ABSTRACT
Background
Following the COVID-19 pandemic, microvascular and macrovascular thrombotic problems emerged that required anticoagulants. Rivaroxaban (RN) is a factor Xa inhibitor that treats deep vein thrombosis and the two forms of artery diseases (coronary artery disease and peripheral artery disease).
Materials and Methods
The study objective was to create fast-disintegrating rivaroxaban Oral Thin Films (OTF) with the help of various super disintegrants to shorten disintegration time and enhance drug release in order to assist patients who have difficulty in swallowing conventional dosage forms and increase bioavailability. OTF was created using the solvent casting method. A 22 factorial design was employed in Design-Expert® software to develop an ideal formula.
Results
The optimized film formula pH, drug content, disintegration time, folding endurance, and dissolution rate were estimated, and the film was subjected to a short-term stability study. The optimized formula exhibited a cumulative drug release of 93.47% in 60 sec.
Conclusion
The drug’s in vitro release pattern shows first-order kinetics and fickian diffusion was the mechanism of drug release. These findings supported that rivaroxaban OTFs offer a quick release of the medication from the administration site into the systemic circulation.
INTRODUCTION
Oral administration is the most effective method for achieving systemic effects. A solid dosage form constitutes around 60% of all formulations. The oral route of administration is widely used due to its ease, absence of pain, and versatility.1 Dysphagia affects people of all ages, but it is more widespread in older persons. The fear of choking inhibits many pediatric and elderly patients from delivering this solid dosage forms.2 Dysphagia is linked to clinical illnesses such as stroke, head and neck thyroid treatment, parkinson’s disease, thyroidectomy, AIDS, and other neurological issues such as cerebral palsy. The most common concern was tablet size, followed by surface area and flavour.3
Oral thin films have variety of approaches to solve the issues associated with the oral route of administration. Oral thin films are one example of a product in which we utilize super disintegrants to disperse the dosage form. These rapid-dissolving drug delivery systems will disperse on the patient’s tongue without water or chewing in minutes or seconds.4
Oral thin films is placed on the dorsum or the floor of the tongue. It confines to the application position, and the drug is absorbed by oral mucosa due to its highly permeable nature into systemic circulation. Due to its high vascularity and permeability, the active substance is delivered for local and systemic uptake. Hydrophilic polymers are chosen since they are easily dissolved when it comes in contact with saliva. This innovative drug delivery method can improve drug solubility/stability, biological half-life, and bioavailability.3,5
Hydroxypropyl methylcellulose (HPMC E 15 LV) was used as a film-forming agent. It contains 28-30% methoxyl, 7-12% hydroxypropyl. It encompasses thermal gelation with outstanding film characteristics (high tensile strength, strength, and elongation). DOACs (Direct Oral Anticoagulants) have been used for various indications- arterial diseases, heart failure, cancer, and the prevention of DVT in acute medical diseases. Post-COVID-19 pandemic, it was clear that the victims have been diagnosed with higher levels of thrombin, fibrin components, and other clotting factors that promote clot formation. This led to an increase in the administration of DOACs. One such anticoagulant is rivaroxaban – a factor Xa inhibitor capable of dissolving clots internally and externally with minimal drug-drug or drug-food interactions. Unlike indirect factor Xa inhibitors such as fondaparinux or heparin, Rivaroxaban suppresses both free and clot-bound factor Xa and prothrombinase, which prolongs the clotting time.6
The aim of the study is to enhance the bioavailability of rivaroxaban at the receptor site by formulating oral thin films. The reason to enhance bioavailability of oral thin films is to over come the disadvanatges of conventional dosage forms.
MATERIALS AND METHODS
Materials
Rivaroxaban was received as a gift sample from Alphamed Formulations Pvt. Ltd., Hyderabad. Propylene glycol was obtained from Thermo Fisher Scientific India Pvt. Ltd., Bengaluru, while HPMC was purchased from Loba Chemie Pvt. Ltd., Mumbai. Sodium starch glycolate and aspartame was supplied by Merck, Mumbai. All other chemicals and compounds utilized were of analytical grade.
Methods
Preformulation studies
The initial stage in the rational development of dosage forms is pre formulation study. It investigates drugs molecular and physical characteristics, both alone and when combined with excipients. Preformulation assessment aims to generate data to help the formulator construct safe, bioavailable, and mass-producible dosage forms.7
Excipient – Drug Compatibility Research
Fourier Transforms Infra-Red (FTIR) Spectroscopy: FT-IR spectra (Bruker alpha, Germany) was obtained to discover possible interactions between the drug and polymers. The ingredients were compressed with a hydraulic press to form a pellet (less than 5 k pas). The disc was put in the centre of the sample holding device and spectrum was recorded using an FT-IR spectrophotometer.8
Differential Scanning Calorimetry (DSC): DSC thermograms of API and physical mixture was recorded by enclosing them in heat-resistant aluminium pans. The surface of the pan lid was crimped by pushing them against a pellet mill. The pans were held in the heating chamber to temperatures ranging from 30 to 300oC at 10oC/min.9
Analytical Method Development
Determination of λmax for rivaroxaban by using acetonitrile: The calibration curve for rivaroxaban was developed using acetonitrile.9
Preparation of standard curve for rivaroxaban by using acetonitrile: From the 1000 μg/mL stock solution, 100μg/mL solution is prepared. Different concentrations of rivaroxaban at various concentrations 2, 4, 6, 8, 10 μg/mL were obtained, and its absorbance was determined.9
Preparation of oral Dissolving Film of Rivaroxaban Using 22 Factorial Designs: A two-factor design has been used for optimization Table 1. The independent factors, as well as the dependent variables, were chosen. Three independent variables has been chosen. The highest and lowest factor values were marked as +1 and -1 Table 2. All samples were quantitatively assessed using ANOVA and Design Expert 11@software to determine the chosen variable significance and non-significance affect on responses such as disintegration time, folding endurance, and thickness. The factor impact was visually represented using 3D response surface plots and contour plots in design expert.10
| Batch code | X1 | X2 |
|---|---|---|
| F1 | + 1 | + 1 |
| F2 | -1 | -1 |
| F3 | + 1 | -1 |
| F4 | -1 | + 1 |
| Coded value | Concentration of Polymer(mg) | concentration of super disintegrant (mg) |
|---|---|---|
| -1 | 350 | 40 |
| + 1 | 500 | 50 |
Preparation of rivaroxaban mouth dissolving film
The solvent casting process involves adding HPMC E15 LV and propylene glycol to the water, keeping the temperature at 60°C and the stirrer rotating at 1000 rpm. Color, flavoring agent, sweetening agent, and disintegrant are dissolved in water with continuous stirring. The resultant solution is combined with the API dissolved in a solvent. To extract the trapped air, a vacuum is employed. The homogenous solution formed is cast as a film and allowed to dry before being cut into the appropriate size.11
Evaluation of mouth dissolving films of rivaroxaban
Physical appearance and surface texture of films: These criterias were confirmed easily by inspecting films visually and by feel or touch. The finding implies the films surface nature and visually appealing or not.12
Organoleptic analysis: Organoleptic assessment of prepared oral disintegrating films are performed with the previous agreement of a group of healthy participants with good organoleptic sensibilities. The flavor, tongue feel (grittiness or smoothness), and the oral disintegrating films outward look was evaluated.13
Thickness: The width of the film is proportionate to its dosage uniformity. The ultimate thickness of the film is checked by calculating the mean thickness at five distinct locations with micrometer screw gauge. It should be between 50 mm to 1000 mm.14
Average weight: About 10 films are individually weighed. The average weight was determined by adding the weight of all films and dividing by 10.15
Surface pH: The surface pH of the films was evaluated by exposing them to 1 mL of distilled water. The surface pH was determined by introducing pH paper close to the surface of the films.16
Folding endurance: Film folding endurance is determined to check the film durability. Folding endurance was determined by repeatedly folding a tiny film strip until it breaks. The endurance value is determined by the number of times it can be folded without tearing.17
Content uniformity: Three films were individually assayed for their drug content. Film was dissolved and diluted with water to get a 1 μg/mL concentration. The film must contain API of 85-115% of the label claim to have content uniformity.18
Disintegration time (petri dish method): This technique was carried out on a petri plate. The oral thin film was placed in the centre of a petri dish filled with 10 mL of distilled water. The time taken for the thin layer to disintegrate is measured, and the procedure is done thrice.19
Moisture loss: The proportion of moisture loss defines a film’s hygroscopicity. Typically, this parameter was found by first determining the original weight of the film, followed by placing it for three days in a desiccator. After three days, the films are removed and reweighed. The following algorithm is used to calculate moisture loss.20–25
Moisture absorption: The moisture absorption is measured by first dividing the film into 2 x 2 cm2 squares. These strips are then subjected to a 75% relative humidity atmosphere at room temperature for a week. Moisture absorption is calculated as a percentage weight increase of the strip.21–25
In vitro dissolution studies
The dissolution study was carried out in a type II USP apparatus. 900 mL of dissolution medium was taken and maintained at 37°C ± 0.5°C. The film was attached to a rotating centre axis. Filtered samples were taken manually at 15, 30, 45, and 60 sec. At the same temperature, the samples were compensated with an equivalent volume of pure water. After appropriate dilution with the dissolving media, the concentration of the drug released in the medium was measured spectrophotometrically at 249 nm.22
Dissolution parameters
- Apparatus: Dissolution apparatus (Type II),
- Dissolution Medium: Purified water, 900 mL,
- RPM: 50,
- Temperature: 37°C ± 0.5°C,
- Withdrawal time: 15, 30, 45 and 60 sec,
- Volume withdrawal: 5 mL.
Release kinetics
The amount of drug released from the pharmaceutical dosage form and its processes is an essential but which is intricate process in matrix systems. Zero order or first order kinetics were used to characterize the sequence of drug release from matrix systems. The Higuchi diffusion model and the Hixon-Crowell erosion model were used to investigate the mechanism of drug release from matrix systems. The Korsmeyer- Peppas equation also categorized drug release mechanisms as Fickian/non- Fickian/anomalous. The release exponent ‘n’ value is utilized in the Korsmeyer-Peppas equation to characterize distinct release processes from the dosage form.22
Stability studies
To examine the stability of the drug formulation, stability experiments were conducted by ICH. The optimized formulation was sealed in a polyethylene-laminated aluminum container. Samples were stored at 40°C and 75% RH for a month. The formulation was examined for any changes in its characteristics.23
RESULTS
Determination of λmax for rivaroxaban by using acetonitrile
The absorption maximum for rivaroxaban by using acetonitrile was noticed at 249 nm.
Preparation of Standard Curve for rivaroxaban by using acetonitrile
The graph was plotted taking absorbance on x- axis and concentration on y-axis. The graph was linear at 2 – 10 μg/mL range and obeys Beer – Lambert’s law Figure 1.
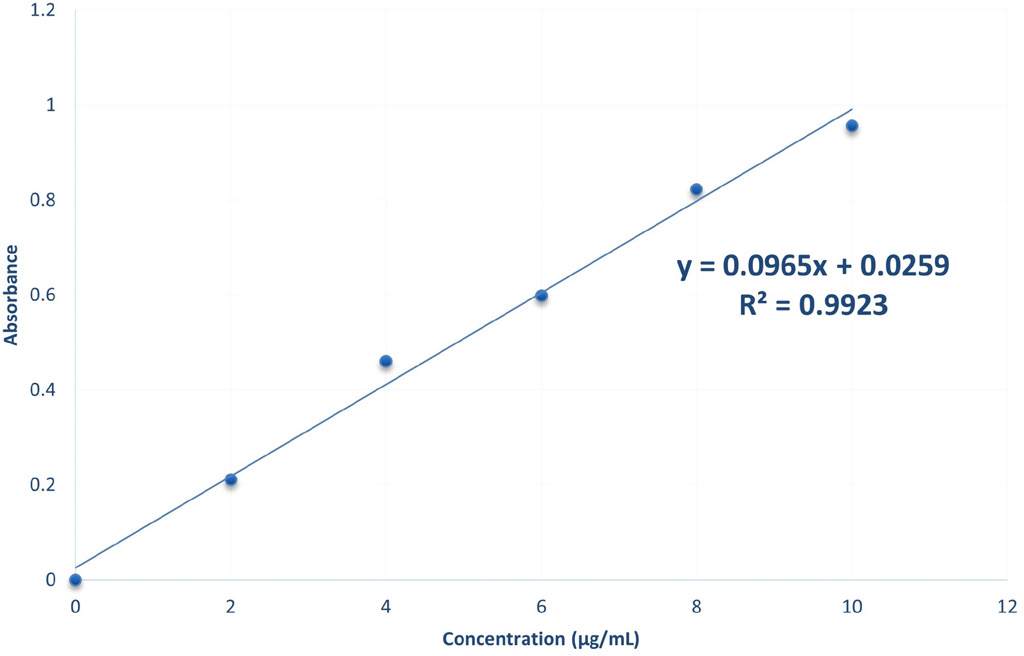
Figure 1:
Standard Graph Of Rivaroxaban in Acetonitrile.
Drug-Excipient Compatibility Studies by FT-IR
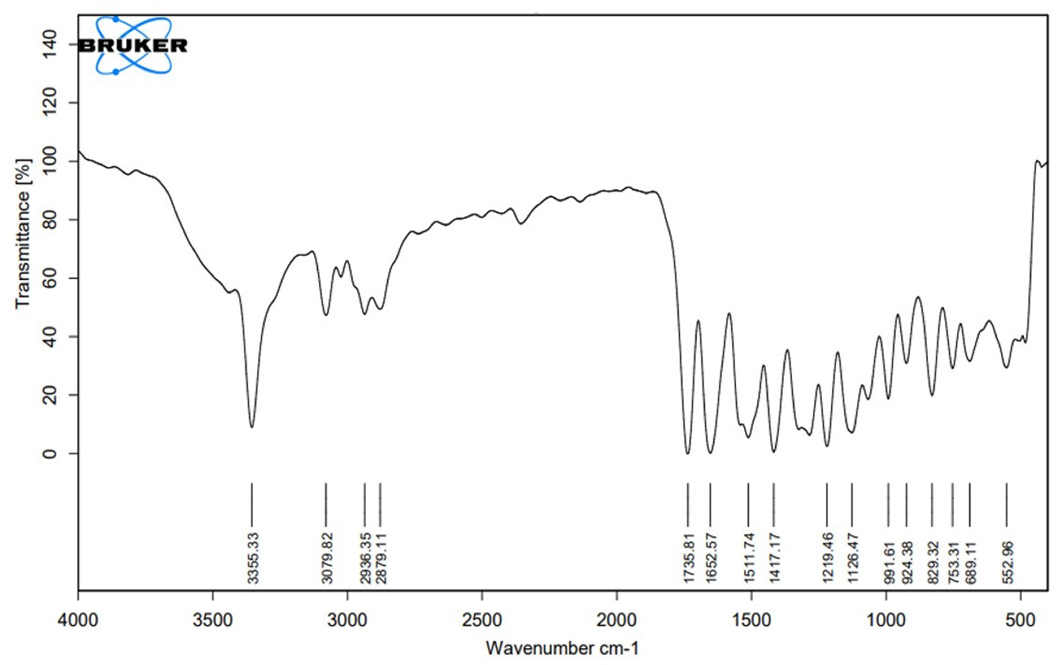
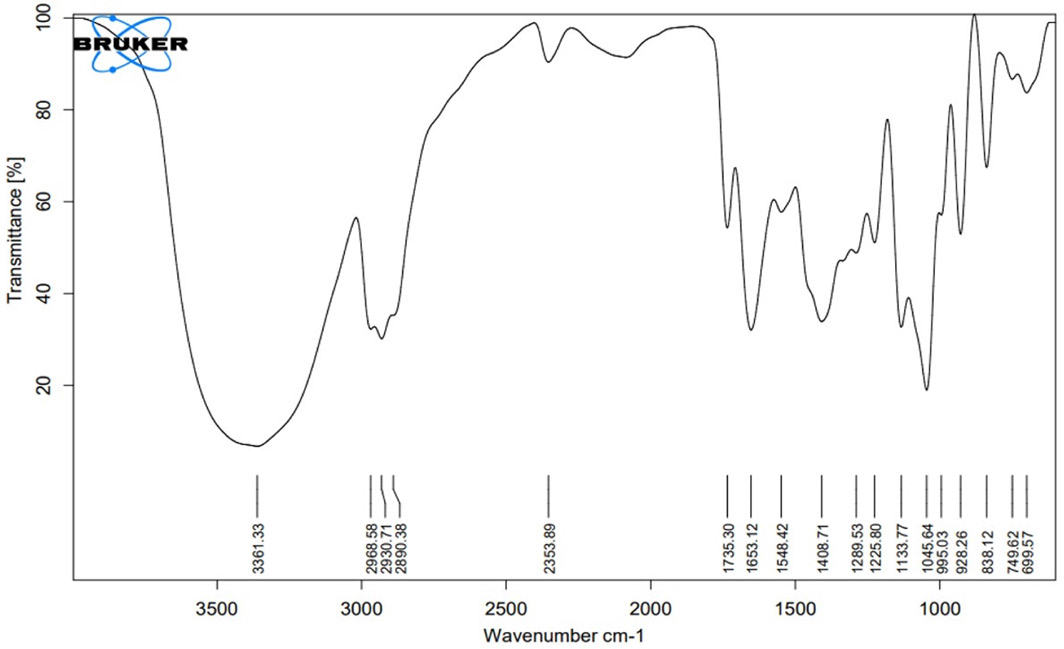
Fourier transforms infrared (FT-IR) spectroscopy
Differential Scanning Calorimetry (DSC)
In spectrum of rivaroxaban Figure 4, there was an endothermic peak at 231.88°C which may be because of oxidation or recrystallization Figure 4. The DSC curve of the physical combination demonstrates the existence of a melting peak at 227.70°C, Figure 5 which correlates to rivaroxaban melting. Comparisons of endothermic peaks of rivaroxaban and the physical mixture showed absence of interaction and presence of drug in unchanged form.
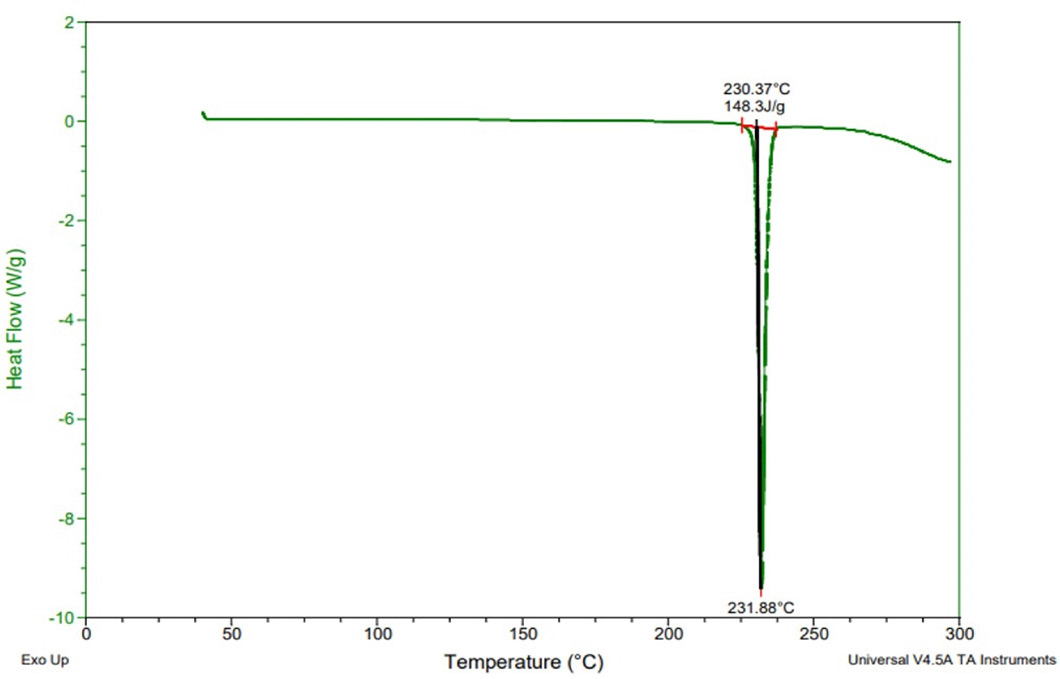
Figure 4:
Thermogram of Rivaroxaban.

Figure 5:
Thermogram of Physical mixture.
Experimental design
The prepared films were subjected to fundamental evaluation tests Table 4. Films are checked for thickness, folding endurance, and disintegration time and evaluated using Design Expert 11@ software for optimization Table 5.
| INGREDIENTS | Formulation | |||
|---|---|---|---|---|
| Formulation code | F1 | F2 | F3 | F4 |
| Drug(mg) | 10 | 10 | 10 | 10 |
| HPMC (mg) | 500 | 350 | 500 | 350 |
| Sodium starch glycolate (mg) | 50 | 40 | 40 | 50 |
| Aspartame (mg) | 15 | 15 | 15 | 15 |
| Propylene glycol (ml) | 1 | 1 | 1 | 1 |
| Water(ml) | 10 | 10 | 10 | 10 |
| Functional Group | Reported Frequencies (in cm-1) | Observed Frequencies in the pure drug (in cm-1) | Observed Frequencies in the Physical mixture (in cm-1) |
|---|---|---|---|
| -NH | 3400-3300 | 3355 | 3361 |
| -CH(-ANE) | 3000-2840 | 2936 | 2930 |
| -CH(-ANE) | 3000-2840 | 2879 | 2890 |
| CH(AROMATIC) | 2000-1650 | 1735 | 1735 |
| C=C | 1662-1626 | 1652 | 1653 |
| -OH(COOH) | 1440-1395 | 1417 | 1408 |
| Run | Factor 1 a:HPMC | Factor 2 b:SSG | Response 1 (Disintegration Time) | Response 2 (Folding Endurance) | Response 3 (Thickness) |
|---|---|---|---|---|---|
| 1 | 500 | 50 | 55 sec | 350 | 0.47 mm |
| 2 | 350 | 40 | 40 sec | 290 | 0.4 mm |
| 3 | 500 | 40 | 62 sec | 324 | 0.51 mm |
| 4 | 350 | 50 | 35 sec | 312 | 0.37 mm |
Optimization of dependent variables
Response 1 (in vitro disintegration time): The formulations indicated a range of 35 to 62 sec. The equation from best suited model to coordinate the answer y and variables (HPMC and SSG) was R1 = +15.50000+0.140000HPMC-0.600000SSG. The equation of the model F value of 238.50 and p value is < 0.05, implying that the model is significant. The predicted r2 0.9979 compared with adjusted r2 0.9937 demonstrated a nicely fit response of factors. R1 Three-Dimensional (3D) response surface plot showed with an increase in factors HPMC and Sodium starch glycolate. There is an increase in the disintegration time Figures 6 and 7.
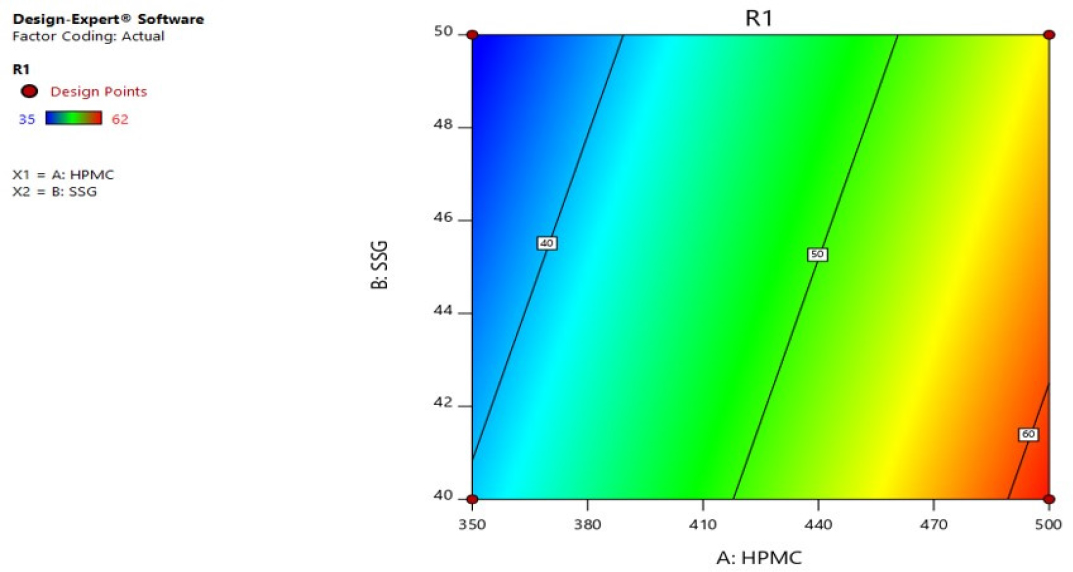
Figure 6:
Contour plot of R1 (disintegration time).
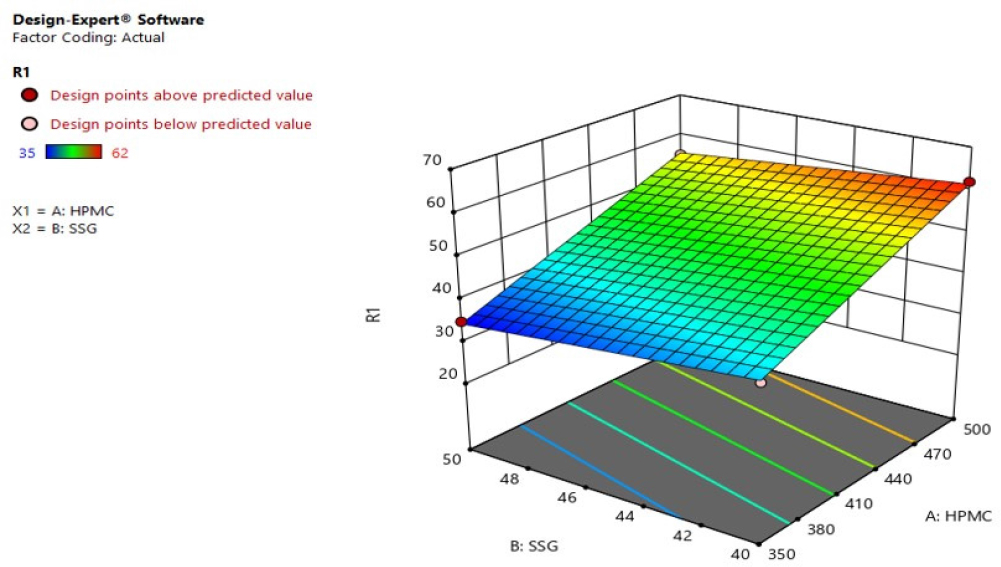
Figure 7:
3D response surface plot of R1 (disintegration time).
Response 2 (Folding Endurance): In the range 290 to 350, the folding endurance values of formulations(F1-F4) were found. R2 = -+109.00000+0.240000HPMC+2.40000SSG was derived from the most appropriate mathematical model for R2 and the independent variables. The model F value 234.00 and P value is < 0.05, implying that the model is significant. And, the r2 0.9970 predicted is in fair competition with the r2 0.9936 adjusted. Variables HPMC and SSG have significantly impacted the folding endurance. Enhanced HPMC and Sodium starch glycolate factors dramatically enhanced folding endurance, according to the R2 three-dimensional response plot Figures 8 and 9.
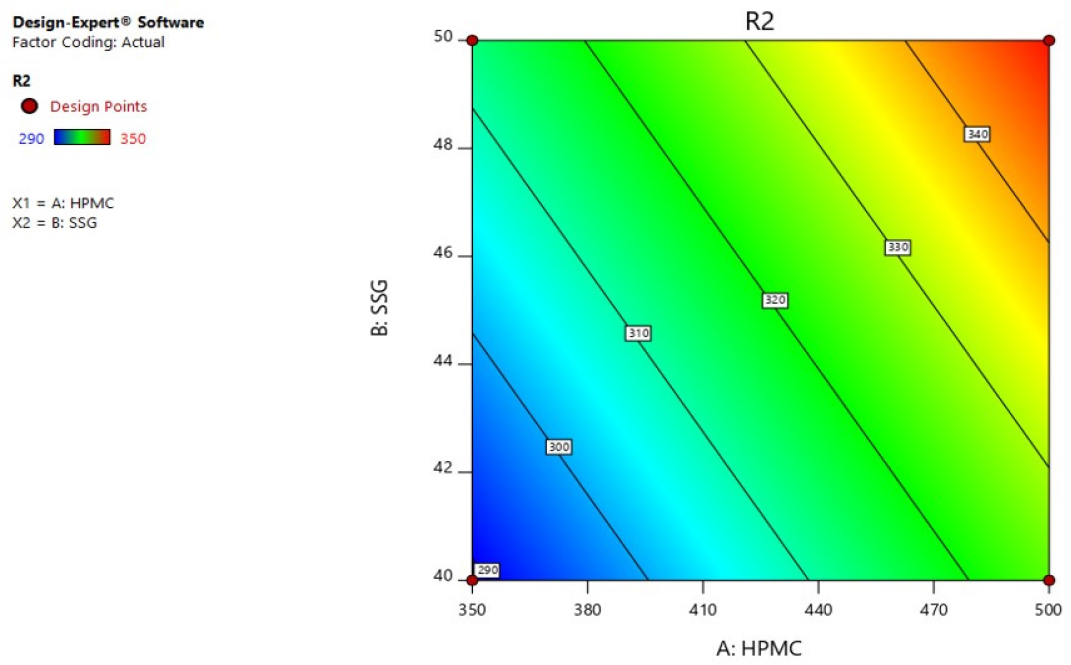
Figure 8:
Contour plot of R2 (Folding Endurance).
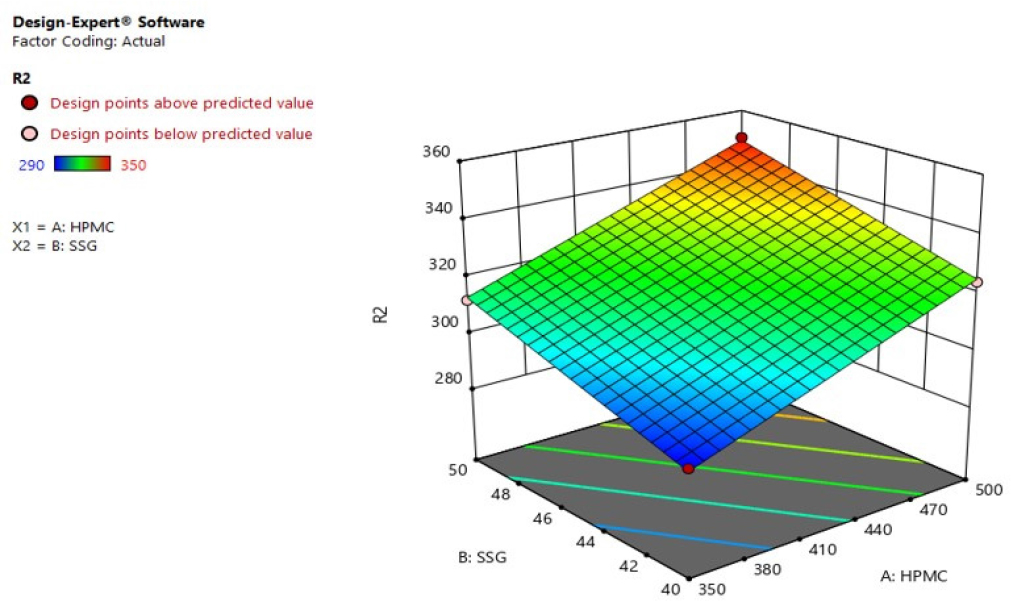
Figure 9:
3D response surface plot of R2 (Folding Endurance).

Figure 10:
Contour plot of R3 (Thickness).
Response 3 (Thickness): Based on 22 factorial designs, Various combinations of parameters (HPMC and SSG) they influenced the thickness response (R3). R3= +0.297500+0.000700HPMC-0 .003500SSG. The independent variables fit the equation derived from the best response R3. The F value 245.00, p<0.05 implying that the model is significant. And, the 0.9980 r2 predicted was comparable to the 0.9939 r2 adjusted. It was discovered that the factors sodium starch glycolate and HPMC had a significant impact on thickness. R3’s 3D response surface plot showed a significant rise in the thickness with increased levels of variables HPMC and Sodium starch glycolate Figure 11.
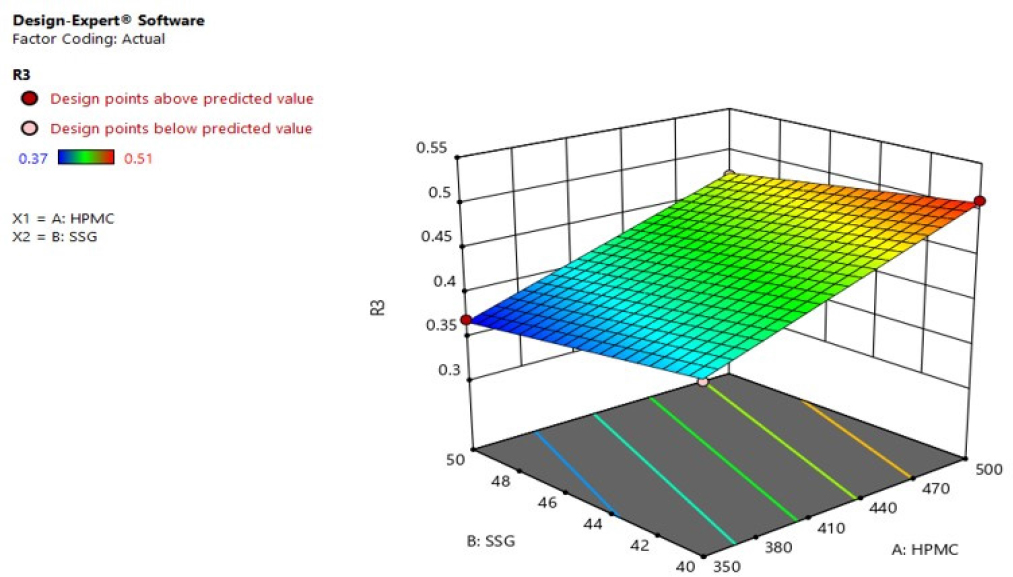
Figure 11:
3D response surface plot of R3 (Thickness).
Checkpoint study and design optimization
By imposing restrictions on the response, such as R1 = 48.5 sec, R2 = 319.9, and R3 = 0.43 mm, the optimized formulation (F5) was discovered using an overlay plot Figure 12. DoE was used to indicate the levels of components from plots which showed a desirability of 0.9428 and the best values of the selected variables, estimated at 0.61 percent for HPMC and 0.67 percent for SSG. The checkpoints were analyzed for the optimized formulation (F5) using thickness (mm), folding endurance, and disintegration time (sec).

Figure 12:
Overlay plot.
Evaluation of Rivaroxaban Oral Thin Film
Thickness, Average weight, and Uniformity of weight: These factors were tested visually and physically through touch or feel. According to the observation, the films have a smooth surface and are visually appealing. Utilizing an electronic balance, the weight of the prepared film was calculated; the average weight was 0.38±0.63 g, respectively. The film had a thickness of 0.45±0.50 mm, respectively.
Surface pH: According to the study, the normal physiological pH of the oral mucosa is 6.2-7.6. pH of the film was found to be around 7, which is an acceptable pH when measured with pH paper, while with that of the pH electrode, it was found to be 7.28. Folding endurance: Flexibility of the film was due to the presence of propylene glycol, and the mechanical properties of film were due to HPMC. Optimized formulation showed a good quality film with folding endurance of 325.
Percentage Moisture loss: Percent moisture loss evaluation can be used to determine the durability and quality of oral films. It was evident from the outcomes that the optimized formulations percentage moisture loss was 1.087±0.264%, clearly showing a correlation between moisture loss and polymer content.
Percentage moisture uptake; Percentage moisture absorption indicates the sustainability of the film. The percentage moisture absorption of the optimized f5 formulation was 1.219± 0.512%. The formulation has been found to absorb less than 2% moisture. Since the films take up moisture, they have to be protected from such an environment.
Drug content and content uniformity: The drug content and homogeneity of the film made with polymer were assessed. The drug concentration was 96.8%. The drug content and homogeneity were judged to be within the pharmacopeial limit. Both results indicate that the API was evenly distributed throughout the film.
Disintegration time: Because there was no formal disintegration test for oral thin films, hence most widely used method was performed. The concentration of super disintegrant influences disintegration time. The time needed for disintegration decreased as the concentration of super disintegrants increased. The disintegration time of the film was found to be 33 ± 1.5 sec.
In vitro dissolution studies
A dissolution study was used to compare the release pattern of drug in dissolution media. The USP type II apparatus was used to estimate the dissolution of oral thin film. The drug release from the film began as soon as it came into contact with the medium, and the drug release was larger than that of the traditional dosage form available. At 60 sec, drug release rapid dissolving films obtained 93.47% (Figure 13 and Table 6).

Figure 13:
In vitro dissolution studies.
| Absorbance | DF | Conc μg/mL | Amt in mg/ mL | Amn in mg/5 mL | Amt mg/900 mL | Cum. DR | %DR | |
|---|---|---|---|---|---|---|---|---|
| 15 | 0.061 | 10 | 0.6321 | 0.0063 | 0.0316 | 5.6891 | 5.7176 | 57.17 |
| 30 | 0.092 | 10 | 0.9533 | 0.0095 | 0.0476 | 8.5803 | 8.6404 | 86.40 |
| 45 | 0.094 | 10 | 0.9740 | 0.0097 | 0.0487 | 8.7668 | 8.8746 | 88.74 |
| 60 | 0.098 | 10 | 1.0155 | 0.0101 | 0.0507 | 9.1398 | 9.3471 | 93.47 |
Kinetic analysis of release data
The optimized formulation in vitro drug release pattern was best described by first order, as the plots exhibited the maximum linearity with R2 =0.9664 Table 7. Higuchi has studied the rate laws anticipated by the various dissolving processes, both alone and in combination. The equation predicts a first order dependency on the concentration gradient between the static liquid layer near to the solid surface and the bulk liquid, similar to the other rate law equations. Noyes and Whitney employed a notion close to the diffusion model to explain their dissolution findings. The data produced by the formulation did not correspond well to zero order kinetics, and this model was not appropriate for explaining the rate kinetics of oral rapid dissolving film formulation. The data was fitted with the peppas equation, which produced n = 0.31 for formulation, showing that fickian diffusion (case I diffusional) was the mechanism of drug release.
| Formulation Code | Zero Order R2 | First Order R2 | Higuchi Matrix R2 | Korsmeyer- Peppas | Hixson- Crowell | Best Fit Model | |
|---|---|---|---|---|---|---|---|
| R 2 | n | R 2 | |||||
| F5 | 0.7891 | 0.9664 | 0.9512 | 0.8734 | 0.3161 | 0.9236 | First order |
Stability studies
According to the findings, there is no substantial change in the surface pH, thickness, folding endurance, drug content, or percentage of drug release Table 8.
| Storage condition | Surface pH | Thickness | Folding endurance | %Drug content | %Drug release |
|---|---|---|---|---|---|
| 25 ± 2°C/60 ± 5% RH | 7.26 | 0.45±0.13 mm | 325±0.02 | 96.8±0.12% | 93.47±1.05% |
| 40 ± 2°C/75 ± 5% RH. | 7.25 | 0.45±0.04 mm | 324±0.03 | 96.68±1.25% | 91.15±1.03% |
DISCUSSION
Rivaroxaban Oral Thin Films (OTF) were developed utilizing the solvent casting method. A full factorial design with 22 experimental factors was employed using Design Expert 11 software. Both independent factors and dependent variables were carefully selected. Quantitative assessment of all samples was performed using Design Expert 11 software to determine the significant and non-significant effects of the chosen components on reactions such as Disintegration Time, Folding Endurance, and Thickness. Through the use of an overlay plot and response surface plot, the optimized formulation (F5) was successfully determined.10
The FT-IR spectra analysis indicated the absence of any detectable chemical interactions between the medication and the polymer. The distinctive bands of the pure drug remained unaffected by the presence of the polymer.8 Additionally, the comparison of melting peaks in the DSC curve between rivaroxaban and the physical combination demonstrated the absence of any interaction, confirming the presence of the drug in its original, unmodified form.9
The observations indicate that the optimized formulation F5 exhibited a visually seamless and attractive surface. The weight of F5 was determined using a digital balance, along with the average weight of the film. The film thickness was measured by calculating the mean value from five different locations using a micrometer screw gauge. The pH of the film was found to be approximately neutral, which is considered acceptable when measured using pH paper. A similar result was obtained when the pH was measured using a pH electrode, indicating a nearly neutral pH.25 The presence of propylene glycol contributed to the flexibility of the film, while the mechanical properties were attributed to HPMC. As the concentration of the polymer increased, the percentage of moisture loss in the film also increased. However, the film was found to absorb less than 2% moisture. Therefore, it is necessary to protect the film from environmental moisture due to its tendency to absorb moisture.26
The drug content and content uniformity of the film were found to be within the acceptable range defined by the pharmacopeia. These results indicate that the medication was uniformly distributed throughout the film. The disintegration time of the film was found to be influenced by the concentration of the super disintegrant. Specifically, as the concentration of the super disintegrant increased, the disintegration time decreased.27 The drug release from the film commenced immediately upon contact with the medium, and the extent of drug release was greater compared to traditional dosage forms currently available. The in vitro release pattern of the drug followed first-order kinetics, and the mechanism of drug release was identified as fickian diffusion.28
CONCLUSION
Solvent casting approach was effectively used to prepare oral thin films that were stocked with rivaroxaban. According to FT-IR spectrum, the polymer and formulation did not change the functional bands of rivaroxaban. A neutral surface pH of optimized formulation of F5 with smooth surface that was sufficiently elegant to be seen. The dose uniformity test’s acceptance value requirement had been met by the optimized F5 formulation, which also showed excellent stability and a dissolution profile. Based on the findings, it can be said that the optimised formulation F5 offers a quick release of the drug from the site of administration into the systemic circulation, thereby enhancing the bioavailability of rivaroxaban.
Cite this article
Kanna S, Nadendla RR, Satyanarayana J, Karthikeya V, Sonu MV, Bhargavi PN. Formulation and Evaluation of Fast-Dissolving Oral Film of Rivaroxaban. J Young Pharm. 2023;15(4):687-95.
ACKNOWLEDGEMENT
The authors express their gratitude to the Chalapathi Institute of Pharmaceutical Sciences, Lam, Guntur, for providing necessary support in due course of the work.
ABBREVIATIONS
| RN | Rivaroxaban |
|---|---|
| OTF | Oral thin films |
| DOACs | Direct oral anticoagulants |
| HPMC | Hydroxypropyl methylcellulose |
| SSG | Sodium starch glycolate |
References
- Rai A, Sharma S. Preparation and evaluation of oral dispersible formulations of amlodipine besylate. Asian J Pharm Res Dev. 1970;7(5):42-55. [CrossRef] | [Google Scholar]
- Ney DM, Weiss JM, Kind AJ, Robbins J. Senescent swallowing: impact, strategies, and interventions. Nutr Clin Pract. 2009;24(3):395-413. [PubMed] | [CrossRef] | [Google Scholar]
- Raju PN, Kumar MS, Reddy CM, Ravishankar K. Formulation and evaluation of fast dissolving films of loratidine by solvent casting method. J Pharm Innov. 2013;2(2):31-5. [PubMed] | [CrossRef] | [Google Scholar]
- Thakur N, Bansal M, Sharma N, Yadav G, Khare P. A novel approach of fast dissolving films and their patients. Adv Biol Res. 2013;7(2):50-8. [PubMed] | [CrossRef] | [Google Scholar]
- Malke S, Shidhaye S, Desai J, Kadam V. Oral films – patient compliant dosage form for pediatrics. J Pediatr Neonatol. 2009;11(2):1-7. [PubMed] | [CrossRef] | [Google Scholar]
- Nadendla RR, Satynarayana J, Burri JK. Rivaroxaban: Compatibilty with pharmaceutical excipients using DSC and FTIR spectrophotometry. J Pharm Res Int. 2022;34(12A):43-50. [CrossRef] | [Google Scholar]
- Ahmed MG, Harish NM, Charyulu RN, Prabhu P. Formulation of chitosan-based ciprofloxacin and diclofenac film for periodontitis therapy. Trop J Pharm Res. 2009;8(1):33-41. [CrossRef] | [Google Scholar]
- Mishra R, Amin A. Formulation and Characterization of Rapidly Dissolving Films of cetirizine hydrochloride using pullulan as a Film Forming Agent. IJPER. 2011;45:71-7. [CrossRef] | [Google Scholar]
- Nadendla RR, Juluri S, Doppalapudi SSS, Pyda VH, Tadiboina A. Physico-chemical characterization of Rivaroxaban and compatibility studies with its pharmaceutical excipients. Int J Life Sci Pharm Res. 2021;11(4):25-32. [CrossRef] | [Google Scholar]
- Gupta NV, Shanmuganathan S, Kanna S, Sastri KT. A 23 Factorial design for formulation and development of doxycycline hydrochloride in situ gel forming solution for wound healing application. Int J Appl Pharm. 2021;13(3):221-32. [CrossRef] | [Google Scholar]
- Saini S, Samta RAC, Gupta S. Optimization of formulation of fast dissolving films made of pullulan polymer. Int J Pharm Sci Rev Res. 2011;9(1):127-31. [CrossRef] | [Google Scholar]
- Yellanki SK, Jagtap S, Masareddy R. Dissofilm: A novel approach for delivery of phenobarbital; design and characterization. J Young Pharm. 2011;3(3):181-8. [PubMed] | [CrossRef] | [Google Scholar]
- Reddy PS, Kumar VA, Deepthi A, Reddy KS, Reddy PVM. Oral films of metoclopramide hydrochloride for pediatric use: formulation and in vitro evaluation. J Chem Pharm Res. 2011;3(4):636-46. [PubMed] | [CrossRef] | [Google Scholar]
- Vishvakarma P. Design and development of montelukast sodium fast dissolving films for better therapeutic efficacy. J Chil Chem Soc. 2018;63(2):3988-93. [CrossRef] | [Google Scholar]
- Gupta MM, Patel MG, Kedawat M. Enhancement of dissolution rate of rapidly dissolving oral film of meclizine hydrochloride by complexation of meclizine hydrochloride with β-cyclodextrine. J Appl Pharm Sci. 2011;1(9):150-3. [CrossRef] | [Google Scholar]
- Prasanthi NL, Krishna CS, Gupta ME, Manikiran SS, Rao NR. Design and development of sublingual fast dissolving films for an antiasthmatic drug. Sch res Libr. 2011;3(1):382-95. [CrossRef] | [Google Scholar]
- Rao NR, Kandhadi SR, Swapna D, Konasree SD, Enugala S. Formulation and evaluation of rapidly dissolving film etophylline. Int J Pharm Biol Sci. 2011;1:145-59. [CrossRef] | [Google Scholar]
- Choudhary DR, Patel V, Patel H, Kundawala A. Formulation and evaluation of quick dissolving film of ondansetron hydrochloride. Int J Pharmacol Res. 2011;3(4):31-50. [CrossRef] | [Google Scholar]
- Shelke PV, Dumbare AS, Gadhave MV, Jadhav SL, Sonawanne AA, Gaikwad DD, et al. Formulation and evaluation of rapidly disintegrating film of amlodipine bedylate. J Drug Deliv Ther. 2012;2(2):72-5. [CrossRef] | [Google Scholar]
- Sapkal NP, Kilor VA, Daud AS, Bonde MN. Development of fast dissolving oral thin films of ambroxol hydrochloride: effect of formulation variables. J Adv Pharm Res. 2011;2(2):102-9. [CrossRef] | [Google Scholar]
- Babu PS, Chowdary KPR. Enhancement of dissolution rate of celocoxib by solid dispersion in super disintegrants. Indian Drugs. 2007;45(7):547-52. [CrossRef] | [Google Scholar]
- Mulla JA, Dasankoppa FS, Vilas GJ, Sholapur HP. Fast dissolving tablets of promethazine: A novel oral formulation for the treatment of fractionated radio therapy induced nausea and emesis. Indian Drugs. 2008;45(4):314-17. [CrossRef] | [Google Scholar]
- Patel DM, Patel MM. Optimization of fast dissolving etorocoxib tablets prepared by sublimation technique. Indian J Pharm Sci. 2008;70(1):71-6. [PubMed] | [CrossRef] | [Google Scholar]
- Irfan M, Rabel S, Bukhtar Q, Qadir MI, Jabeen F, Khan A, et al. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm J. 2016;24(5):537-46. [PubMed] | [CrossRef] | [Google Scholar]
- Chaudhary H, Gauri S, Rathee P, Kumar V. Development and optimization of fast dissolving oro-dispersible films of granisetron HCl using Box-Behnken statistical design. Bull Fac Pharm Cairo Univ. 2013;51(2):193-201. [CrossRef] | [Google Scholar]
- Ghadermazi R, Hamdipour S, Sadeghi K, Ghadermazi R, Khosrowshahi Asl A. Effect of various additives on the properties of the films and coatings derived from hydroxypropyl methylcellulose-A review. Food Sci Nutr. 2019;7(11):3363-77. [PubMed] | [CrossRef] | [Google Scholar]
- Zhang L, Aloia M, Pielecha-Safira B, Lin H, Rajai PM, Kunnath K, et al. Impact of super disintegrants and film thickness on disintegration time of strip films loaded with poorly water-soluble drug microparticles. J Pharm Sci. 2018;107(8):2107-18. [PubMed] | [CrossRef] | [Google Scholar]
- Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7(4):429-44. [PubMed] | [CrossRef] | [Google Scholar]