ABSTRACT
Background
Concerns regarding the detrimental consequences of oxidative stress on human health have grown recently on a global scale and herbal treatment approaches to treat oxidative stress-related illnesses in humans are now a hot topic in traditional medicine. Diplocyclos palmatus is one such herbal remedy, it is utilised to treat a wide range of illnesses because of its antioxidant qualities. To provide scientific evidence for this claim the study was planned to evaluate the antioxidant capabilities of D. palmatus Seed Methanolic Extract (SME) using both in vitro and in vivo investigations.
Materials and Methods
SME prepared with cold percolation method was first evaluated for phytochemical constituents. In vitro assays were carried out to get a preliminary indication of the antioxidant capacity of extract in a regulated environment whereas, in vivo analysis was done using an induced Wistar rat model to justify its biological relevance. The main bioactive components in SME were identified using the RP-HPLC technique.
Results
SME has proven to be an effective antioxidant in both in vitro and in vivo experiments. The RP-HPLC analysis has revealed the presence of quercetin and vanillic acid as the main antioxidants in SME. All the results were obtained as Mean±Standard Deviation. Tests including Student’s T-test (unpaired) and ANOVA were applied to compare the Statistical difference. A ‘p’ value of less than 0.05 was considered significant.
Conclusion
The research findings indicate that D. palmatus SME demonstrates noteworthy antioxidant properties. Thus, the findings could contribute to developing a safe and effective natural health supplement.
INTRODUCTION
Medicinal plants are widely used in both developed and developing countries for their healing properties (Bajpayeeet al., 2024). Before the 18th century, the therapeutic qualities of many plants were recognized, though their active ingredients were not yet understood (Salmerón-Manzanoet al., 2020). Modern techniques now allow for the extraction and identification of these compounds. (Balkrishnaet al., 2024). Phytochemicals can help combat oxidative stress caused by reactive oxygen species, which poses a significant global health concern (Symanet al., 2024; Jomovaet al., 2024). Plant antioxidants can neutralize free radicals, reducing the risk of serious diseases and serving as potential therapeutic agents (Tiwanaet al., 2024; Chaachouayet al., 2024). India’s diverse climate makes it a prominent source of medicinal plants (Atanasovet al., 2021; Varahet al., 2023). D. palmatus (Shivlingi), from the Cucurbitaceae family, is traditionally used by tribal communities for various chronic ailments (Patelet al., 2020; Kauravet al., 2021). This study aimed to investigate the antioxidant potential of D. palmatus extract through in vitro and in vivo assays and to evaluate its bioactive compounds using RP-HPLC.
MATERIALS AND METHODS
Plant collection and identification
Plants were collected in October and November 2022 from Badheri, Uttarakhand, India (29°53’54.3″N 77°59’08.0″E) and matched with a specimen (Accession No. 14405, Patanjali Research Institute, Panchayanpur) and identified as D. palmatus.
Chemicals used
Analytical grades of standards and other chemicals were procured from Sigma Aldrich Chemical Pvt. Ltd., India.
Extract preparation
Air-dried seeds from healthy fruits were used to prepare SME by cold percolation method.
In vitro antioxidant assays
Three experimental replicates were used to calculate the mean value for the given below in vitro antioxidant assays.
DPPH (2,2-diphenyl-1-picrylhydrazyl) assay
Different concentrations (20-100 μg/mL) of extract and Ascorbic Acid (AA) were prepared from a stock solution (1 mg/mL) by dilution with methanol. A mixture of 1 mL of extract or AA and 3 mL of 0.2 mM DPPH was incubated for 30 min in the dark. Absorbance was measured at 517 nm against a methanol blank, with DPPH alone serving as the control. The percentage radical scavenging activity (% RSA) was calculated using the formula: % RSA=(abs. of control – abs. of sample)/abs. of control×100. The IC50 value was determined for both standard and extract using their calibration curve equation “y=mx+c.” (Delarosaet al., 2023).
ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) assay
The assay involved mixing 7 mM ABTS solution with 2.45 mM potassium per sulfate and letting it sit for 12-16 hr at room temperature. After dilution with ethanol (1:89 v/v) and equilibrating at 30ºC, the absorbance was set to 0.700±0.02 at 734 nm. Then, 1 mL of the extract and antioxidant (from 1 mg/mL stock) were combined with 3.5 mL of ABTS, and absorbance was read at 734 nm (Hussenet al., 2023). % RSA and IC50 values were calculated as given in DPPH assay.
NO (Nitric Oxide) assay
To perform the assay, a 5 mM sodium nitroprusside solution was prepared, which generates nitric oxide in the presence of oxygen, forming nitrite ions that react with Griess Reagent to produce a colored compound. Antioxidants in the extract compete with oxygen, decreasing nitrite ion formation and colored compound levels. A reaction mixture was created using 5 mM nitroprusside in phosphate-buffered saline (0.2 mM, pH 7.4) along with varying concentrations of the extract and Gallic Acid (GA) as a standard, and incubated at 25ºC for 150 min. After adding 0.5 mL of Griess reagent, absorbance was measured at 546 nm (Daset al., 2023) and % RSA and IC50 values were calculated as given in the DPPH assay.
Total Phenolic and Flavonoid Content Assay
The TPC and TFC of the extract were measured at 100 μg/mL. For TPC, 5 mL of 10% Folin-Ciocalteu’s reagent was added to the extract and Gallic Acid (GA) standard, incubated in the dark for 5-10 min, followed by 4 mL of 7% Na2CO3 and a 90-min incubation. Absorbance was recorded at 750 nm, with a blank sample for comparison. The GA calibration curve “y=mx+c” determined mg GA Equivalent (GAE) per gram dry weight of extract.
For TFC, 0.3 mL of 5% NaNO2 was mixed with the extract and quercetin standard, allowed to sit for 5-6 min, then 0.3 mL of 10% AlCl3, 4 mL of 1M NaOH, and 10 mL of water was added and shaken. Absorbance was measured at 510 nm, again using a blank for comparison. The quercetin calibration curve “y=mx +c” calculated mg Quercetin Equivalent (QE) per gram dry weight of the extract (Islamet al., 2023).
In vivo antioxidant assay
The study was approved by the Institutional Animal Ethical Committee (IAEC) of M.D. University, Rohtak (ref: IAEC/2023/46-60, dated 02/03/23). 15 rats were divided into Negative control, Positive control, Metformin and SME groups as shown in Figure 1. 12 rats received Letrozole (1 mg/kg/day) for 21 days to induce PCOS (Kakadia et al., 2021), which causes oxidative stress (Senguptaet al., 2024). After sacrificing one rat and confirming induction, each group contained 3 experimental replicates. Induced groups were treated with metformin (500 mg/kg) (Desaiet al., 2012) and SME (100 mg/kg) (Reddyet al., 2010; Chauhanet al., 2010; Sivakumaret al., 2004) for 21 days. Post-dissection, liver and kidney homogenates were prepared to evaluate the impact of treatments on oxidative stress, compared with the untreated PCOS group. Following assays were conducted to assess in vivo antioxidant activity. In all the assays three experimental replicates were used to calculate the mean.
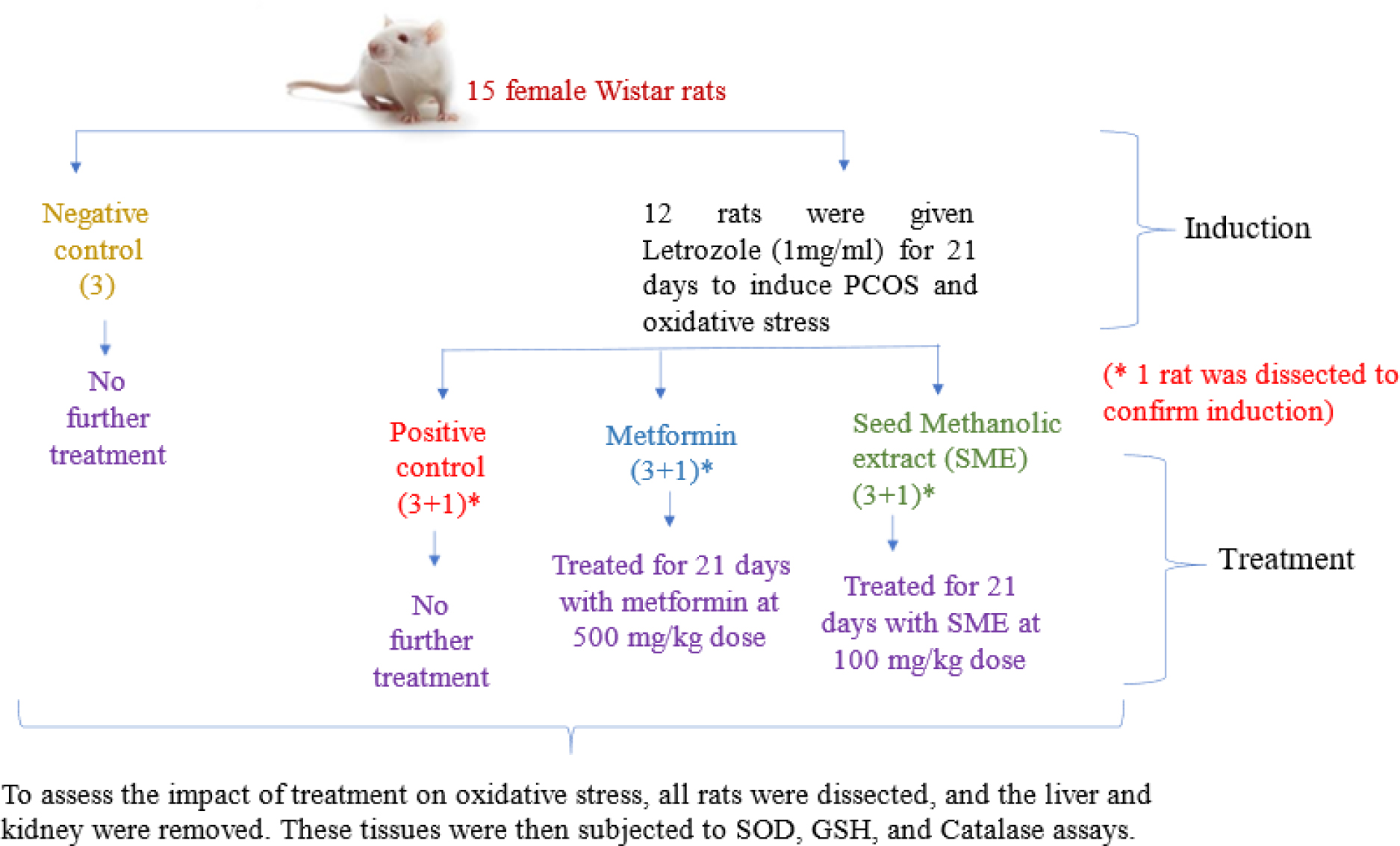
Figure 1:
Experimental setup for in vivo oxidative stress evaluation.
Superoxide Dismutase (SOD) assay
The main reagent in the assay was Pyrogallol, which auto-oxidizes to form free radicals and a brown Pyrogallol-quinone complex that absorbs at 420 nm. This auto-oxidation is inhibited by SOD, with one unit defined as the amount of enzyme that prevents 50% of the reaction. A reaction mixture (3 mL) was prepared with 1 mM EDTA, 50 mM Tris-HCl buffer (pH 8.2), 100 µL tissue homogenate, and 0.4 mM pyrogallol, topped with distilled water. Blanks excluded pyrogallol, while controls included it. Absorbance was measured at 420 nm for 2 min at 30±2ºC. Following steps were used to calculate SOD activity (Siswoyoet al., 2021):
The rate of absorbance/min was calculated for the control and extract by using the equation:
SOD activity was calculated by using the equation.
SOD activity (U/min/mg protein)=% inhibition/0.5.
Catalase Assay
Hydrogen peroxide is converted into oxygen and water by the enzyme catalase, found in the peroxisomes of eukaryotic cells. The reduction in absorbance at 240 nm correlates with the decomposition rate of hydrogen peroxide. For the assay, a mixture was prepared with 50 µL of tissue homogenate, 1 mL of 30 mM H2O2, and 2 mL of phosphate buffer (pH 7.0). Phosphate buffer served as Blank, while the controls excluded the sample. Absorbance was measured at 240 nm for 1 min. Catalase activity (U/mg) was calculated by dividing the absorbance by the molar extinction coefficient (43.6 M–¹cm–¹), indicating the degradation of 1 mM H2O2 per min (Tekinet al., 2022).
GSH (reduced glutathione) Assay
GSH is an intracellular reductant that alleviates oxidative stress. When oxidized by DTNB (5,5′-dithio-bis-2-nitrobenzoic acid), it forms the yellow compound TNB, which has an absorbance peak at 412 nm. For the assay, 50 µL of tissue homogenate in 0.1 M phosphate buffer (pH 7.4) was mixed with an equal volume of 20% TCA and 1 mM EDTA to precipitate proteins. After standing for 5 min, the mixture was centrifuged at 2000 rpm for 10 min, and the supernatant was collected. Then, 1.8 mL of 0.1 mM Ellman’s reagent was added, and the volume was adjusted to 2 mL before measuring absorbance at 412 nm against a blank. GSH enzyme activity (U/mg protein) was calculated using the standard curve for GSH as shown in Figure 2 (Alahmadiet al., 2023).
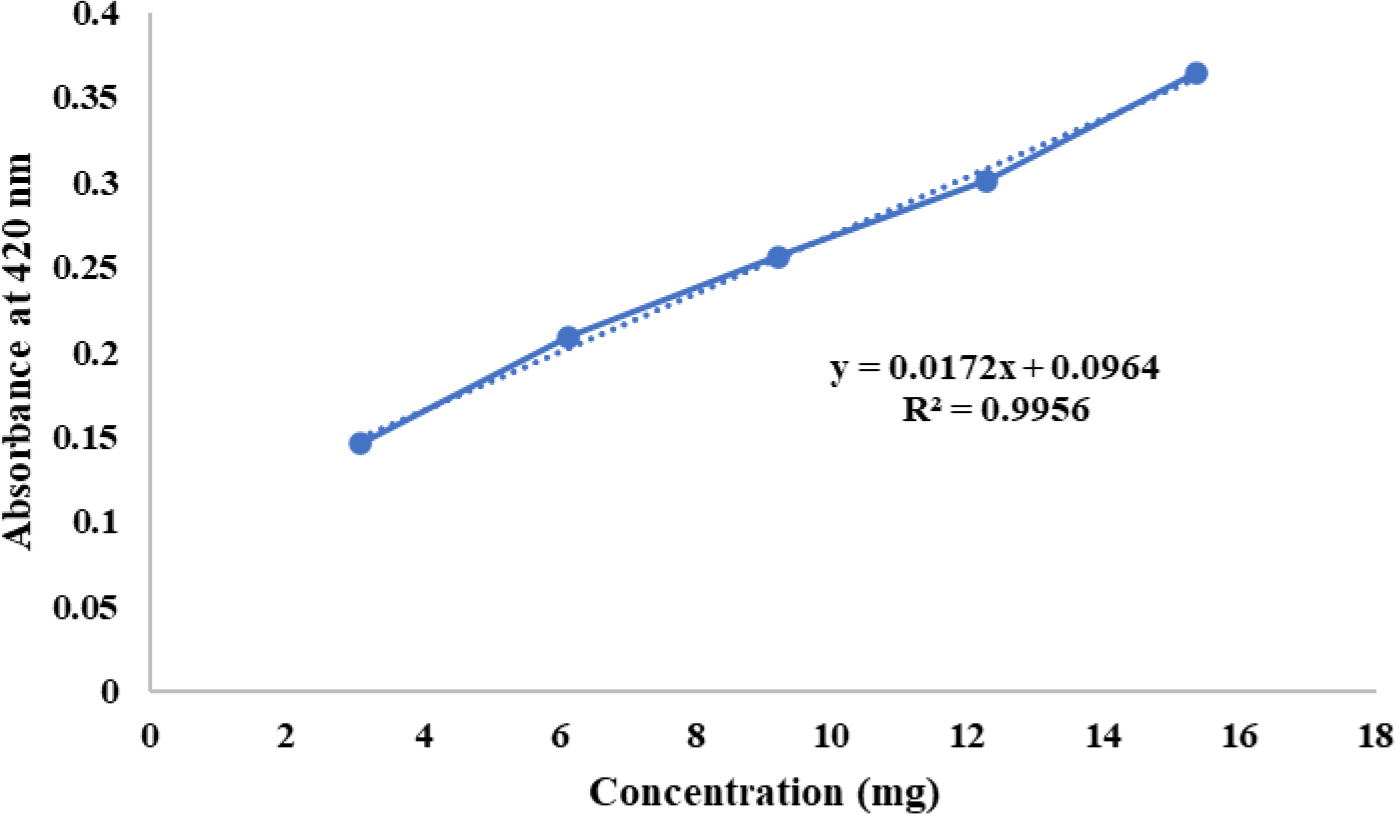
Figure 2:
Standard curve for GSH.
Chromatographic identification and quantification of bioactive Compounds
RP-HPLC instrumentation
HPLC (Agilent 1260 Infinity model, Agilent, USA), which was outfitted with a DAD G4212B detector, a G1311B quaternary pump, and an automatic injector (G1329B). The HPLC was operated by ChemStation software (Agilent, USA).
Preparation of solutions A standard stock solution of Quercetin and vanillic acid was prepared in methanol at 1 mg/mL. A calibration curve was created using a concentration range of 0.10-25 μg/mL. For each experiment, three experimental replicates were used. Extract solutions were made at 10 mg/mL in methanol. Both standard and sample solutions were homogenized for thirty seconds, and then filtered through a 0.22 μM membrane. An aliquot was placed in an amber vial and sealed before being injected into the HPLC unit.
Preparation of mobile phase Quercetin was separated from SME using an isocratic mobile phase of HPLC-grade acetonitrile and 0.3% Trichloroacetic Acid (TCA) in water (50:50, v/v) at a flow rate of 0.9 mL/min for 13 min, detected at 254 nm. Vanillic acid was separated using a mixture of distilled water, methanol, and acetic acid (700:300:10 mL) at a flow rate of 1.0 mL/min for 14 min, detected at 260 nm.
Method validation Following ICH requirements, the method’s accuracy, linearity, specificity, Limit of Detection (LOD), precision and Limit of Quantification (LOQ) were all validated.
Statistical analysis
Results were recorded as Mean±SD. Graph Pad (Version 3.0) was used to analyse the statistical data. Student’s t-test determined the significant differences between the outcomes. p<0.05 was considered as significant.
RESULTS
In vitro antioxidant assay
The DPPH, ABTS, and NO assays showed an increase in % RSA with increasing concentrations of the standard and extract (Figures 3–5). The estimated IC50 values were 28.69 µg/mL for AA and 53.82 µg/mL for SME in the DPPH assay, 21.2 µg/mL for AA and 37.43 µg/mL for SME in the ABTS assay, and 13.37 µg/mL for GA and 26.16 µg/mL for SME in the NO assay.
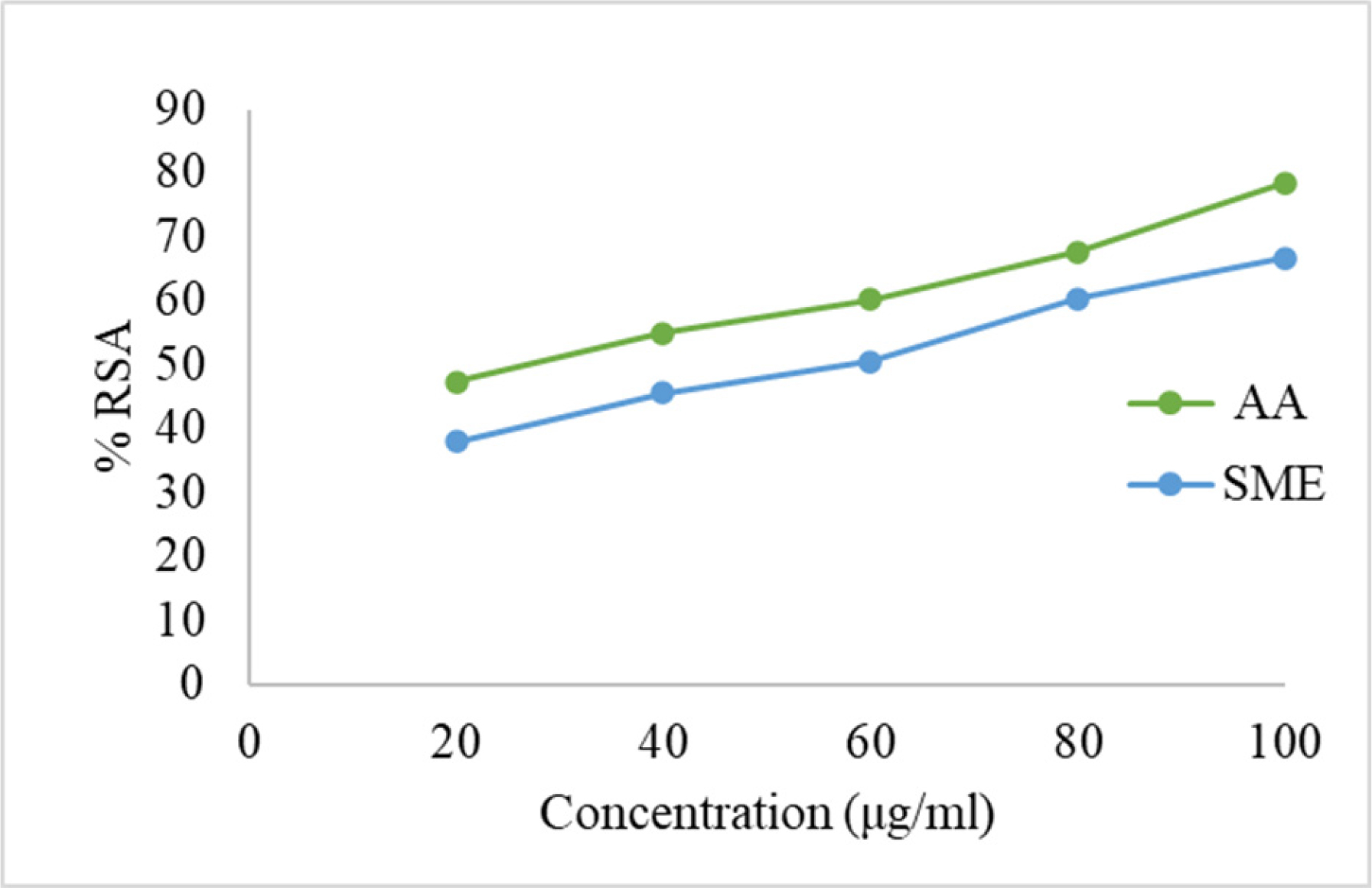
Figure 3:
Calibration curve for AA and SME by DPPH assay.
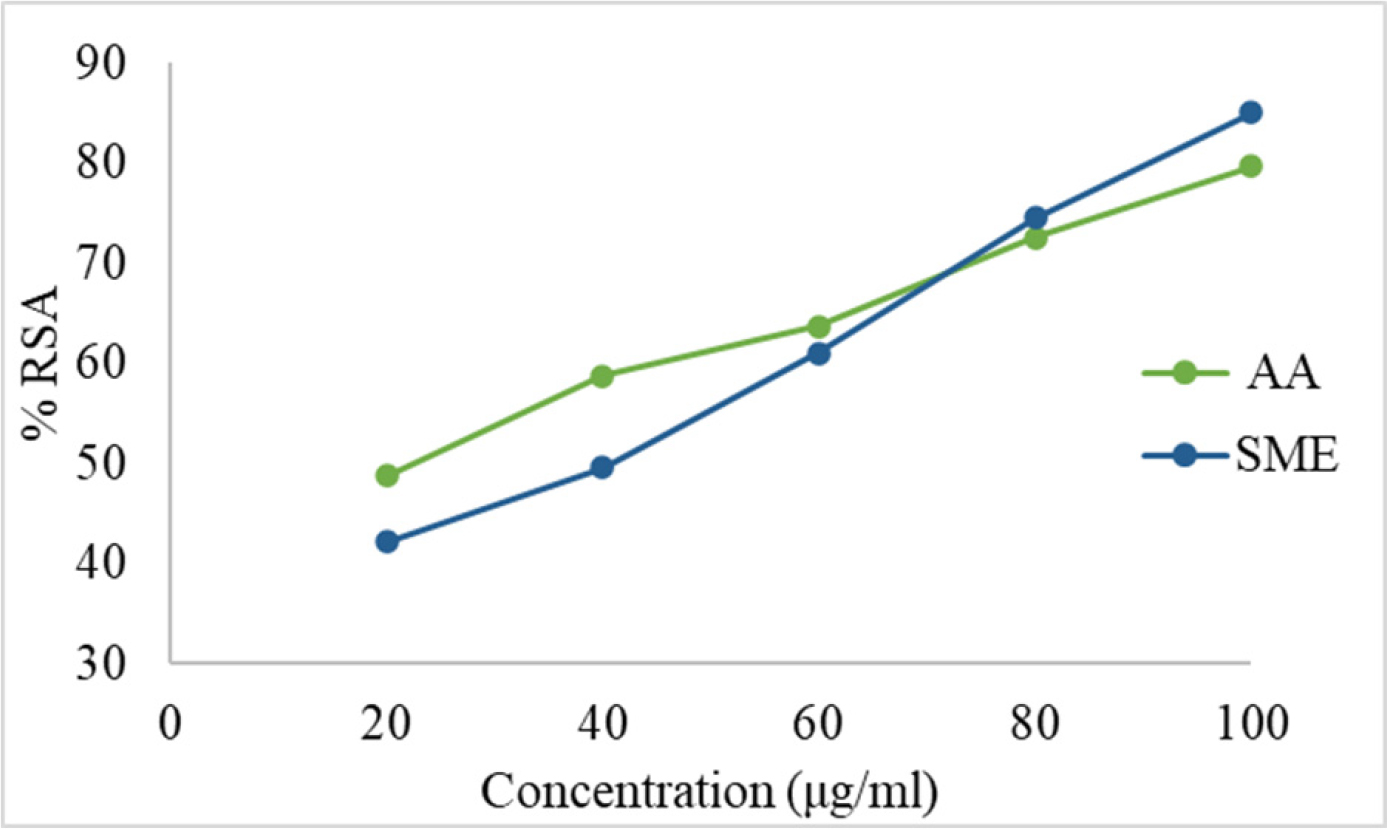
Figure 4:
Calibration curve for AA and SME by ABTS assay.
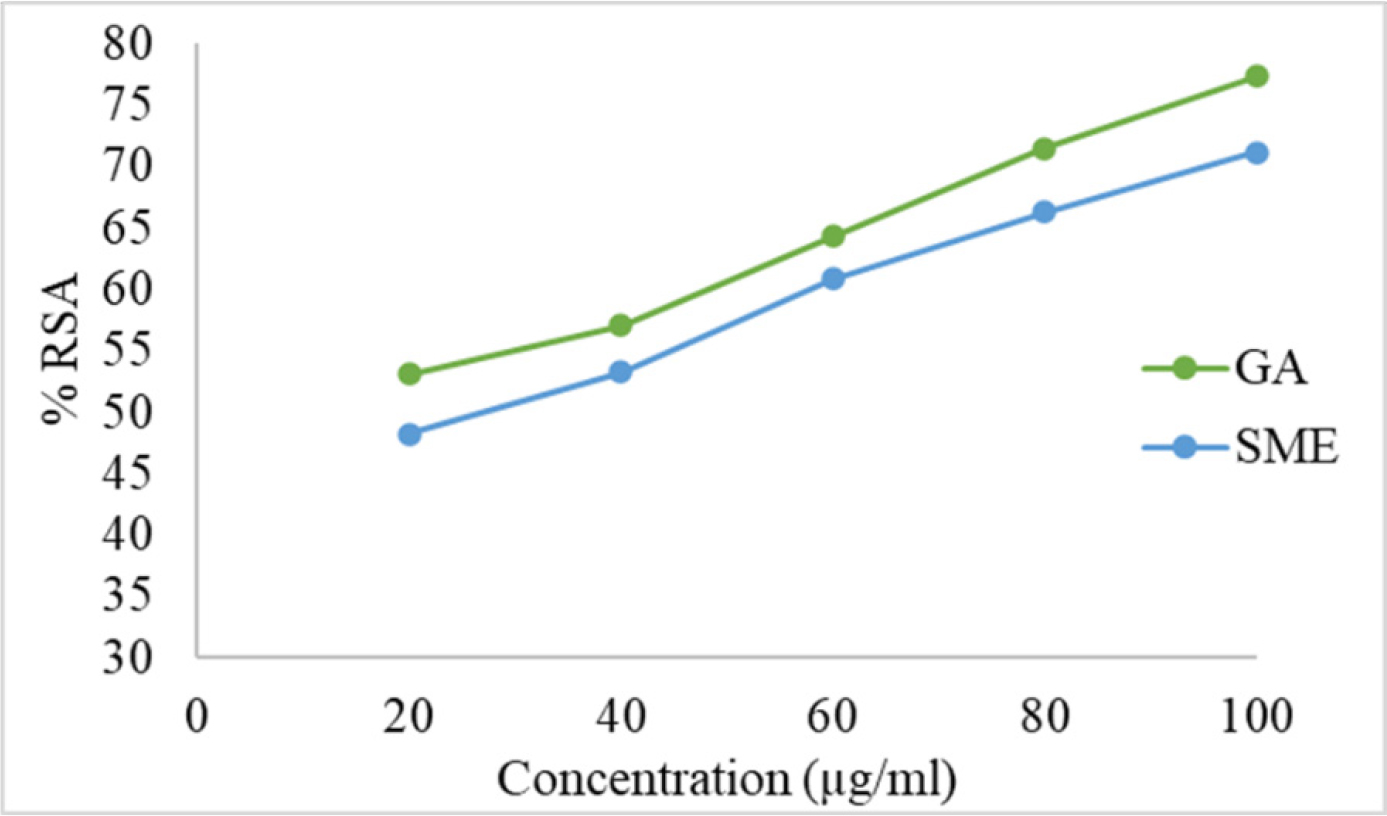
Figure 5:
Calibration curve for GA and SME by NO assay.
In TPC analysis, the calibration curve for Gallic Acid as in Figure 6(a) yielded the equation “y=0.001x+0.0492” (R²=0.9901) for calculating mg GAE/g of extract. For TFC analysis, the Quercetin calibration curve as in Figure 6(b) provided “y=0.0873x+0.0579” (R²=0.996) for calculating mg QE/g of extract. The results indicated that SME contains 28.13±0.2 mg GAE/g and 10.51±0.01 mg QE/g of dry extract
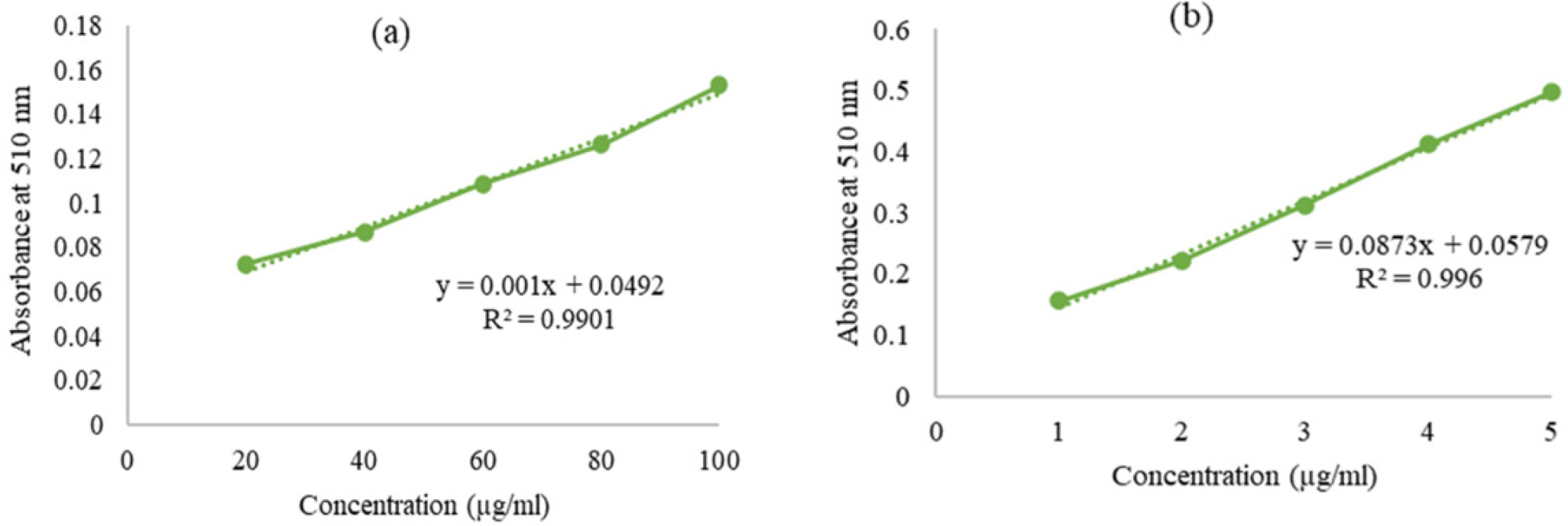
Figure 6:
Calibration curve for (a) Gallic Acid (TPC assay) and (b) Quercetin (TFC assay).
In vivo antioxidant assay
The PCOS induction in female Wistar rats has reduced the levels of oxidative stress markers SOD, GSH, and catalase in all groups except the Negative control. kidney and Liver tissue homogenate of different groups when subjected to SOD, GSH and Catalase assay the results indicated that the 21 days extract treatment had significantly raised the levels of these markers in all the treated groups compared to PC as shown in Tables 1 and 2 respectively.
| Group | Oxidative stress marker levels (Kidney Tissue Homogenate) (After treatment) | ||
|---|---|---|---|
| Mean SOD (U/mg-protein) | Mean GSH (U/mg-protein) | Mean Catalase (U/mg- protein) | |
| NC | 12.23 ± 0.6*a | 124.46 ± 0.002*a1 | 3.4 ± 0.04*a2 |
| PC | 5.47 ± 0.7*b | 35.502 ± 0.001*b1 | 1.7 ± 0.03*b2 |
| Met | 10.96 ± 0.3*c | 109.70 ± 0.004*c1 | 3.15±0.03*c2 |
| SME | 10.319 ± 0.3*d | 88.543 ± 0.004*d1 | 2.61 ± 0.004*d2 |
| Group | Oxidative stress marker levels (liver Tissue Homogenate) (After treatment) | ||
|---|---|---|---|
| Mean SOD (U/mg-protein) | Mean GSH (U/mg-protein) | Mean Catalase (U/mg- protein) | |
| NC | 10.27 ± 0.8*a | 110.50 ± 0.003*a1 | 3.1 ± 0.08*a2 |
| PC | 3.80 ± 0.5*b | 27.26 ± 0.007*b1 | 1.6 ± 0.01*b2 |
| Met | 9.20 ± 0.7*c | 96.15 ± 0.004*c1 | 2.96±0.01*c2 |
| SME | 7.00 ± 0.6*d | 90.24 ± 0.005*d1 | 2.90 ± 0.004*d2 |
RP- HPLC
Linearity A range of 0.10-25 μg/mL was taken as the calibration range. Vanillic acid and quercetin were determined to have 0.997 and 0.994 as coefficients of determination (R2), respectively.
Specificity According to the specificity test, the well-shaped peak showed that the main peak of bioactive chemicals is not impacted by other components found in the extract.
LOD and LOQ Quercetin’s LOD and LOQ were determined to be 0.184±0.6 and 0.723±0.9 µg/mL, respectively, while that for vanillic acid were 0.171±0.2 and 0.732±0.5 µg/mL, respectively.
Accuracy: The high recovery values demonstrated the method’s accuracy for vanillic acid and quercetin, which ranged from 99.40 to 99.91% and 99.53 to 99.90%, respectively.
Precision: The remarkable repeatability of the procedure is confirmed by the finding that the percentage RSD of both intra-day and inter-day precision was less than 0.20%.
Robustness: The robustness was assessed by taking the standard solution (n=3) of quercetin and vanillic acid with slight variations of ± 2 in flow rate, column temperature, pH, and wavelength. However, no appreciable variations in the recovery, peak area, or retention time were noted.
The RP-HPLC analysis of SME showed Quercetin peak at a retention time of 2.643 min with a peak area of 24424 and the calculated value of the bioactive compound was 3.342%. Figure 7 represents the peaks for (a) Quercetin standard and (b) SME. Figure 8 represents the peaks for (a) Vanillic acid standard and (b) SME in similar chromatographic conditions. SME showed a vanillic acid peak at a retention time of 6.258 with a peak area of 3283 and the calculated value of the bioactive compound was 2.312%.
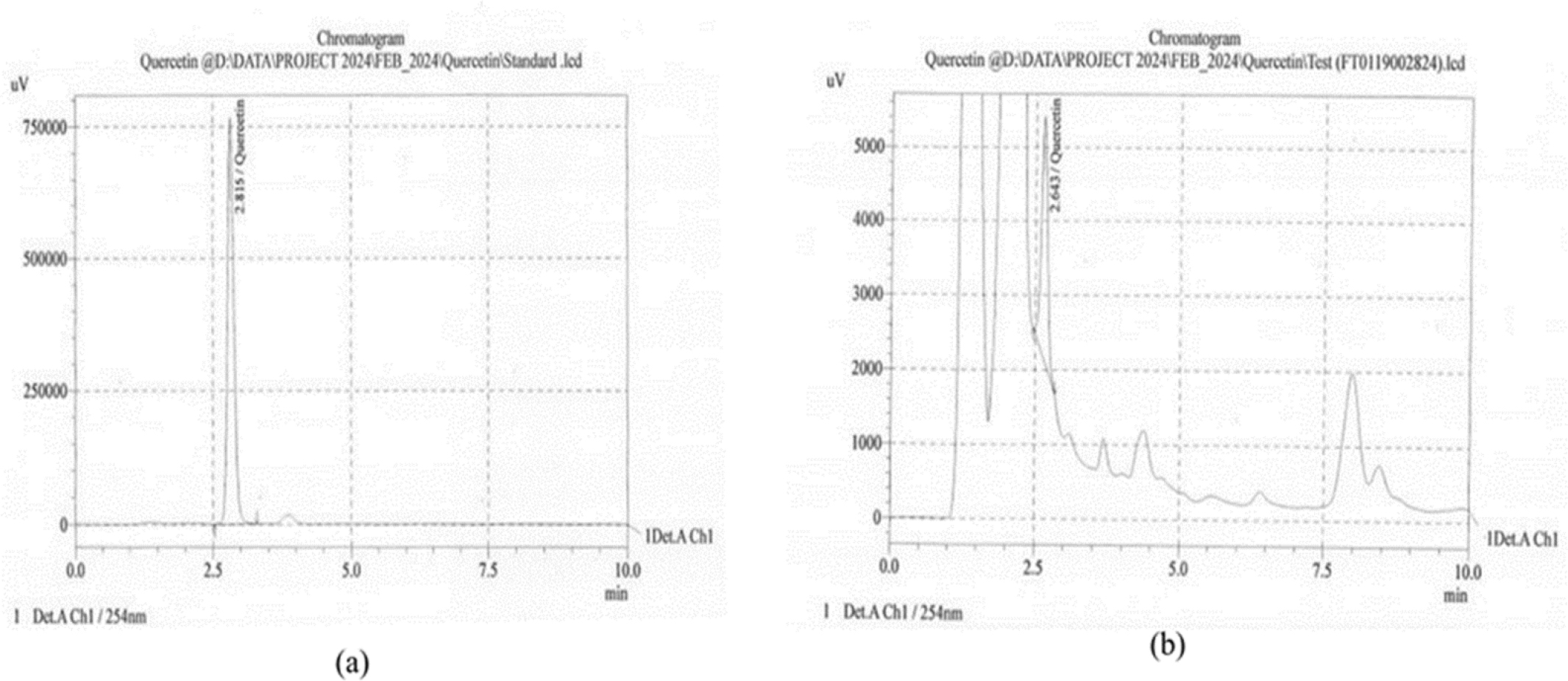
Figure 7:
HPLC Peak for (a) Quercetin standard (b) SME.
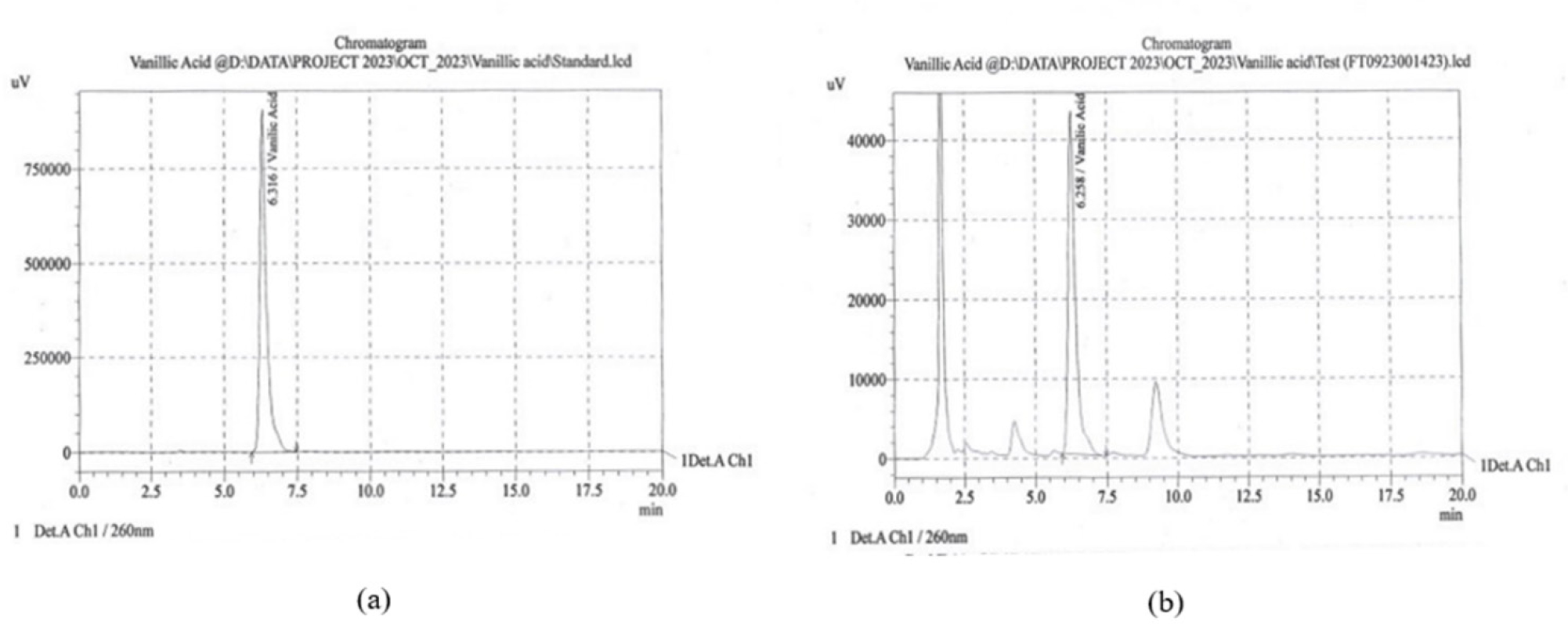
Figure 8:
HPLC peak for (a) Vanillic acid standard (b) SME.
DISCUSSION
Phyto-antioxidants protect cells from oxidative stress by scavenging free radicals, with various phytochemicals playing a role in health promotion and disease prevention (Dibaet al., 2021; Mishraet al., 2020; Pandeyet al., 2009; Bhattacharjeeet al., 2022). Research indicates essential phytochemicals are present in the methanolic extract of D. palmatus (Attaret al., 2017). In vitro assays (DPPH, ABTS, Nitric oxide) evaluated the antioxidant potential of SME, revealing IC50 values comparable to standards. TPC and TFC analysis showed SME has 28.13±0.2 mg GAE/g and 10.51±0.01 mg QE/g dry weight. The results were consistent with the earlier research demonstrating the presence of polyphenols, a class of antioxidants in the methanolic extract of D. palmatus (Upadhyayet al., 2023). In vivo assays demonstrated reduced oxidative stress markers in PCOS female Wistar rats treated with SME, along with increased levels in liver and kidney tissue homogenate. RP-HPLC analysis confirmed vanillic acid and quercetin as the primary antioxidants in SME, supporting their high antioxidant capacity.
CONCLUSION
The study concluded that the SME of D. palmatus exhibits significant antioxidant activity with vanillic acid and quercetin identified as the main antioxidants by RP-HPLC. Therefore, the research promises to create a safe, all-natural health supplement.
Cite this article:
Yadav R, Yadav JP. Exploring the Antioxidant Properties and Bioactive Compounds of Methanolic Extract of Diplocyclos palmatus Seeds. J Young Pharm. 2025;17(3):551-8.
ACKNOWLEDGEMENT
The authors are thankful to the Head, Department of Genetics and Animal House, Maharshi Dayanand University, Rohtak, Haryana, India, for providing the facilities to carry out the trial.
ABBREVIATIONS
| SME | Seeds Methanolic Extract |
|---|---|
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| NO | Nitric Oxide |
| FRAP | Ferric Reducing Antioxidant Power |
| ABTS | 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| TFC | Total Flavonoid Content |
| TPC | Total Phenolic Content |
| SOD | Superoxide Dismutase |
| RP-HPLC | Reversed-phase high-performance liquid chromatography |
| TLC | Thin Layer Chromatography |
| ANOVA | Analysis of Variance |
| ROS | Reactive Oxygen Species |
| TPTZ | 2,4,6-Tripyridyl-S-triazine |
| SNP | Sodium Nitroprusside |
| AA | Ascorbic acid |
| GA | Gallic acid |
| EDTA | Ethylenediamine Tetra acetic Acid |
| DTNB | 5,5’-dithio-bis-2-nitrobenzoic acid |
| TCA | Trichloroacetic Acid |
| IC50 | Half-maximal inhibitory concentration |
| PCOS | Polycystic Ovary Syndrome |
| Cont. | Control |
| LOD | limit of detection |
| LOQ | limit of quantification |
| ICH | International Conference on Harmonisation |
| LQC | Low-quality control |
| MQC | Medium-quality control |
| HQC | High-quality control |
| RSD | Relative Standard Deviation |
| SD | Standard Deviation. |
References
- Alahmadi T. A., Alharbi S. A., Ravindran B., Saravanan K.. (2023) Evaluation of Antioxidant and Oxidative Stress Activity of L. Extract in lung cancer A549 cells. Indian Journal of Pharmaceutical Education and Research 57: 1112-1118 https://doi.org/10.5530/ijper.57.4.134 | Google Scholar
- Atanasov A. G., Zotchev S. B., Dirsch V. M., Supuran C. T.. (2021) Natural products in drug discovery: Advances and opportunities. Nature Reviews. Drug Discovery 20: 200-216 https://doi.org/10.1038/s41573-020-00114-z | Google Scholar
- Attar U. A., Ghane S. G.. (2017) Phytochemicals, antioxidant activity and phenolic profiling of (L.) C. Jeffery. International Journal of Pharmacy and Pharmaceutical Sciences 9: 1-6 https://doi.org/10.1038/s41573-020-00114-z | Google Scholar
- Bajpayee K. K., Ashaq M.. (2024) Medicinal plants used in different diseases. Journal of Research in Science Teaching 3: 55-68 https://doi.org/10.1038/s41573-020-00114-z | Google Scholar
- Balkrishna A., Sharma N., Srivastava D., Kukreti A., Srivastava S., Arya V., et al. (2024) Exploring the safety, efficacy, and bioactivity of herbal medicines: Bridging traditional wisdom and modern science in healthcare. Future Integrative Medicine 3: 35-49 https://doi.org/10.14218/FIM.2023.00086 | Google Scholar
- Bhattacharjee S., Deb P. K., Devi R.Rudrapal M.. (2022) Phytoantioxidants and Nanotherapeutics : 77-98 https://doi.org/10.1002/9781119811794.ch4 | Google Scholar
- Chaachouay N., Zidane L.. (2024) Plant-derived natural products: A source for drug discovery and development. Drugs and Drug Candidates 3: 184-207 https://doi.org/10.3390/ddc3010011 | Google Scholar
- Chauhan N. S., Dixit V. K.. (2010) Effects of seeds on sexual behaviour of male rats. International Journal of Impotence Research 22: 190-195 https://doi.org/10.1038/ijir.2009.62 | Google Scholar
- Das S., Hallur R. L., Mandal A. B.. (2023) Protective activity and antioxidant potential. Biomedicine 43: 595-602 https://doi.org/10.1038/ijir.2009.62 | Google Scholar
- delaRosa A., Hendrawan R. P., Halimah E.. (2023) Screening of and determination of antioxidant potential using DPPH method: A review. European Journal of Medicinal Plants 34: 17-28 https://doi.org/10.9734/ejmp/2023/v34i71146 | Google Scholar
- Desai B. N., Maharjan R. H., Nampoothiri L. P.. (2012)
Aloe barbadensis Mill. formulation restores lipid profile to normal in a letrozole-induced polycystic ovarian syndrome rat model. Phcog Res 4: 1-7 https://doi.org/10.9734/ejmp/2023/v34i71146 | Google Scholar - Diba M., Seghatoleslam A., Namavari M., Assadi S., Vakili S. N., Babaei Z., Akmali M., et al. (2021) Potential Protective Role of in Antioxidant Parameters in Serum and Liver of Rats with 5-FU-Induced Oxidative Damage. Archives of Razi Institute 76: 95-105 https://doi.org/10.22092/ari.2019.126702.1356 | Google Scholar
- Hussen E. M., Endalew S. A.. (2023)
In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina. BMC Complementary Medicine and Therapies 23: 146 https://doi.org/10.1186/s12906-023-03923-y | Google Scholar - Islam M. R., Kamal M. M., Kabir M. R., Hasan M. M., Haque A. R., Hasan S. M. K., et al. (2023) Phenolic compounds and antioxidants activity of banana peel extracts: Testing and optimization of enzyme-assisted conditions. Measurement: Food 10: 1-7 https://doi.org/10.1016/j.meafoo.2023.100085 | Google Scholar
- Jomova K., Alomar S. Y., Alwasel S. H., Nepovimova E., Kuca K., Valko M., et al. (2024) Several lines of antioxidant defence against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Archives of Toxicology 98: 1323-1367 https://doi.org/10.1007/s00204-024-03696-4 | Google Scholar
- Kakadia N., Patel P., Deshpande S., Shah G.. (2019) Effect of L. seeds in letrozole induced polycystic ovarian syndrome. Journal of Traditional and Complementary Medicine 9: 336-345 https://doi.org/10.1016/j.jtcme.2018.03.001 | Google Scholar
- Kaurav H., Choudhary S., Chaudhary G.. (2021)
Bryonia laciniosa an ayurvedic herbal plant “Bryonia laciniosa” with its ethnomedicinal significance. Journal of Drug Delivery and Therapeutics 11: 137-141 https://doi.org/10.22270/jddt.v11i3-S.4889 | Google Scholar - Mishra T., Kondepati A. K., Pasumarthi S. D., Chilana G. S., Devabhaktuni S., Singh P. K., et al. (2020) Phytotherapeutic antioxidants. Asian Journal of Medical Sciences 11: 96-100 https://doi.org/10.3126/ajms.v11i2.26465 | Google Scholar
- Pandey K. B., Rizvi S. I.. (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity 2: 270-278 https://doi.org/10.4161/oxim.2.5.9498 | Google Scholar
- Patel S. B., Attar U. A., Sakate D. M., Ghane S. G.. (2020) Efficient extraction of cucurbitacins from (L.) C. Jeffrey: Optimization using response surface methodology, extraction methods, and study of some important bioactivities. Scientific Reports 10: 1 https://doi.org/10.4161/oxim.2.5.9498 | Google Scholar
- Reddy J., Gnanasekaran D., Vijay D., Ranganathan T. V.. (2010)
In vitro studies on antiasthmatic, analgesic and anticonvulsant activities of the medicinal plant Bryonia laciniosa. Linn. International Journal of Drug Discovery 2: 01-10 https://doi.org/10.4161/oxim.2.5.9498 | Google Scholar - Salmerón-Manzano E., Manzano-Agugliaro F.. (2020) Bibliometric studies and worldwide research trends on global health. International Journal of Environmental Research and Public Health 17: Article 5748 https://doi.org/10.3390/ijerph17165748 | Google Scholar
- SenGupta P., Dutta S., Fallah Hassan M. F.. (2024) Polycystic ovary syndrome (PCOS) and oxidative stress. Journal of Integrated Science and Technology 12: 752-752 https://doi.org/10.62110/sciencein.jist.2024.v12.752 | Google Scholar
- Siswoyo T. A., Arum L. S., Sanjaya B. R. L., Aisyah Z. S.. (2021) The growth responses and antioxidant capabilities of melinjo ( L.) in different durations of drought stress. Annals of Agricultural Sciences 66: 81-86 https://doi.org/10.1016/j.aoas.2021.05.003 | Google Scholar
- Sivakumar T., Perumal P., Kumar R. S., Vamsi M. L., Gomathi P., Mazumder U. K., Gupta M., et al. (2004) Evaluation of analgesic, antipyretic activity and toxicity study of in mice and rats. The American Journal of Chinese Medicine 32: 531-539 https://doi.org/10.1142/S0192415X0400217X | Google Scholar
- Syman K., Turpanova R., Nazarova G. A., Berdenkulova A. Z., Tulindinova G. K., Korogod N. P., Utegaliyeva R., et al. (2024) Role of oxidative stress enzymes in abiotic and biotic stress. Casp J. Environ. Sci. 22: 521-528 https://doi.org/10.1142/S0192415X0400217X | Google Scholar
- Tekin S., Seven E.. (2022) Assessment of serum catalase, reduced glutathione, and superoxide dismutase activities and malondialdehyde levels in keratoconus patients. Eye 36: 2062-2066 https://doi.org/10.1038/s41433-021-01753-1 | Google Scholar
- Tiwana J. K., Shah A. K., Dhalla N. S.. (2024) The involvement of reactive oxygen species in causing chronic cardiovascular and neurodegenerative diseases and some cancers. Scripta Medica 55: 199-217 https://doi.org/10.5937/scriptamed55-48730 | Google Scholar
- Upadhyay P., Chaturvedi P., Dwivedi S.. (2023) Evaluation of anti-inflammatory activity of Hydro-alcoholic extract (L.) Jeffry. roots in experimental animals. International Journal of Pharmaceutical Sciences 14 https://doi.org/10.5937/scriptamed55-48730 | Google Scholar
- Varah F., Desai P. N.. (2023) Mapping the emerging innovation system in the Indian ethnobotany genomics. Technology Analysis and Strategic Management 35: 352-364 https://doi.org/10.1080/09537325.2021.1975035 | Google Scholar
