Randomised, Double-blind, Placebo-controlled clinical trial to assess the efficacy of plasmalogens in improving cognition in patients with mild AD and MCI (Mild Cognitive Impairment) for 28 weeks. Plasmalogens were derived from the Scallops in the study. The cognitive ability was measured using the MMSE-J (Mini-Mental State Examination – Japanese) scale and WMS-R (Wechsler Memory Scale-Revised). The WSM-R score significantly improved in the treatment compared to the placebo group. The study showed significant results in female patients and individuals below 77 years of age. These results indicated the importance of plasmalogens in improving memory functions with mild AD. Studies have also suggested the involvement of Docosahexaenoic acid (DHA) and Eicosapentaenoic acid (EPA) in brain functions. The scallop-derived plasmalogens have large amounts of DHA and EPA, which may be responsible for improving cognitive functions in mild AD. However, the mechanisms involved in plasmalogen-mediated changes in brain function are yet to be determined.26 Further understanding of how plasmalogens improve human cognition may open new therapeutic avenues for neurodegenerative disorders.
REGULATION OF SCHWANN CELL DIFFERENTIATION IN PNS BY PLASMALOGENS
Plasmalogens can regulate schwann cell differentiation. Silva et al. conducted studies in PNS where plasmalogens controlled schwann cells and were crucial for developing and differentiating schwann cells.27 The deficiency of plasmalogen has been shown to cause impaired radial sorting by schwann cells, thus affecting myelination patterns. Impaired myelination, defective compartmentalization of schwann cells, and increased non-compact myelin highlight the role of plasmalogens in the organization of schwann cells. Studies also showed the absence of plasmalogens could lead to depletion of myelin. In a study using double mutant mice (DM Mice), i.e., mutations in PEX7 and GNPAT (glycerophosphate O-acyltransferase), were created to assess the cruciality of plasmalogens in myelination.28 The biosynthesis of ether phospholipids is initiated in peroxisomes by the activity of GNPAT and Alkylglycerone phosphate synthase (AGPS).29 Mutations in GNPAT, AGPS, or PEX7, which targets AGPS in peroxisomes, lead to highly reduced levels of plasmalogens.1 The DM mice showed impaired nerve conduction and myelination, indicating the importance of plasmalogens in myelination. Studies on GNPAT – Knock Out mice revealed dysfunctional AKT activation. The study showed that plasmalogen deficiency might impair Schwann cells’ ability to differentiate and mature, which results in defective myelination and nerve condition.27
PLASMALOGEN IN DISEASES
Zellweger Syndrome
Zellweger Syndrome (ZS) is classified as a peroxisomal disorder. Peroxisomes play an essential role in modulating a variety of signaling pathways. Hence, mutations in genes involved in peroxisomal biogenesis can decrease plasmalogen, resulting in this syndrome.2 Zellweger syndrome is also an autosomal recessive disorder characterized by severe neurological dysfunction, hepato-renal disease, various metabolic abnormalities, and unusual physical features. Peroxisomal deficits have been observed in the liver of ZS patients.30
The patients with ZS are deficient in plasmalogen precursor, DHAP-AT (Dihydroxy acetophosphate acyl transferase), which is present in the peroxisomes of the liver and leucocytes.31 Heymans et al., performed experiments on children who died of this disease and found the percentage of PlsEtn to be less than 10% of the average level of choline plasmalogens.32 It was concluded that peroxisomes play a vital role in the brain. Peroxisomal dysfunction has severe neuropathological consequences, while the loss of plasmalogens is considered to be a strong pathological factor in ZS.33 Plasmalogen deficiency is known to be the direct cause of RCDP (Rhizomelic chondrodysplasia punctate), a peroxisomal disorder that shows up with abnormalities in the skeletal muscle, joint immobility, congenital cataracts, and severely impaired growth. Plasmalogen precursor 1-O-Alkylglycerol has been proposed as a therapeutic intervention in Zellweger patients.34
Parkinson’s Disease
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by loss of nigrostriatal dopaminergic neurons, the presence of Lewy bodies, and alpha-synuclein aggregation.40 Plasma and erythrocytic plasmalogen levels were found to be lower than 30% in PD patients.2 Clinical studies by Dragonas et al., report oxidative stress as the predominant factor for the decrease in plasmalogens in PD.41 Vinyl ether bond present at the sn-1 position of plasmalogens makes it susceptible to oxidative stress. Oral ingestion of scallop-derived purified plasmalogens improved some non-motor clinical symptoms of PD.42
Alzheimer’s Disease
The role of plasmalogens in Alzheimer’s disease has been reported; however, the exact mechanism of action is yet to be determined. Studies have stated that it can improve cognitive function as a neuroprotective agent.43 AD is one of the most common neurodegenerative disorders, with millions of cases worldwide characterized by the deposition of amyloid plaques and phosphorylated tau proteins. Growing research has indicated lipid dysregulations as the cause of Alzheimer’s disease. It includes the Phosphoinositide pathway (PI3K), where the pathology of Tau proteins is associated with lipid metabolism.44
The glycerophospholipids, i.e., ethanolamine plasmalogens, play a role in the pathophysiology of AD. A study by Kling et al., about plasmalogens in AD suggested that reducing plasmalogens in the brain is associated with an increased risk of AD.45 It also indicated the reduction of plasmalogen occurs at the stage of MCI and is subsequently related to the development of dementia. There is a 70% reduction of plasmalogens in the brain of AD patients as compared to a healthy brain, while the administration of Pls can improve cognitive functions in AD patients.25,46
Multiple Sclerosis
Multiple sclerosis (MS) is a neurodegenerative autoimmune disorder characterized by demyelination.47 MS patients show lower amounts of plasmalogens. This study, conducted by Ferriera et al., found a lower abundance of PC and PE species of Pls compared to healthy controls. The reduction of Pls within MS patients is due to an increase in ROS, which can degrade plasmalogens in the body. ROS has been found to aggravate the symptoms of MS.48 While plasmalogens can also act as antioxidants reducing the oxidative-stress-induced demyelination and axonal degeneration.37
PLASMALOGENS AS A THERAPEUTIC AGENT
Multiple trials using plasmalogens, i.e., both PlsEtn and choline plasmalogens have looked at their effect on brain functions. An increase in their levels within the brain is neuroprotective while increasing cognitive function. The deficiency of plasmalogens is reported in various conditions. Whether the deficit is the cause or the result in that particular state must be determined. At the same time, replenishing these plasmalogens can improve the condition of these disease states is also being studied.
Dietary incorporation of plasmalogens was performed in an experiment back in 1992 by Das et al. where plasmalogen replacement therapy was performed.48 Here, dietary alkylglycerol was applied to different tissues in rats where plasmalogen levels increased significantly. They also concluded that membrane alkylglycerols and plasmalogens could synthesize dietary ether lipids.49 This led to the brain forming fewer plasmalogens than predicted. In a study by Mawatari et al., dietary plasmalogens were used to replenish plasmalogens obtained from chicken skin.50
PlsEtn obtained from the source was given in the diet to Zucker Diabetic Fatty (ZDF) rats for four weeks. It was observed that erythrocytic PlsEtn, along with phosphatidylethanolamine, increased in normal rats. There were no adverse effects. In another study by Wood et al., a severe decrease in plasmalogens was observed in Alzheimer’s. An orally bioavailable compound was developed, i.e., PPI-1011 which aimed for the same, i.e., Plasmalogen Replacement Therapy.51 They used DHA and DHA-containing ethanolamine plasmalogens (PlsEtn) for the desired results in rabbits. Rabbits were orally dosed with the compound delivered in hard gelatin capsules resulting in increased levels of circulating PlsEtn and PtdEtn (Phosphatidylethanolamine). Those results concluded in the successful use of plasmalogen precursors by lipid remodeling (dealkylation-alkylation reaction). This product was also able to cross the blood-brain barrier as well as the blood-retinal barrier. Since, in previous studies, PlsEtn decrease has been seen in patients with PD, it was concluded that a reduction in PlsEtn is due to systemic oxidative stress. PPI-1011 was used to evaluate the role of plasmalogen in reversing a dopaminergic loss in PD. The experimental mice showed the neuroprotective activity of PPI-1011 on striatal DA markers in the PD mouse model.52
CONCLUSION
Plasmalogens are endogenous molecules that are important in neuroprotection, oxidative stress, and vesicular fusion. It has a prominent role in neurodegenerative disorders such as Parkinson’s and Alzheimer where there has been a decrease. Replenishment of PlsEtn and choline plasmalogens has benefited several neurodegenerative and cardiovascular disease cases. In AD, membrane fusion can lead to new opportunities where plasmalogen contributes to increased fusion. In PD, neuroprotective activity has also been observed in PD mice (Figure 3). Future in-vivo studies would help develop PlsEtn into a successful treatment for various neurological and other unexplored diseases.
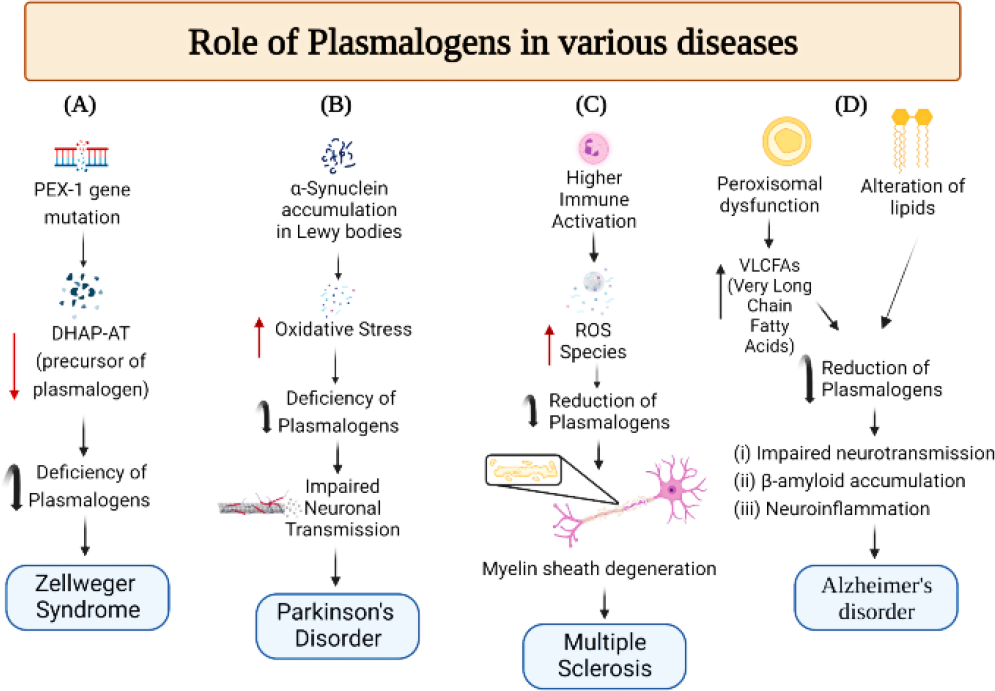
Figure 3.
Plasmalogens and neurological disorders: (A) Mutations in the PEX-1 gene35 lead to the deficiency of Dihydroxyacetone phosphate acyltransferase (DHAP-AT ), which is required to form plasmalogens.31 A reduction of DHAP-AT leads to a shortage of plasmalogens causing Zellweger’s syndrome. (B) Alpha-synuclein leads to increased oxidative stress and impaired mitochondrial functions,36 which makes plasmalogens susceptible to destruction. Reduced amounts of plasmalogen impair neuronal transmission associated with Parkinson’s disease. (C) Excess immune cell activation in multiple sclerosis37 leads to increased reactive oxygen species, which reduces plasmalogens.38 Plasmalogens are involved in maintaining myelin sheath integrity.39 (D) Peroxisomal dysfunction increases VLCFAs and alteration of lipids leads to a decrease in plasmalogens resulting in impaired neurotransmission and accumulation of β-amyloid plaques in Alzheimer’s disease.8
References
- Bozelli JC, Azher S, Epand RM. Plasmalogens and chronic inflammatory diseases. Front Physiol. 2021;12:730829 [PubMed] | [CrossRef] | [Google Scholar]
- Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim biophys acta. 2012;1822(9):1442-52. [PubMed] | [CrossRef] | [Google Scholar]
- West A, Zoni V, Teague WE, Leonard AN, Vanni S, Gawrisch K, et al. How do ethanolamine plasmalogens contribute to order and structure of neurological membranes?. J Phys Chem B. 2020;124(5):828-39. [PubMed] | [CrossRef] | [Google Scholar]
- Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, et al. Peripheral ethanolamine plasmalogen deficiency: A logical causative factor in Alzheimer’s disease and dementia. J Lipid Res. 2007;48(11):2485-98. [PubMed] | [CrossRef] | [Google Scholar]
- Brites P, Waterham HR, Wanders RJ. Functions and biosynthesis of plasmalogens in health and disease. Biochim Biophys Acta. 2004;1636(2-3):219-31. [PubMed] | [CrossRef] | [Google Scholar]
- Messias MCF, Mecatti GC, Priolli DG, De Oliveira Carvalho P. Plasmalogen lipids: Functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018;17(1):41 [PubMed] | [CrossRef] | [Google Scholar]
- Kytikova OY, Novgorodtseva TP, Antonyuk MV, Gvozdenko TA. [Plasmalogenes in the pathophysiology and therapy of age-specific diseases]. Adv Gerontol. 2019;32(6):948-58. [PubMed] | [Google Scholar]
- Su XQ, Wang J, Sinclair AJ. Plasmalogens and Alzheimer’s disease: A review. Lipids Health Dis. 2019;18(1):100 [PubMed] | [CrossRef] | [Google Scholar]
- Zhou Y, Yu N, Zhao J, Xie Z, Yang Z, Tian B, et al. Advances in the biosynthetic pathways and application potential of plasmalogens in medicine. Front Cell Dev Biol. 2020;8:765 [PubMed] | [CrossRef] | [Google Scholar]
- Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40(3):199-229. [PubMed] | [CrossRef] | [Google Scholar]
- Dr. Tanmay K, Dr. Alpana K, Dr. Varun A, Dr. Radha G. Zellweger syndrome: A Downs syndrome mimic. Pediatric review. Int J Pediatr Res. 2019;6(2) [PubMed] | [CrossRef] | [Google Scholar]
- Guan Z, Wang Y, Cairns NJ, Lantos PL, Dallner G, Sindelar PJ, et al. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(7):740-7. [PubMed] | [CrossRef] | [Google Scholar]
- Dorninger F, Forss-Petter S, Wimmer I, Berger J. Plasmalogens, platelet-activating factor and beyond – ether lipids in signaling and neurodegeneration. Neurobiol Dis. 2020;145:105061 [PubMed] | [CrossRef] | [Google Scholar]
- Koivuniemi A. The biophysical properties of plasmalogens originating from their unique molecular architecture. FEBS Lett. 2017;591(18):2700-13. [PubMed] | [CrossRef] | [Google Scholar]
- Betteridge DJ. What is oxidative stress?. Metabolism. 2000;49(2) Suppl 1:3-8 [PubMed] | [CrossRef] | [Google Scholar]
- Brosche T, Platt D. The biological significance of plasmalogens in defense against oxidative damage. Exp Gerontol. 1998;33(5):363-9. [PubMed] | [CrossRef] | [Google Scholar]
- Zoeller RA, Grazia TJ, LaCamera P, Park J, Gaposchkin DP, Farber HW, et al. Increasing plasmalogen levels protects human endothelial cells during hypoxia. Am J Physiol Heart Circ Physiol. 2002;283(2):H671-9. [PubMed] | [CrossRef] | [Google Scholar]
- Kuczynski B, Reo NV. Evidence that plasmalogen is protective against oxidative stress in the rat brain. Neurochem Res. 2006;31(5):639-56. [PubMed] | [CrossRef] | [Google Scholar]
- Ford DA, Hale CCJFl. Plasmalogen and anionic phospholipid dependence of the cardiac sarcolemmal sodium-calcium exchanger. FEBS Lett. 1996;394(1):99-102. [PubMed] | [CrossRef] | [Google Scholar]
- Vemuri R, Philipson KD. Phospholipid composition modulates the Na+-Ca2+ exchange activity of cardiac sarcolemma in reconstituted vesicles. Biochim Biophys Acta. 1988;937(2):258-68. [PubMed] | [CrossRef] | [Google Scholar]
- Meletis CD. Alkyl-acylglycerols and the important clinical ramifications of raising plasmalogens in dementia and Alzheimer’s disease. Integr Med (Encinitas). 2020;19(3):12-6. [PubMed] | [Google Scholar]
- Yamashita S, Hashimoto M, Haque AM, Nakagawa K, Kinoshita M, Shido O, et al. Oral administration of ethanolamine glycerophospholipid containing a high level of plasmalogen improves memory impairment in amyloid β-infused rats. Lipids. 2017;52(7):575-85. [PubMed] | [CrossRef] | [Google Scholar]
- Hossain MS, Tajima A, Kotoura S, Katafuchi T. Oral ingestion of plasmalogens can attenuate the LPS-induced memory loss and microglial activation. Biochem Biophys Res Commun. 2018;496(4):1033-9. [PubMed] | [CrossRef] | [Google Scholar]
- Hossain MS, Ifuku M, Take S, Kawamura J, Miake K, Katafuchi T, et al. Plasmalogens rescue neuronal cell death through an activation of AKT and ERK survival signaling. PLOS ONE. 2013;8(12):e83508 [PubMed] | [CrossRef] | [Google Scholar]
- Wood PL, Mankidy R, Ritchie S, Heath D, Wood JA, Flax J, et al. Circulating plasmalogen levels and Alzheimer disease Assessment Scale-Cognitive scores in Alzheimer patients. J Psychiatry Neurosci. 2010;35(1):59-62. [PubMed] | [CrossRef] | [Google Scholar]
- Fujino T, Yamada T, Asada T, Tsuboi Y, Wakana C, Mawatari S, et al. Efficacy and blood plasmalogen changes by oral administration of plasmalogen in patients with mild Alzheimer’s disease and mild cognitive impairment: A multicenter, randomized, double-blind, placebo-controlled trial. EBiomedicine. 2017;17:199-205. [PubMed] | [CrossRef] | [Google Scholar]
- da Silva TF, Eira J, Lopes AT, Malheiro AR, Sousa V, Luoma A, et al. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J Clin Invest. 2014;124(6):2560-70. [PubMed] | [CrossRef] | [Google Scholar]
- Readhead C, Hood L. The dysmyelinating mouse mutations shiverer (shi) and myelin deficient (shimld). Behav Genet. 1990;20(2):213-34. [PubMed] | [CrossRef] | [Google Scholar]
- Razeto A, Mattiroli F, Carpanelli E, Aliverti A, Pandini V, Coda A, et al. The crucial step in ether phospholipid biosynthesis: structural basis of a noncanonical reaction associated with a peroxisomal disorder. Structure. 2007;15(6):683-92. [PubMed] | [CrossRef] | [Google Scholar]
- Wilson GN, Holmes RG, Custer J, Lipkowitz JL, Stover J, Datta N, et al. Zellweger syndrome: Diagnostic assays, syndrome delineation, and potential therapy. Am J Med Genet. 1986;24(1):69-82. [PubMed] | [CrossRef] | [Google Scholar]
- Zoeller RA, Raetz CR. Isolation of animal cell mutants deficient in plasmalogen biosynthesis and peroxisome assembly. Proc Natl Acad Sci U S A. 1986;83(14):5170-4. [PubMed] | [CrossRef] | [Google Scholar]
- Heymans HSA, Schutgens RBH, Tan R, van den Bosch H, Borst P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (). Nature. 1983;306(5938):69-70. [PubMed] | [CrossRef] | [Google Scholar]
- Crane DI. Revisiting the neuropathogenesis of Zellweger syndrome. Neurochem Int. 2014;69:1-8. [PubMed] | [CrossRef] | [Google Scholar]
- Soppert J, Lehrke M, Marx N, Jankowski J, Noels H. Lipoproteins and lipids in cardiovascular disease: From mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. 2020;159:4-33. [PubMed] | [CrossRef] | [Google Scholar]
- Walter C, Gootjes J, Mooijer PA, Portsteffen H, Klein C, Waterham HR, et al. Disorders of peroxisome biogenesis due to mutations in PEX1: Phenotypes and PEX1 protein levels. Am J Hum Genet. 2001;69(1):35-48. [PubMed] | [CrossRef] | [Google Scholar]
- Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol. 2009;41(10):2015-24. [PubMed] | [CrossRef] | [Google Scholar]
- Ferreira HB, Melo T, Monteiro A, Paiva A, Domingues P, Domingues MR, et al. Serum phospholipidomics reveals altered lipid profile and promising biomarkers in multiple sclerosis. Arch Biochem Biophys. 2021;697:108672 [PubMed] | [CrossRef] | [Google Scholar]
- Hu C, Zhou J, Yang S, Li H, Wang C, Fang X, et al. Oxidative stress leads to reduction of plasmalogen serving as a novel biomarker for systemic lupus erythematosus. Free Radic Biol Med. 2016;101:475-81. [PubMed] | [CrossRef] | [Google Scholar]
- Luoma AM, Kuo F, Cakici O, Crowther MN, Denninger AR, Avila RL, et al. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic Biol Med. 2015;84:296-310. [PubMed] | [CrossRef] | [Google Scholar]
- Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;86:109-27. [PubMed] | [CrossRef] | [Google Scholar]
- Dragonas C, Bertsch T, Sieber CC, Brosche T. Plasmalogens as a marker of elevated systemic oxidative stress in Parkinson’s disease. Clin Chem Lab Med. 2009;47(7):894-7. [PubMed] | [CrossRef] | [Google Scholar]
- Mawatari S, Ohara S, Taniwaki Y, Tsuboi Y, Maruyama T, Fujino T, et al. Improvement of blood plasmalogens and clinical symptoms in Parkinson’s disease by oral administration of ether phospholipids: A preliminary report. Parkinsons Dis. 2020. 2020:2671070 [PubMed] | [CrossRef] | [Google Scholar]
- Feng T, Hu X, Fukui Y, Tadokoro K, Bian Z, Morihara R, et al. Neuroprotective effects of Scallop-derived plasmalogen in a mouse model of ischemic stroke. Brain Res. 2021;1766:147516 [PubMed] | [CrossRef] | [Google Scholar]
- Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat Rev Neurosci. 2011;12(5):284-96. [PubMed] | [CrossRef] | [Google Scholar]
- Kling MA, Goodenowe DB, Senanayake V, Mahmoudian Dehkordi S, Arnold M, Massaro TJ, et al. Circulating ethanolamine plasmalogen indices in Alzheimer’s disease: Relation to diagnosis, cognition, and CSF tau. Alzheimers Dement. 2020;16(9):1234-47. [PubMed] | [CrossRef] | [Google Scholar]
- Onodera T, Futai E, Kan E, Abe N, Uchida T, Kamio Y, et al. Phosphatidylethanolamine plasmalogen enhances the inhibiting effect of phosphatidylethanolamine on γ-secretase activity. J Biochem. 2015;157(5):301-9. [PubMed] | [CrossRef] | [Google Scholar]
- Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. 2019;26(1):27-40. [PubMed] | [CrossRef] | [Google Scholar]
- Das AK, Holmes RD, Wilson GN, Hajra AK. Dietary ether lipid incorporation into tissue plasmalogens of humans and rodents. Lipids. 1992;27(6):401-5. [PubMed] | [CrossRef] | [Google Scholar]
- Dean JM, Lodhi IJ. Structural and functional roles of ether lipids. Protein Cell. 2018;9(2):196-206. [PubMed] | [CrossRef] | [Google Scholar]
- Mawatari S, Katafuchi T, Miake K, Fujino T. Dietary plasmalogen increases erythrocyte membrane plasmalogen in rats. Lipids Health Dis. 2012;11(1):161 [PubMed] | [CrossRef] | [Google Scholar]
- Wood PL, Smith T, Lane N, Khan MA, Ehrmantraut G, Goodenowe DB, et al. Oral bioavailability of the ether lipid plasmalogen precursor, PPI-1011, in the rabbit: A new therapeutic strategy for Alzheimer’s disease. Lipids Health Dis. 2011;10(1):227 [PubMed] | [CrossRef] | [Google Scholar]
- Miville-Godbout E, Bourque M, Morissette M, Al-Sweidi S, Smith T, Mochizuki A, et al. Plasmalogen augmentation reverses striatal dopamine loss in MPTP mice. PLOS ONE. 2016;11(3):e0151020 [PubMed] | [CrossRef] | [Google Scholar]
