ABSTRACT
Background
An over-prescription of opioid analgesics triggered the opioid epidemic. Repeated opioid administrations are needed to treat chronic pain, leading to tolerance and physical dependence.
Aim
Marrubium vulgare Extract (MVE) was used in this study to investigate its effects on reducing symptoms of Morphine Tolerance (MT) and Withdrawal Syndrome (WS).
Materials and Methods
To evaluate MT, 6 rat groups (n=8) were studied in which first group received only morphine (10 mg/kg/day) and the second, third, fourth and fifth ones received morphine with different doses of extract (E20, 40 and80 mg/kg/day) or vehicle (0.25 mL/ rat DMSO) intraperitoneally. The sixth group received only extract (80 mg/kg, i.p as the most effective dose of extract). The hot-plate test was done every other day, 90 min after the injections. In order to study WS, 49 rats were divided into seven groups (n=7), including: morphine, vehicle + morphine, saline, extract (10, 20 and 40 mg/kg) + morphine, and effective dose of extract without morphine (20 mg/kg).
Results
The rats were rendered dependent by injection of additive doses of morphine for 9 days. In 9th day, naloxone (4 mg/kg) was injected. Withdrawal signs were calculated for an hour. Complete tolerance was induced in the morphine group on day 7. The groups receiving extract doses of 20, 40, and 80 mg/kg showed complete tolerance on days 9, 11, and 19, respectively. MVE significantly attenuated withdrawal symptoms.
Conclusion
At last, the obtained results suggest that M. vulgare extract inhibits morphine tolerance and decreases morphine withdrawal syndrome when co-administered with morphine.
INTRODUCTION
Pain is the defense mechanism of body during tissue damage, which causes a person to illustrate reaction and eliminate pain agent. Insufficient relief of pain can delay healing process. Opioids (due to their superior analgesic effectiveness) have been used for many years for pain control related to different clinical situations. However, the increase in pain-relieving tolerance (which indicates a loss of efficacy over time) and physical addiction have limited their usage.1 In some cases of tolerance, even the highest dosage of an opioid cannot provide desired analgesic effect. In other words, higher doses of opioids are required by pain-afflicted patients for maintaining a regularly pain-free state, elevating the risk of fatal overdose, dependence, and addiction.2 The other limitation is withdrawal syndrome which is considered an important problem in the cure of dependent patients.3 Tolerance to morphine is multifaceted and its accurate mechanism is still not recognized.
Since many years ago, natural products have been prescribed widely for relieving and treating diseases especially due to their effectiveness, low cost and insignificant side effects. In recent years, some herbal drugs with anti-inflammatory features like Corydalis yanhusuo4 to delay morphine-induced tolerance and decrease withdrawal syndrome are getting a great deal of attention.5
Marrubium vulgare, a well-known herb related to the Lamiaceae family and Marrubium genus is also called “Marrubia” in Tunisia, “horehound” in Europe, and “ Faracion or Gandenaye kouhi “ in Iran.6 This plant can grow up to 60 cm tall. Precise analysis represented that the plant included sterols, alkaloids, steroids, terpenoids (especially diterpenoids like marrubiin and premarrubiin), saponins, flavonoids (which ameliorate hyperglycaemia and dyslipidemia in rats),7 phenolic compounds8 and many other bioactive ingredients.
According to the pharmacological assessments, the plant exerted anti-oedematogenic, anti-microbial,9 anti-fungal,10 larvicidal,11 anti-inflammatory,12 antioxidant,13 analgesic,14 anti-diabetic,15 anti-spasmodic,16 cardiovascular hypolipidemic, and several other biological effects.
In a study, it was revealed that terpenes and steroids of M. vulgare relieve inflammatory pain in a non-opioid way. Premarrubiin, β-sitosterol, and marrubiin have analgesic and anti-inflammatory activities by modulation of B2 receptors against prostaglandin and bradykinin receptors.13 Also, phenylpropanoid esters available in this herb had a key role in the anti-inflammatory activity by affecting selective inhibition of the cyclooxygenase 2 enzyme and neutrophil’s oxidative metabolism.12
There are at least two ways for opioid receptor desensitization through phosphorylation by second messenger kinases like Protein Kinase C (PKC) and phosphorylation by GRK.17 Tolerance induced by opioid receptor agonists with low and moderate efficacy is based on PKC, while high-efficacy agonists-induced tolerance is based on GRK. PKC is activated by the chronic administration of morphine.18 Therefore, opioid tolerance can be delayed by PKC inhibitors. One phenylpropanoid non-glycosidic ester (caffeoyl-L-malic acid) showed antioxidant activity by interfering with the PKC path.19
In present research, the effects of using MVE on the development of MT and WS were assessed in male rats. As we know, no reports have been presented about MVE on delaying morphine-induced tolerance and withdrawal syndrome attenuation. Thus, we made our best effort to clarify the effects of MVE on attenuating withdrawal syndrome and delaying tolerance induced by morphine for the first time.
MATERIALS AND METHODS
Animals
Male Wistar rats (225-275 g) were acquired from the Pasteur Institute (Tehran, Iran) and kept at relative humidity (50±10%) and standard temperature (22±2°C) in polypropylene cages (10 per cage with appropriate water and food). After that, they were separated randomly into numerous experimental groups. The 7-9 rats of all groups were separated in two cages (4 per cage). It must be noted that, 2 days before the experiment, the rats were adapted to testing situation (such as transporting to the experimental laboratory, management to adapt the animals, and weighing to minimize the nonspecific stress responses).
All experiments were done by the Guide for Care and Use of Laboratory Animals of Tabriz University of Medical Sciences (National Institutes of Health Publication No 85-23). Number of approved ethics certificate for the use of animals was IR.TBZMED. REC.1395.169 (letter number was 5/D/19649) and the name of the institution that has provided it was The Ethics Committee of Tabriz University of Medical Sciences.
Drugs
Morphine sulfate (Darupakhsh Company, Iran) in the normal saline was injected subcutaneously (s.c) or intraperitoneally (i.p) according to the experiment protocol. Naloxone hydrochloride (Darupakhsh Company, Iran) in the normal saline was injected i.p, injection of the plant extracts was performed as i.p in various regimes and doses.
Plant extract
M. vulgare plant was gathered in appropriate time from the north part of Iran and its species was confirmed by the relevant expert. After grinding the dried aerial parts and extraction (with methanol) via maceration at room temperature, a rotary evaporator was utilized to remove solvent at 40°C. A greenish residue was kept in a refrigerator (in an airtight bottle) till use. Chemical components were identified by subjecting the final methanolic extract to phytochemical screening. The silica gel was subjected to GF254 Thin-Layer Chromatography (TLC) (95:5 chloroform: methanol). Detecting spots by spraying H2SO4 10% in ethanol indicated the existence of Marrubiin at Rf=0.63 in the extract.20
Morphine Tolerance (MT)
Male Wistar rats were injected once daily and the hot plate test was performed on day 7 for tolerance- like behavior. They were allocated to 6 experimental groups (n=8) including morphine (10 mg/kg, i.p) (group 1), morphine (10 mg/kg, i.p) in combination with three various doses of the extract (20, 40, and 80 mg/kg, i.p) (groups 2, 3 and 4) and morphine (10 mg/kg, i.p) in combination with vehicle (0.25 mL/rat DMSO 50% in saline, i.p) (group 5) and the last group was only extract (80 mg/kg, i.p) (the most effective dose) without morphine (group 6). Nociception was examined every other day 30 min after injection via a hot plate test (55±0.5°C).21
Rats were located on a stainless-steel surface (23×23 cm) surrounded by a plexiglass wall (height of 20 cm) (maintained at 55 ± 2°C) and when the animal licked its hind paw, latency time was measured (40 sec was considered as a cut-off time to keep away from any possible tissue injury).
Morphine withdrawal
49 rats were separated into 7 experimental groups (n=7) randomly. In 5 groups, morphine was injected subcutaneously for 9 days every 12 hr according to the following protocol: the first day (5 mg/kg, sc), the second and third days (10 mg/kg, sc), the 4th and 5th days (15 mg/kg, sc), the 6th and 7th day (20 mg/kg, sc) and on the eighth and ninth day (25 mg/kg, sc) was injected. On the ninth day, 2 hr after morning dose of morphine, a dose of naloxone (4 mg/kg, i.p) was injected.
First group received only morphine as discussed. Groups II, III, and IV received various doses of extract (10, 20, and 40 mg/kg, i.p) 1 hr after morphine. In group V, vehicle (0.25 mL/rat DMSO 50% in saline, i.p) was injected 1 hr after morphine. Group VI was injected saline (1 mL/kg/12 hr, i.p) 1 hr after morphine administration. Extract (20 mg/kg/12 hr, i.p) (effective dose) was injected alone in group VII.
To precipitate withdrawal signs, naloxone (4 mg/kg, i.p) was injected 2 hr after the last morphine administration. After naloxone challenge, rats were transferred immediately to a filter paper individually in an open plexiglass chamber. Recorded withdrawal signs include jumping, pulling the stomach on the ground, head shakes, genital grooming and other remarkable signs. The number of withdrawal signs was counted for 60 min after the naloxone injection and then based on the adjusted Rasmussen technique22 was divided by their weighing factor and 11 Total Withdrawal Scores (TWS) were obtained by the summation of these values so, the values attained for each parameter were divided by standard values (Table 1). Summing up the numbers, an average was determined for each group, which was reported as a Total Withdrawal Score (TWS). TWS can help to accurately estimate the overall impact of the extract on the withdrawal signs. Some features like: rearing and body grooming are not specified for the withdrawal syndrome and these behaviors can be occurred by the animal in normal condition. They have a great weight factor therefore the number of each symptom is divided by a larger weight factor. The obtained value will be smaller and will have less impact on TWS. However, some behaviors like jumping and wet-dog shakes can be rarely occurred by the animal in normal state. So, these features will be divided by a small weight factor and the resulting number will be larger and will be more valuable in TWS.
| Withdrawal symptoms | Weighting factor |
|---|---|
| Jumping | 4 |
| Pulling the stomach on the ground | 5 |
| Wet -dog shakes | 5 |
| Head shakes | 5 |
| Paw tremor | 5 |
| Genital grooming | 5 |
| Body grooming | 10 |
| Face wiping | 10 |
| Teeth chattering | 10 |
| Swallowing | 10 |
| Rearing | 20 |
Locomotor activity
In a group receiving the effective dose of extract, locomotor activity (open field test) was performed on day 1 and 9 before morphine injection. A box made of plywood with dimensions of 100 cm × 100 cm × 40 cm painted in white was used. The box was divided into 25 squares (20 cm × 20 cm). Each rat was placed in the middle square of the field and the number of the crossing lines was counted within 30 min (Total locomotion).
Statistical analysis
All analyses were conducted via Sigma plot ver. 12.2 software. To evaluate the normality of obtained data, Shapiro-wilk test was performed. If data was not normally distributed, Kruskal Wallis test was preferred as a non-parametric method and equivalent of ANOVA to compare the means in variables under study of the groups (to find complete tolerance day). Furthermore, comparison of the groups with morphine + vehicle group was done by post hoc Tukey’s test. p values< 0.05 were considered significant.
RESULTS
Morphine tolerance
To measure anti-nociceptive activities, foot withdrawal latency was used in the hot plate assay. Rats were tested first prior to injection for their basal foot withdrawal latency response which was similar within the range of 12-15 sec. The rats were then intraperitoneally injected (i.p) with the vehicle, morphine and variable doses of plant extract. The results obtained from Shapiro-wilk test are presented in Table 2. As can be seen, data was not normally distributed in many groups.
| Variables | Groups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E80 | M+E80 | M+E40 | M+E20 | M | M+V | |||||||
| d f | p-value | d f | p-value | d f | p-value | d f | p-value | d f | p-value | d f | p-value | |
| BL | 9 | 0/04* | 9 | 0*** | 9 | 0/96 | 9 | 0/06 | 9 | 0/13 | 9 | 0/82 |
| TL1 | 9 | 0/2 | 9 | 0/02* | 9 | 0/09 | 9 | 0/04* | 9 | 0*** | 9 | 0*** |
| TL3 | 9 | 0/02* | 9 | 0*** | 9 | 0/03* | 9 | 0/01** | 9 | 0/02* | 9 | 0/1 |
| TL5 | 9 | 0/34 | 9 | 0*** | 9 | 0*** | 9 | 0*** | 9 | 0/06 | 9 | 0/1 |
| TL7 | 9 | 0/42 | 9 | 0*** | 9 | 0*** | 9 | 0*** | 9 | 0*** | 9 | 0/37 |
| TL9 | 9 | 0/07 | 9 | 0/87 | 9 | 0*** | 9 | 0/17 | 9 | 0/5 | 9 | 0/49 |
| TL11 | 9 | 0/02* | 9 | 0/23 | 9 | 0/01** | 9 | 0/34 | 9 | 0/14 | 9 | 0/44 |
| TL13 | 9 | 0/13 | 9 | 0/78 | 9 | 0/21 | 9 | 0/1 | 9 | 0*** | 9 | 0/47 |
| TL15 | 9 | 0/07 | 9 | 0/91 | 9 | 0/3 | 9 | 0/19 | 9 | 0*** | 9 | 0/29 |
| TL17 | 9 | 0/04* | 9 | 0/27 | 9 | 0/27 | 9 | 0/02* | 9 | 0/1 | 9 | 0/4 |
| TL19 | 9 | 0/4 | 9 | 0/17 | 9 | 0/69 | 9 | 0/17 | 9 | 0/29 | 9 | 0/21 |
No remarkable differences were found between the mean latency time of morphine group and morphine + vehicle group over the test days. To assess complete tolerance, morphine was injected along with vehicle, plant extract, or a combination of them daily for 23 days. Complete tolerance was induced in the morphine group on day 7. As can be seen in Figure 1, by administering morphine and different doses of plant extract, a delay occurred in complete tolerance against morphine antinociceptive effects (p<0.01) and complete tolerance in these groups happened on days 9, 11, and 19, respectively. No analgesic effect was observed in the group with an only injection of plant extract (80 mg/kg) when evaluating in the hot-plate test. The results showed that morphine tolerance is merely delayed by the extract as would be expected with synergistic analgesia.
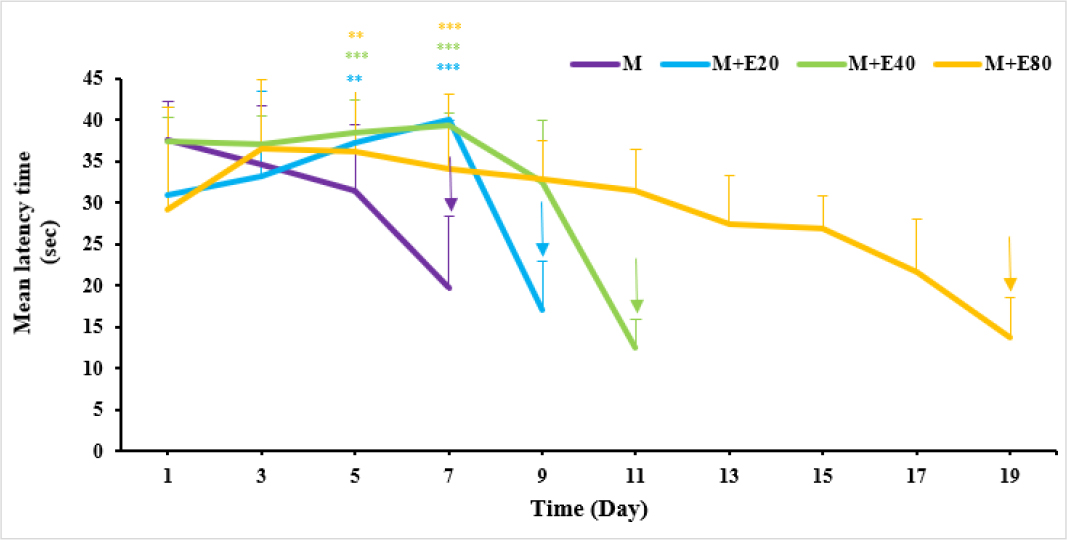
Figure 1:
The effects of administration vehicle and 3 different doses of M. vulgare extract on morphine-induced tolerance in rats. Post hoc Tukey’s test was used to compare groups.
Shown statistical differences are (* p<0.05, ** p<0.01, and *** p<0.001) compared to the morphine + vehicle group. M: Morphine; E20, E40 and E80: 20, 40 and 80 mg/kg extract.
Morphine withdrawal
The effects of morphine and plant extract on withdrawal were investigated, by observing chronically treated animals followed by injection of naloxone. Withdrawal behavior, like body grooming, head shakes, jumping, genital grooming, teeth chattering, face wiping, rearing, and chewing were scored over 60 min period. According to the results, no remarkable differences were observed between the morphine group and vehicle-treated+morphine group. The TWS in morphine group was increased significantly in comparison to the saline group (p<0.001) and the group receiving an effective dose of extract alone (p<0.001). The extract made a significant difference versus morphine + vehicle group (p<0.01 for 40 mg/kg and p<0.001 for 20 mg/kg) as depicted in Table 3. It was found that the M. vulgare extract significantly attenuated withdrawal signs (Figure 2).
| Signs | M | M+E10 | M+E20 | M+E40 | Saline | M+V | E20 |
|---|---|---|---|---|---|---|---|
| Jumping | 15.75±4.88 | 24.25±1.079 | 9±2.701 | 3.75±0.8 | 0 | 8.25±1.714 | 0 |
| Pulling the stomach | 18.8±5.468 | 27.8±3.043 | 3.2±1.063 | 3±0.962 | 1±0.286* | 14.2±3.474 | 0.6±0.202* |
| Wet dog shakes | 8.6±1.045 | 12±0.533** | 6.2±2.399 | 3.2±0.993* | 0*** | 4.6±0.68 | 0.2±0.143 |
| Head shakes | 22.8±3.914 | 4.4±1.72** | 1.8±0.644** | 3.6±1.192** | 3.6±0.747** | 8.2±1.088** | 0.6±0.202** |
| Paw tremor | 13±2.552 | 2.06±1.304 | 14±2.526 | 29.2±5.68 | 0.8±0.184** | 71.2±8.826*** | 4.8±0.481* |
| Genital grooming | 6±0.837 | 5.6±1.291 | 5.8±1.01 | 3.8±0.94 | 1±0.286** | 12.6±2.95 | 5.4±0.634 |
| Body grooming | 2.9±1.503 | 1.7±1.088 | 1.9±0.644 | 1.4±0.617 | 1.2±0.747 | 3.1±1.525 | 3±0.36 |
| Face wiping | 8.4±4.88 | 11±1.079 | 6.6±2.701 | 5.8±0.8 | 5.3±1.875 | 12.3±1.714 | 6.2±0.508 |
| Teeth chattering | 5±0.962 | 5.3±1.122 | 7.9±0.962 | 10.7±4.247 | 9.7±3.663 | 5.5±2.224 | 1.5±2.844** |
| Swallowing | 7.7±3.281 | 6.6±1.974 | 4.6±1.36 | 3.2±2.244 | 17.9±1.494** | 9.7±3.097 | 13.4±1.378 |
| Rearing | 11.2±2.398 | 6.2±1.523*** | 4.55±1.877*** | 6.05±1.874*** | 5.1±1.998*** | 9.75±1.317* | 4.2±1.902*** |

Figure 2:
Effects of intraperitoneal injection of Marrubium vulgare on the expression of naloxone-induced TWS in morphine-dependent rats in comparison to morphine + vehicle group.
Shown statistical differences are (* p<0.05, ** p<0.01, and *** p<0.001) compared to the morphine + vehicle group. TWS: Total withdrawal scores; M: Morphine; V: Vehicle; E10, E20 and E40: 10, 20 and 40 mg/kg extract.
The outcomes of locomotor activity indicated that there was not significant difference between total locomotion on days 1 and 9. Therefore, it can be concluded that the extract did not have a key role on impaired locomotion style or limb motor function.
DISCUSSION
The use of opioids has limitations due to the effects it has on a person using them. These effects can cause irreparable damage to a person in the short term and even in the long term. Researchers are making their effort to reduce consumption of opioids as much as possible. To reach this goal, the US Centers for Disease Control and Prevention (CDC) has recommended using Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) alone or combined with opioid drugs for reducing their use. The person using opioids expects that their use will not lead to addiction or tolerance. To extract from M. vulgare plant, there are three methods (ethanolic, methanolic and aqueous). The obtained results indicated that acute administration of M. vulgare methanolic extract could delay morphine-induced tolerance dose-dependently and attenuate withdrawal syndrome. Ethanolic and aqueous extract of M. vulgare reduced physical activity as a morphine withdrawal sign.6 The important point when using this extract or similar extracts is that they must indicate same antinociceptive effectiveness level (comparing with only using morphine) while reducing the consumption of morphine. Hence, the addiction risk will be reduced in this situation. In trying to comprehend M. vulgare mechanism of action, it must be noted that M. vulgare is a complicated extract and its effects may depend on numerous components. The essential mechanisms of opioid tolerance and dependency are still unclear but recent studies showed a glial inflammatory response is important in dependence pathogenesis. Activation of the glia can help pain transmission. Morphine binds to Toll-Like Receptor 4 (TLR4) and releases proinflammatory cytokines like TNF. These cytokines excite neurons and simplify pain transmission.23 Subsequently, by reducing the pro-inflammatory cytokines, morphine-induced tolerance can be delayed and withdrawal syndrome can be attenuated. Methanolic extract of M. vulgare showed a good anti-inflammatory effect through myeloperoxidase activation in rats.24 Results obtained by a same research demonstrated that oral administration of methanolic extract of M. vulgare (200 mg/Kg) in rats decreased inflammation significantly (87%) compared to diclofenac as a positive control.25
In another research, the anti-inflammatory effect of M. vulgare was compared to indomethacin and the obtained outcomes illustrated that the extract and the reference drug had a similar anti-inflammatory effect against prostaglandin E2 and carrageenan. In the present work, the analgesic activity of the extract was assessed by p– benzoquinone inducing. The extract’s analgesic activity was equal to acetylsalicylic acid.26 In the other study, neutrophil count and TNF-α level were decreased by M. vulgare extract in rats with Myocardial Infarction (MI).27 Vulgarcoside A and 11-oxomarrubiin of M. vulgare inhibit Nitric Oxide (NO) production.28
M. vulgare is a key source of flavonoids. These flavonoids exert several biological roles. The flavonoids’ anti-inflammatory actions inhibit the synthesis and activities of different pro-inflammatory mediators like TNF-α, IL-1β, and IL-6. Vulgarcoside A inhibits proinflammatory cytokines like TNF-α.28 Also, glycosidic phenylpropanoid esters like forsythoside B, acteoside, and arenarioside showed an inhibitory activity toward the COX-2 enzyme. Moreover, β-sitosterol, marrubiin, and premarrubiin modulate the B2 receptors against prostaglandin and bradykinin receptors and have anti-inflammatory and analgesic activities.12 Also, rosmarinic acid, oleanolic acid, β-sitosterol, luteolin-7-O-β- glucopyranoside, and apigenin-7-O-β-glucopyranoside had a suppressive effect on the production of prostaglandins, peroxasalandine, and hormones by COX enzyme inhibition.29 Marrubiin and marrubiinic acid showed a good analgesic effect in the pain model in mice.30 Steroids and terpenes of hydroalcoholic extract of M. vulgare reduced acute pain.16
Phenolic acids (syringic acid, sinapic acid, 2- hydroxycinnamic acid, rosmarinic acid and coumaric acid) and flavonoids (Quercetin, kaemferol, luteolin) of M. vulgare have antioxidant properties. Antioxidants avoid radical-mediated cell damage and suppress apoptosis indirectly.
CONCLUSION
Despite several studies on the other therapeutic plants for different ailments, there is no clear information about the integration of herbal medicine and opioids to manage addiction and pain. The obtained results from the current research indicated that M. vulgare extract could decrease the doses of morphine required in the management of pain and can block the development of morphine tolerance successfully through synergistic analgesic effect. Furthermore, M. vulgare extract could reverse a formerly established opioid withdrawal. Thus, this plant extract displayed beneficial features in the current study to curb the opioid epidemic. Also, according to the safety information of M. vulgare methanolic extract, it can be used to decrease tolerance to morphine and suppress the morphine withdrawal syndrome without any signs of toxicity like palpitation and mortality.
Cite this article
Sajjadi S, Niknam R, Charkhpour M, Fathiazad F, Moradi R, Parvizpur A. The Effect of Marrubium vulgareon Morphine Tolerance and Withdrawal Syndrome in Male Rats. J Young Pharm. 2023;15(4):704-10.
ACKNOWLEDGEMENT
We wish to thank the authority of the Faculty of Pharmacy, Tabriz University of Medical Sciences for the grant supporting this work based on two Pharm. D theses (No 3937 and 3938).
References
- Roeckel LA, Le Coz GM, Gavériaux-Ruff C, Simonin F. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience. 2016;338:160-82. [PubMed] | [CrossRef] | [Google Scholar]
- Baldo BA. Toxicities of opioid analgesics: respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch Toxicol. 2021;95(8):2627-42. [PubMed] | [CrossRef] | [Google Scholar]
- Zhou J, Ma R, Jin Y, Fang J, Du J, Shao X, et al. Molecular mechanisms of opioid tolerance: from opioid receptors to inflammatory mediators (Review). Exp Ther Med. 2021;22(3):1004 [PubMed] | [CrossRef] | [Google Scholar]
- Alhassen L, Nuseir K, Ha A, Phan W, Marmouzi I, Shah S, et al. The Extract of Corydalis yanhusuo Prevents morphine Tolerance and Dependence. Pharmaceuticals (Basel). 2021;14(10):1034 [PubMed] | [CrossRef] | [Google Scholar]
- Al-Snafi AE, Al-Saedy HA, Talab TA, Majid WJ, El-Saber Batiha GJ-SA. The bioactive ingredients and therapeutic effects of Marrubium vulgare-A review. IJBPSA. 2021;1(2):9-21. [PubMed] | [CrossRef] | [Google Scholar]
- Aćimović M, Jeremić K, Salaj N, Gavarić N, Kiprovski B, Sikora V, et al. Marrubium vulgare L.: A phytochemical and pharmacological overview. Molecules. 2020;25(12):2898 [PubMed] | [CrossRef] | [Google Scholar]
- Gavarić A, Vladić J, Vujetić J, Radnović D, Volarić A, Živković J, et al. The application of ultrasonic waves and microwaves to improve antihyperglycaemic and antimicrobial activities of Marrubium vulgare extracts. Antibiotics (Basel). 2022;11(11):1475 [PubMed] | [CrossRef] | [Google Scholar]
- Hayat J, Akodad M, Moumen A, Baghour M, Skalli A, Ezrari S, et al. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon. 2020;6(11):e05609 [PubMed] | [CrossRef] | [Google Scholar]
- Cravens A. Antimicrobial activity of extracts from the leaves of Marrubium vulgare. 2020 [PubMed] | [CrossRef] | [Google Scholar]
- Rezgui M, Majdoub N, Mabrouk B, Baldisserotto A, Bino A, Ben Kaab LB, et al. Antioxidant and antifungal activities of marrubiin, extracts and essential oil from Marrubium vulgare L. against pathogenic dermatophyte strains. J Mycol Med. 2020;30(1):100927 [PubMed] | [CrossRef] | [Google Scholar]
- Amel A, Sélima B. Larvicidal effect of Marrubium vulgare on Culexpipiens in eastern Algeria. Energy Procedia. 2015;74:1026-31. [CrossRef] | [Google Scholar]
- Bousselsela H, Ghedadba N, Hambaba L, Hachemi M, Dassamiour S, Mouffouk C, et al. In vivo anti-inflammatory activities of Marrubium vulgare L. and Marrubium deserti de Noé species Growing in Algeria. Phytothérapie. 2022;20(4-5):214-23. [CrossRef] | [Google Scholar]
- . Edible plants in health and diseases. Phytochemical and Pharmacological Properties. 2022;2:349-72. [CrossRef] | [Google Scholar]
- Singh G, Valecha R, Shukla G, Kaushik D, Rahman MA, Gautam RK, et al. Neurobehavioral and biochemical evidences in support of protective effect of marrubiin (furan labdane diterpene) from Marrubium vulgare Linn. and its extracts after traumatic brain injury in experimental mice. eCAM. 2022:2022 [CrossRef] | [Google Scholar]
- Kamyab R, Namdar H, Torbati M, Ghojazadeh M, Araj-Khodaei M, Fazljou SMB, et al. Medicinal plants in the treatment of hypertension: a review. Adv Pharm Bull. 2021;11(4):601-17. [PubMed] | [CrossRef] | [Google Scholar]
- Lodhi S, Vadnere GP, Sharma VK, Usman MR. Marrubium vulgare L.: a review on phytochemical and pharmacological aspects. J Intercult Ethnopharmacol. 2017;6(4):429-52. [CrossRef] | [Google Scholar]
- Lemel L, Lane JR, Canals M. GRKs as key modulators of opioid receptor function. Cells. 2020;9(11):2400 [PubMed] | [CrossRef] | [Google Scholar]
- Lemos Duarte ML, Devi LA. Post-translational modifications of opioid receptors. Trends Neurosci. 2020;43(6):417-32. [PubMed] | [CrossRef] | [Google Scholar]
- Liang Y, Zhang T, Zhang J. Natural tyrosine kinase inhibitors acting on the epidermal growth factor receptor: their relevance for cancer therapy. Pharmacol Res. 2020;161:105164 [PubMed] | [CrossRef] | [Google Scholar]
- Rezgui M, Basma M, Neng N, Nogueira JM, Bettaieb Ben-Kaab L, Machado Araújo ME, et al. Evaluation of Marrubium vulgare growing wild in Tunisia for its potential as a dietary supplement. Foods. 2021;10(11):2864 [PubMed] | [CrossRef] | [Google Scholar]
- Ghavimi H, Hassanzadeh K, Maleki-Dizaji N, Azarfardian A, Ghasami S, Zolali E, et al. Pioglitazone prevents morphine antinociception tolerance and withdrawal symptoms in rats. Naunyn-Schmiedeberg Arch Pharmacol. 2014;387(9):811-21. [PubMed] | [CrossRef] | [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10(7):2308-17. [PubMed] | [CrossRef] | [Google Scholar]
- Eidson LN, Murphy AZ. Inflammatory mediators of opioid tolerance: implications for dependency and addiction. Peptides. 2019;115:51-8. [PubMed] | [CrossRef] | [Google Scholar]
- Fathiazad F, Rameshrad M, Asghari S, Hamedeyazdan S, Garjani A, Maleki-Dizaji N, et al. Phytochemical screening and anti-inflammatory effect of Marrubium vulgare L. methanol extract on carrageenan-induced paw inflammation in rats. Pharm Sci. 2016;23(1):3-11. [CrossRef] | [Google Scholar]
- Ghedadba N, Hambaba L, Bousselsela H, Hachemi M, Drid A, Abd-Essmad A, et al. Evaluation of in vitro antioxidant and in vivo anti-inflammatory potential of white horehound (Marrubium vulgare L.) leaves. Int J Pharm Sci Rev Res. 2016;41:252-9. [CrossRef] | [Google Scholar]
- Kanyonga P, Faouzi M, Meddah B, Mpona M, Essassi E, Cherrah Y, et al. Assessment of methanolic extract of Marrubium vulgare for anti-inflammatory, analgesic and anti-microbiologic activities. J Chem Pharm Res. 2011;3(1):199-204. [CrossRef] | [Google Scholar]
- Yousefi K, Fathiazad F, Soraya H, Rameshrad M, Maleki-Dizaji N, Garjani A, et al. Marrubium vulgare L. methanolic extract inhibits inflammatory response and prevents cardiomyocyte fibrosis in isoproterenol-induced acute myocardial infarction in rats. Bioimpacts. 2014;4(1):21-7. [PubMed] | [CrossRef] | [Google Scholar]
- Shaheen F, Rasoola S, Shah ZA, Soomro S, Jabeen A, Mesaik MA, et al. Chemical constituents of Marrubium vulgare as potential inhibitors of nitric oxide and respiratory burst. Nat Prod Commun. 2014;9(7):903-6. 1934578X1400900705 [PubMed] | [CrossRef] | [Google Scholar]
- Neamah S, Sarhan IA. Al-Shaye’a ON. Extraction and evaluation of the anti-inflammatory activity of six compounds of Marrubium vulgare L. Biosci Res. 2018;15(3):2393-400. [PubMed] | [CrossRef] | [Google Scholar]
- Meyre-Silva C, Yunes RA, Schlemper V, Campos-Buzzi F, Cechinel-Filho V. Analgesic potential of marrubiin derivatives, a bioactive diterpene present in Marrubium vulgare (Lamiaceae). Farmaco. 2005;60(4):321-6. [PubMed] | [CrossRef] | [Google Scholar]
