ABSTRACT
Background
Santha Chandrodaya Mathirai (SSM) [Cāntacantirōtaya māttirai] is a classical tablet formulation in Siddha medicine used in various types of fevers. Because of limited research validation in terms of the product compactness, quality and safety, the present study focuses on the formulation, characterization and standardization of the tablet dosage. The objective of the study is to prepare SSM as per Standard operating Procedures (SoPs) mentioned in classical text and to characterize it chemically using modern analytical techniques.
Materials and Methods
The tablet dosage was prepared from the In-house R&D GMP Pharmacy facility of Siddha central research Institute and validated through analytical measures like pre-compression, post-compression parameters, physiochemical analysis, analytical studies like High Performance Thin Layer Chromatography (HPTLC), Special Edition Microscopy (SEM) with Energy Dispersive X-Ray (EDAX), Fourier Transform Infrared Spectroscopy (FT-IR) and UV Absorption Spectroscopy (UV-AS).
Results
As per the reference standards, the mean flow property of the Tablet granules (31°) was fair enough, the mean compressibility index (17.3%) and Hausner’s ratio (1.452) indicates its good flow character. The tablet passed the USP standards of weight variation in %. The friability test reported the maximum weight loss to be 0.06 %, a good acceptable value. The highest disintegration time was observed at 60 min. The samples were devoid of Heavy metals, microbial and aflatoxin contamination. At short UV of 254 nm, long UV of 366 nm, and post derivatized plate in white light there were observation of 6 spots, 8 spots and 12 spots respectively in TLC photo documentation. EDAX reported the presence of Carbon, Oxygen, Sodium, Chlorine, Potassium, and Niobium molecules in the sample with no traces of heavy metals. FTIR spectra showed three high peak areas at the range of band 2917.08, 2849.10, and at 1717.08 that corresponds to carboxylic acid, alkane bond, and α, β-unsaturated ester.
Conclusion
The In-house samples of SSM reported standard values in terms of quality and safety. Further clinical trials are warranted to validate its efficacy.
INTRODUCTION
Siddha Medicines are mainly based on plants and plant products besides animal products metal, minerals and products of marine origin from various resources.1 Each product should comply with the regulatory guidelines set forth by Pharmacopeial Laboratory of Indian Medicine (PLIM).2 Standardization is a mandate process resulting from the consensus based on scientific finding from an orderly approach that validates the entire process starting from the identity and purity of the raw material used, the phases of manufacture, operating procedures and analytical tests for authenticating the formulation.3 In the current scenario, Siddha tablet dosages are getting much attention in medical practice owing to its compatible dose fixation, and ease of usage. But there are limited studies of its validation in terms of the product compactness, quality and safety. Quality Standards for Tablets, the parameters for assuring it are the need of present research as it leads more stringent lines to cut the probabilities of batch-to- batch variations.
The present study focuses on the formulation, characterization and standardization of a classical Antipyretic Siddha tablet dosage Cāntacantirōtaya māttirai (SSM). In accordance with traditional Siddha literature references, and with the guidelines put forth by various regulatory bodies like PLIM, IP, and international guidelines (WHO), the Tablet dosage was prepared, and validated.
MATERIALS AND METHODS
Description of Master formulation
SSM is a herbo mineral blend containing 4 ingredients (Table 1 and Figure 1).4
| Sl. No. | Ingredients | Botanical name / Scientific name | Part used | Quantity mentioned in the literature | Quantity used in the formulation |
|---|---|---|---|---|---|
| 1. | Mañcaļ | Curcuma longa | Rhizome (dried) | 3 parts | 300 g |
| 2. | Veńkāram | Borax (purified) | – | ½ Part | 50 g |
| 3. | Pūram | Calomel (purified) | – | 1 Part | 100 g |
| 4. | Elumiccaicāŗu | Citrus aurantiifolia (Christm.) Swingle | Fruit juice | Q.S | Q.S |
| 5. | Excipients | – | – | Q.S | Q.S |

Figure 1:
Ingredients of Cāntacantirōtaya māttirai.
Raw drug collection and Authentication
The raw drugs were purchased from an authentic country drug merchant, Chennai and were authenticated from the Department of Pharmacognosy and the Department of Chemistry, Siddha Central Research Institute, Chennai, Tamil Nadu.
Standard Operating Procedure (Textual reference)
Ingredients 1-3 are detoxified as per traditional procedures (Table 2). Weigh the detoxified ingredients, powder and mix in the proper ratio. Grind the powder mixture with Item 4 in a traditional mortar for 12 hr. When pill rolling consistency is achieved, pills are made in the size of black pepper and shade dried.5
| Raw drug | Purification as per Traditional procedures |
|---|---|
| Mañcal | Clean and dried. |
| Venkāram (Borax) | Roast till dehydration, powder and preserve. |
| Pūram (Calomel) | Paste is prepared with Betel leaves and black pepper which is mixed in water to prepare a solution. The material to be purified is coarsely powdered and suspended in a bundle and boiled in the above prepared mixture till it is reduced to 3/4th of the volume. Then washed anddried. |
Standard Operating Procedure-Formulation of Cāntacantirōtaya māttirai
Cutti (Purification and pre-processing)
Preparation of Cāntacantirōtaya māttirai Curnam (granule for tablet processing)
The purified ingredients were finely powdered separately and ground with Elumiccai cāru (lemon juice) for 12 hr.
Then dehydrate the powder mixture by drying in shade or a drier.
Formulation of Cāntacantirōtaya māttirai
The tablet form of Cānta cantirōtayam (100 mg) was prepared from Cāntacantirōtaya māttirai Curnam by dry granulation method.
The powder blend is then weighed accurately and passed through sieve # 40.
Tablets of 100 mg were compressed from the herbal powder of Cāntacantirōtaya māttirai Curnam blend using Parle ECO series III tableting machine (Figure 2).7

Figure 2:
Cāntacantirōtaya māttirai – Finished product.
Standardization of Cāntacantirōtaya māttirai
The granules of Cāntacantirōtaya māttirai were evaluated for quality standardization (Precompression parameters including Test for Angle of Repose (θ), Compressibility Index (%), and Hausner’s Ratio). The final tablets were analyzed for post compression studies (Weight Variation %, Width (in mm), Thickness (in mm), Hardness (in Newton), Friability (%), and Disintegration Time (min/sec)) and Physiochemical analysis (Organoleptic characters, Moisture, Aqueous extractive value in (%), Alcohol extractive value in (%), Ash value, Fluorescence, Heavy metal analysis, Total viable aerobic count, and aflatoxins).7,8 Analytical studies like Special Edition Microscopy (SEM) with Energy Dispersive X-Ray (EDAX), Fourier Transform Infrared Spectroscopy (FT-IR) and UV absorption Spectroscopy (UV- AS) were also performed in the final product sample of SSM.9 The parameters were carried out with reference to Ramulu et al., Brinda et al., and as per the guidelines of PLIM, IP and WHO (International guidelines).7,10 For the final Tablet samples, High performance Thin Layer Chromatography (HPTLC) were carried out as per the procedures. The Ethanolic extract of SSM were prepared in Toluene: Ethyl acetate: Formic acid (5:1.5:0.5, v/v/v) solvent system. Analysis was performed on (5cm×10cm) silica gel 60F254 precoated aluminium plate 10 μL of ethanolic extract of SSM was applied by ATS4 sampler with band width 8 mm and 10 mm from the bottom of the plate. Other procedures for development, visualization/photo documentation, scanning before and after derivation were followed as per procedure mentioned in previous literature.10
RESULTS
Pre-compression and Post compression studies of Master formulations
Pre-compression parameters
With the three batches of granules (SSM A, B, and C) assessed, the mean flow property was fair enough (31°) as per the reference standards. The mean compressibility index of 17.3% and Hausner’s ratio of 1.452 indicates its flow character to be good (Table 3).
| Sample code | Angle of Repose (θ) | Bulk density (gm/ mL) | Tap density (gm/ mL) | Compressibility Index(%) | Hausner’s Ratio |
|---|---|---|---|---|---|
| SSM A | 33 | 0.8 | 1.162 | 17.3 | 1.452 |
| SSM B | 29 | 0.8 | 1.162 | 17.3 | 1.452 |
| SSM C | 32 | 0.8 | 1.162 | 17.3 | 1.452 |
| Mean Value | 31 | 0.8 | 1.162 | 17.3 | 1.452 |
Post-compression parameters
The mean weight of 20 tablets was found to be 130 mg, and the tablet passed the USP standards of weight variation in %. The friability test reported the maximum weight loss to be 0.06 %, a good acceptable value. The highest disintegration time of tablets tested was found to be 60 min (Tables 4 and 5).
| Sample Code | Weight (mg) | Weight Variation % | Width (in mm) | Thickness (mm) | Hardness (in Newton) |
|---|---|---|---|---|---|
| Code SSM1 | 128 | -1.53 | 5.95 | 3.45 | 89 |
| Code SSM2 | 133 | 2.30 | 5.96 | 3.46 | 83 |
| Code SSM3 | 133 | 2.30 | 5.90 | 3.47 | 87 |
| Code SSM4 | 126 | -3.07 | 5.92 | 3.57 | 85 |
| Code SSM5 | 130 | 0 | 5.93 | 3.36 | 86 |
| Code SSM6 | 129 | -0.76 | 5.99 | 3.38 | 79 |
| Code SSM7 | 131 | 0.76 | 5.92 | 3.39 | 91 |
| Code SSM8 | 125 | -3.84 | 6.03 | 3.42 | 93 |
| Code SSM9 | 126 | -3.07 | 5.97 | 3.43 | 87 |
| Code SSM10 | 129 | -0.76 | 5.86 | 3.47 | 86 |
| Code SSM11 | 130 | 0 | 5.97 | 3.53 | 84 |
| Code SSM12 | 128 | -1.53 | 6.17 | 3.51 | 94 |
| Code SSM13 | 130 | 0 | 6.12 | 3.58 | 91 |
| Code SSM14 | 131 | 0.76 | 6.13 | 3.59 | 89 |
| Code SSM15 | 131 | 0.76 | 5.95 | 3.41 | 79 |
| Code SSM16 | 135 | 3.85 | 5.97 | 3.49 | 77 |
| Code SSM17 | 128 | -1.54 | 5.99 | 3.45 | 76 |
| Code SSM18 | 129 | -0.76 | 6.03 | 3.47 | 84 |
| Code SSM19 | 132 | 1.53 | 5.91 | 3.46 | 85 |
| Code SSM20 | 136 | 4.62 | 5.41 | 3.47 | 79 |
| Sl. No. | Name of the test | Results |
|---|---|---|
| 1. | Mean weight – 20 Tablets | 130 mg |
| 2. | Mean Width (in mm) – 20 Tablets | 5.95 mm |
| 3. | Mean Thickness (in mm) – 20 Tablets | 3.47 mm |
| 4. | Mean Hardness (in Newton)-20 Tablets | 85N |
| 5. | Friability (%) | 0.06 % |
| 6. | Disintegration Time (min/sec) | 60 min |
Physiochemical Analysis
Physiochemical Analysis of final samples of SSM reported standard values in terms of quality, and safety. The samples were devoid of Heavy metal, microbial and aflatoxin contamination (Table 6).
| Sl. No. | Parameters | Results |
|---|---|---|
| 1. | Description | A brown coloured circular shaped slightly biconvex uncoated Tablet |
| 2. | Moisture | 7.9118% w/w |
| 3. | Aqueous extractive value in (%) | 43.8% w/w |
| 4. | Alcohol extractive value in (%) | 87.45% w/w |
| 5. | Ash value | 7.9118% w/w |
| 6. | Fluorescence | No fluorescence particles are observed |
| 7. | Heavy metals | |
| 8. | Lead (Pb) | Less than 2 ppm |
| 9. | Arsenic (As) | Absent |
| 10. | Cadmium (Cd) | Absent |
| 11. | Mercury (Hg) | Absent |
| 12. | Total viable aerobic count | 20 cfu/gm |
| 13. | Escherichia coli | Absent |
| 14. | Salmonella sps | Absent |
| 15. | Aflatoxins | |
| 16. | B1 | Nil |
| 17. | B2 | Nil |
| 18. | G1 | Nil |
| 19. | G2 | Nil |
SEM -EDAX analysis
The Special Edition Microscopy (SEM) images of the finished product of SSM were observed at 1000 X and 2000 X magnification range (Figure 3). The Energy Dispersive X-Ray Microanalysis System (EDAX) results indicated the presence of carbon, oxygen, sodium, chlorine, potassium, and niobium molecules in the sample. There are no traces of heavy metals in the sample.
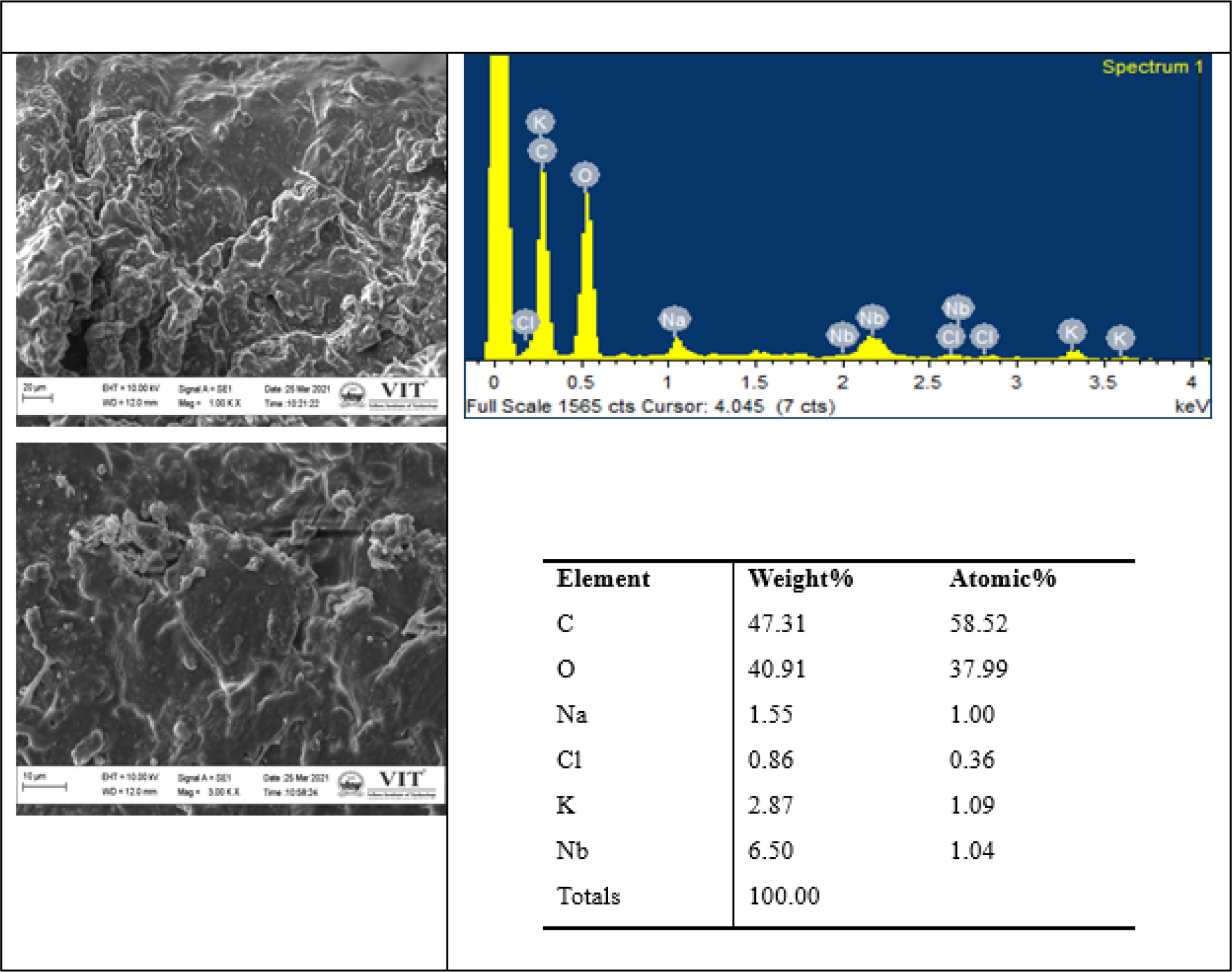
Figure 3:
SEM and EDAX Reports of Finished product-Cāntacantirōtaya māttirai.
FT-IR analysis
FT-IR spectrum of finished product of SSM is shown in (Figure 4). The obtained spectra showed three high peak areas at the range of band 2917.08, 2849.10, and at 1717.08. The band corresponds to the presence of following groups in the sample. The band at 2917.08 corresponds to carboxylic acid or intramolecular alcohol bond (O-H stretching) / amine salt (N-H stretching) / alkane bond (C-H stretching), band at 2849.10 to alkane bond (C-H stretching) / amine salt bond (N-H stretching), and band at 1717.08 corresponds α, β-unsaturated ester or formates (C=O stretching)/ aliphatic ketone or cyclohexanone or cyclopentenone C=O stretching / carboxylic acid dimer bond (C=O stretching).
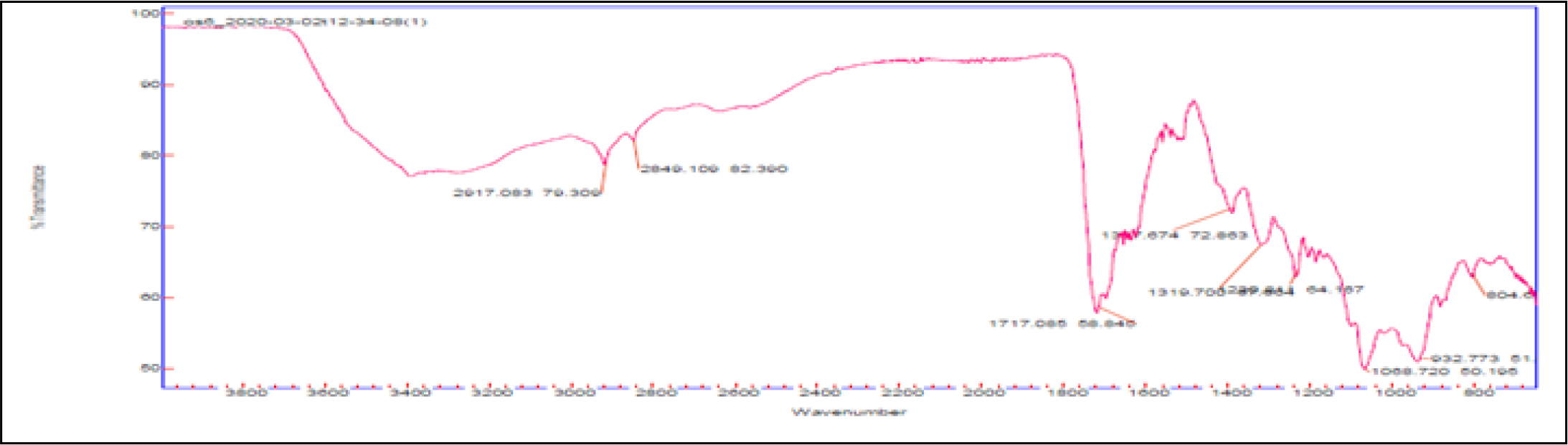
Figure 4:
FTIR spectrum of Finished product-Cāntacantirōtaya māttirai.
Uv-vis analysis
The UV absorption maxima peaks were observed between the range of 200-300 nm and one minor peak was observed between 300-400 nm, and 400-500 nm (Figure 5).
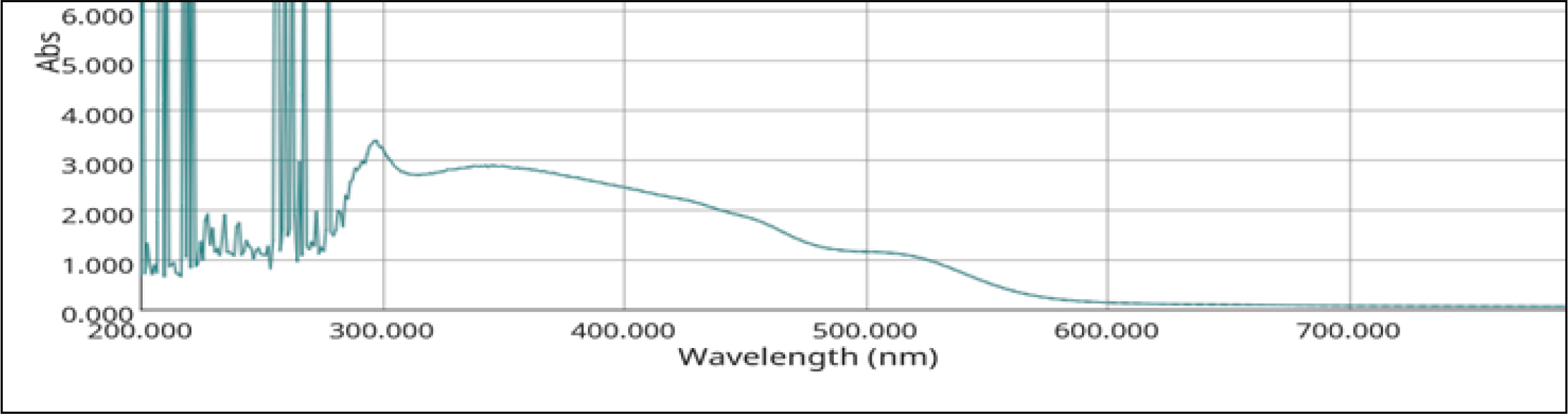
Figure 5:
UVS spectrum of Finished product-Cāntacantirōtaya māttirai.
Analytical Specifications of Finished product
TLC photo documentation
The pictures of TLC photo documentation, HPTLC fingerprint profile with the Rf values and colour of spots in different wavelength regions are presented in Table 7 and Figure 6. At short UV of 254 nm, long UV of 366 nm and post derivatized plate in white light, there were observation of 6 spots, 8 spots and 12 spots respectively in TLC photo documentation. The Rf values under 254 nm were recorded at 0.16 (light green), 0.26 (dark green), 0.44 (light green), 0.51 (light green), 0.59 (light green) and 0.90 (dark green). The Rf values for 366 nm was recorded at 0.02 (yellow), 0.19 (green), 0.33 (blue), 0.42 (blue), 0.49 (yellow), 0.63 (fluorescent blue), 0.75 (fluorescent blue), and 0.84 (green). The Rf values in white light for post derivatized plate were recorded at 0.06 (pink), 0.16 (light green), 0.26 (green), 0.34 (pink), 0.49 (yellow), 0.59 (light pink), 0.65 (pink), 0.74 (violet), 0.81 (light blue), 0.90 (blue) and 0.98 (violet).
| λ254 nm | λ366 nm | λ520 nm (Derivitized) | |||
|---|---|---|---|---|---|
| Rf | Colour | Rf | Colour | Rf | Colour |
| 0.16 | Light | 0.02 | Yellow | 0.06 | Pink |
| 0.26 | Dark | 0.19 | Green | 0.16 | Light green |
| 0.44 | Light | 0.33 | Blue | 0.26 | Green |
| 0.51 | Light | 0.42 | Blue | 0.34 | Pink |
| 0.59 | Light | 0.49 | Yellow | 0.40 | Pink |
| 0.90 | Dark | 0.63 | Fluorescent blue | 0.49 | Yellow |
| – | – | 0.75 | Fluorescent blue | 0.59 | Light pink |
| – | – | 0.84 | Green | 0.65 | Pink |
| – | – | – | – | 0.74 | Violet |
| – | – | – | – | 0.81 | Light blue |
| – | – | – | – | 0.90 | Blue |
| – | – | – | – | 0.98 | Violet |

Figure 6:
TLC/HPTLC of ethanol extract of SSM.
HPTLC densitometric scan
The densitometric scanning of ethanolic extract of SSM at λ254 nm revealed 13 peaks at Rf 0.02 (area 29.27%), 0.18 (10.20%), 0.28 (19.76%), 0.92 (10.69%), 0.11 (4.28%) as major peaks; 0.21 (4.36%), 0.36 (3.39%), 0.42 (3.03%), 0.46 (4.34%), 0.53 (5.08%), 0.61 (3.72%), 0.72 (0.32%), 0.85 (0.15%) and 0.98 (1.41%) as other peaks. At λ366 nm, revealed 10 peaks at Rf 0.04 (area 8.67%), 0.32 (15.86%), 0.36 (10.03%), 0.44 (14.11%), 0.51 (26 %), 0.66 (10.10%), 0.78 (8.83%) as major peaks; 0.86 (5.37%), 0.95 (0.53%), 0.97 (0.50%) as other peaks. Derivatized plate under white light at λ520 nm revealed 13 peaks at Rf 0.03 (area 57.75%) as major peak and 0.19 (4.37%), 0.26 (4.00%), 0.29 (6.70%), 0.37 (5.38%), 0.43 (7.06%), 0.53 (0.85%), 0.64 (1.12%), 0.70 (2.68%), 0.79 (3.55%), 0.85 (0.16%), 0.95 (5.99%) and 0.98 (0.40%) as minor peaks.
DISCUSSION
SSM prepared as per the SOP were evaluated for precompression, post compression tests, physiochemical analysis and characterization. The flow properties of the pre-compressed mixture were fair enough, and the compressibility index and Hausner’s ratio indicated its good flow property in par with the reference standards. The post compression studies elucidated the physical quality and compactness of the tablet. Physiochemical analysis of final samples of CCM reported standard values in terms of quality, and safety. The samples were devoid of Heavy metal, microbial and aflatoxin contamination. SEM-EDAX reported neither of any presence of heavy metals or other trace elements in the sample. Other base periodic elements were observed in the sample and considering its biological role this may indicate a positive value in promoting health. HPTLC reported distinct separation of bands in both the wavelength regions under 254 nm, 366 nm and after spray with VSR reagent for ethanol extract of CCM. Each band represents a distinct compound of the ingredients in it. Curcumin is the major compound in the chief ingredient Curcuma longa Linn. in CCM. As per previous report (Anonymous, 2010), curcumin appears as dark reddish-brown band at Rf 0.53 but in the present study, in CCM formulation curcumin was detected in less percentage at λ254 nm and λ520 nm which appeared at same Rf at 0.53 whereas at λ254 it appeared in 0.51. May be during the preparation process, most of the curcumin might have undergone chemical change due to borax.
CONCLUSION
The anti-pyretic tablet dosage, SSM was formulated, and characterized as per the quality standards of the regulatory bodies. The reports in various levels may help in the pharmaceutical research field to replicate the tablet batches without batch-to-batch variations. The works fulfils the preliminary level standardization of the tablet, and this may be identified as a reference for future considerations.
Cite this article
Devi SMS, Sathiyarajeswaran P, Vinayak S, Kirubakaran N, Allu R, Shakila R, et al. Analytical Standardization and Profiling of Santha Chandrodaya Mathirai – A Siddha Herbo-Mineral Tablet Formulation. J Young Pharm. 2023;15(4):679-86.
ACKNOWLEDGEMENT
The Authors express heartfelt thanks and would like to acknowledge Dr. Siva Ramamoorthy, Professor and Dean, School of Bio Sciences and Technology, Vellore Institute of Technology, C. George Priya Doss, Department of Integrative Biology, School of Biosciences and Technology, Vellore Institute of Technology Vellore, TN, India for helping in data analysis and interpretation of the results.
ABBREVIATIONS
| EDAX | Energy Dispersive X-Ray Spectroscopy |
|---|---|
| FTIR | Fourier-Transform Infra Red Spectroscopy |
| HPTLC | High-performance thin-layer chromatography |
| IP | Indian Pharmacopeia |
| PLIM | Pharmacopeial Laboratory of Indian Medicine |
| SEM | Scanning Electron Microscopy |
| SSM | Cānta cantirōtaya māttirai |
| TLC | Thin Layer Chromatography |
| Uv-Vis | Ultraviolet visible spectroscopy |
References
- Kandaswamy Pillai N. History of Siddha medicine. 2012
- Anonymous. Pharmacopeial laboratory for Indian medicine. Protocol for testing ayurvedic, Siddha and Unani medicines. Ghaziabad. :29 [Google Scholar]
- Central Council for Research in ayurvedic sciences (India). General guidelines for drug development of Ayurvedic formulations. 2018 [Google Scholar]
- Kuppusami Muthaliyar U, Thirattu SV. 2006:21 [Google Scholar]
- Anonymous. The Siddha formulary of India (SFI) (Ministry of Health and Family Welfare, Govt. of India, New Delhi) Part I. 1992:86 [Google Scholar]
- Anonymous. The Siddha formulary of India (SFI) (Ministry of Health and Family Welfare, Govt. of India, New Delhi) Part I. 1992 [Google Scholar]
- Ramulu SD, Sathiyarajeswaran P, Narayanan K, Vinayak S, Ganesan K, Kanakavalli K, et al. Formulation and Standardization of Siddha Pediatric Tablet dosage: Bala Sanjeevi Mathirai. Int J Nutr Pharmacol Neurol Dis. 2021;11(4):267 [Google Scholar]
- Bhowmik D, Duraivel S, AN R, Kumar KS. Tablet manufacturing process and defects of tablets. Elixir Pharm. 2014;70:24368-74. [Google Scholar]
- Patwardhan B, Vaidya AD, Chorghade M. Ayurveda and natural products drug discovery. Curr Sci. ;2004:789-99. [Google Scholar]
- Sampangiramulu SDM, Sundaramoorthy B, Mandal AK, Soman V, Narayana SKK, Ramachandran S, et al. Standardization of Kirampu Kutinir decoction and extract granules using pharmacognostic, physicochemical and HPTLC studies. Int J Pharm Investig. 2021;11(4):362-7. [CrossRef] | [Google Scholar]
