ABSTRACT
Objectives
The consumption of fruits, vegetables, dietary supplements, and herbal goods has skyrocketed, which has greatly increased human exposure to phytochemicals. Phytochemicals can alter the activity of drug-metabolizing enzyme systems such cytochrome P450, resulting in clinically significant drug-phytochemical interactions and changed plasma levels of drugs. Ertugliflozin represents a sodium-glucose co-transporter 2 inhibitor explored to treat type 2 diabetes. Sinapic acid, a bioactive phytoconstituent, treats many diseases as a dietary supplement. This study explored Sinapic acid’s effects on ertugliflozin’s pharmacodynamics as well as pharmacokinetics in Streptozotocin induced type-2 diabetic rats.
Materials and Methods
A validated RP-HPLC method was employed in order determine the ertugliflozin concentration present in the plasma samples. Ertugliflozin, both on its own and in combination with sinapic acid administered to different groups of normal rats as well as rats with diabetes that were experimentally induced and the results of the experiment concerning single dose and multiple dose interactions were analysed. In diabetic rats, the concentrations of glucose on average were measured.
Results
Ertugliflozin co-administration with sinapic acid resulted in a marginally significant increase in Cmax, t1/2, AUC, and MRT, Vd but a marginally significant decrease in CL was observed when compared to rats that were treated ertugliflozin alone. The enhancement in Cmax, AUC, t1/2, MRT, Vd and the reduction in CL may be responsible for the inhibition of CYP metabolising enzymes.
Conclusion
Intake of ertugliflozin with sinapic acid may require a minor dose adjustment, and caution should be used when the two medications are administered together for their clinical benefits in diabetic patients.
INTRODUCTION
Our modern era’s biggest metabolic threat is diabetes. Prehistoric India described Diabetes by many terms like premeha means excess urine, madhumeha-sweet urine. It was mentioned that ants gathering near a patient’s urine may be used to diagnose the illness.1 Hyperglycaemia is the defining characteristic of diabetes, a group of metabolic diseases that are characterised by the fact that they cause both microvascular and macrovascular issues within the body. These issues are the root cause of a diminished quality of life as well as a shortened life expectancy.2 Type 2 diabetes can be identified by its hallmark symptoms, which include insulin insensitivity brought on by insulin resistance, a decrease in insulin production, and subsequently the death of beta cells in the pancreas. Because of this, the process by which glucose is transported inside the liver, muscle cells, and fatty tissue is slowed down. An increase in the breakdown of fat is brought on by hyperglycaemia. Recent studies have shown that a decrease in alpha-cell activity play a role in the pathogenesis of type 2 diabetes. As a result of this malfunction, eating does not have effect of lowering the levels of glucagon and hepatic glucose that rise during periods of fasting. Hyperglycaemia can occur when insulin levels are low and insulin resistance is increasing in the body.3 According to estimates, there will be 93 million people living with diabetes in India by the year 2030. It would be necessary to spend an exponentially greater amount of money on efforts to avoid, detect, and treat this disease that cannot be reversed if there were a greater number of diabetic patients.4
As a novel class of oral hypoglycaemics, Sodium-Glucose Cotransporter Type-2 (SGLT2) inhibitors are recommended for their beneficial effects on patients with type 2 diabetes. This is particularly selected in risk of cardiovascular or renal challenges. Ertugliflozin (ERT) is a novel SGLT2 inhibitor that is highly selective and potent. Ertugliflozin lowers the renal threshold over glucose, results in an increase in glucose excretion via urine. This is because Ertugliflozin lowers glucose reabsorption in the kidney tubules. Ertugliflozin also reduced plasma glucose and glycated Haemoglobin (HbA1c) levels in patients with type 2 diabetes without causing an excessive amount of insulin secretion. Ertugliflozin underwent very little phase I oxidative metabolism triggered by the Cytochrome P450 (CYP) isoforms CYP3A5, and CYP2C8.5 Sinapic acid, also known as sinapinic acid, is a polyphenol that is derived from hydroxycinnamic acid and contains 3,5-dimethoxy,4-hydroxyl substitutions in the phenyl group of cinnamic acid. Sinapic acid can be found in a variety of fruits and vegetables, including wheat, rice, spices, oil seeds, citrus fruits, cereals, broccoli cabbage, turnip, radish, and vinegar. It is also possible to extract sinapic acid from oil seeds. Sinapic acid was found to have powerful antioxidant, anti-inflammatory, anticancer, hepatoprotective, anti-diabetic activity, anti-hypertensive, cardioprotective, reno-protective, neuroprotective, anxiolytic, sedative, anti-convulsant, anti-depressant, anti-epileptic, and anti-epileptic effects as well as antibacterial properties.6
The use of herbal remedies, phytochemicals that are derived from them as fringe medicine, nutritional supplements has significantly increased the level of concern regarding the possibility of interactions between phytochemicals-pharmaceuticals. Interactions between phytochemicals – pharmaceuticals could be to blame for the modulatory effects that prescription drugs have leading to unexpected clinical outcomes. Phytochemicals have the potential to either increase or decrease the activity of drug metabolising enzymes, which can then affect the metabolism and disposition of concomitant drugs, leading to changes in the concentration of the drug in plasma and in particular tissues. There is not, to the best of our knowledge, any information that has been reported on how the pharmacodynamics-pharmacokinetics of ertugliflozin are affected by the presence of sinapic acid. The goal of this particular study was to investigate the interaction with one another.
MATERIALS AND METHODS
Drugs and Chemicals
Streptozotocin, Acetonitrile, Methanol (HPLC grade) was purchased from Taranath Scientific and Chemicals (Hanamkonda, Telangana). We purchased Sinapic acid from (BLD Pharmatech, Hyderabad, India), Ertugliflozin (gifted from MSN Laboratories Pvt. Ltd., Hyderabad), MilliQ water prepared in our laboratory using a purification system (Pharmacology Lab, Kakatiya University, Hanamkonda, Warangal, Telangana).
Animals for study
Adult Wistar albino rats (180-200g) bought from Vyas Enterprises, Hyderabad. Animals housed in polypropylene cages in a room at 27 ±1°C temperature with 12 hr light and dark cycles. We fed animals with standard pellet diet and water ad libitum for seven days so they could acclimatise to their new environment. The institution Animal Ethical Committee, Kakatiya University, Warangal approved protocol as per CPCSEA guidelines (approval no. 01/IAEC/UCPSc/K U/2022: CPCSEA).
HPLC Analysis of Ertugliflozin: Instrumentation and chromatographic conditions
Ultra-Fast Liquid Chromatography (UFLC) with SPD-M20A PDA binary LC-20AD pumps (Shimadzu Corporation, Kyoto, Japan) outfitted with RP C18 column comprised of micro-gradient mixer 250 4.6 mm, 5 m, Phenomenex Luna as the stationary phase,20µL loop volume injector with solvent flow of 1mL min–1 used for sample investigation, LC lab solutions software is applied for data accuracy in tandem Chromatogram analysis. Mixer of Potassium dihydrogen ortho phosphate and acetonitrile (60:40v/v) adjusted pH 4.8 utilised as mobile phase.7 Methanol was used for sample dilutions. Mobile phase, stock and working solutions were sonicated for 30 min prior to analysis. The sample (ertugliflozin) detection was carried out at 240 nm.
Preparation of sample, extraction and Analysis
In a centrifuge tube containing 250 µL plasma, 50 µL of different concentrations (0.05-5 µg /mL) of prepared standard and 500 µL internal standard (100 µg/mL) were added along with 2mL of acetonitrile, vortexed for 2 min, centrifuged at 3200rpm for 3 min. After separating, drying and reconstituting the residue with mobile phase, a 20-µL aliquot was injected into the HPLC system to perform chromatographic analysis.7–9 Ratio of ertugliflozin peak areas to those of an internal standard was used to quantify ertugliflozin in plasma samples. HPLC technique was used to the quantification of ertugliflozin in rat plasma after being verified in terms of repeatability, system appropriateness, recovery, accuracy, and precision. Ertugliflozin and the IS were respectively eluted at 4.1 and 5.3 min. Using the Kinetica TM software (version 4.4.1, Thermo Fisher Scientific Corporation, USA), pharmacokinetic parameters such as maximum concentration of drug in plasma (Cmax), time required to achieve Cmax (Tmax), Area Under the curve (AUC), Mean Residence Time (MRT), Volume of Distribution (Vd), and total drug clearance (CL) were calculated.
Study design
Overnight fasted Wistar albino rats received a single intraperitoneal injection of 120 mg/kg nicotinamide 15 min after Streptozotocin (in citrate buffer, pH 4.5) was injected intraperitoneally at a dose of 55 mg/kg to induce diabetes. Using a glucometer, the elevated glucose levels in blood from the tail vein at 72 hr confirmed hyperglycemia. To conduct this experiment, diabetic rats with fasting blood glucose levels greater than 250 mg/dL were chosen.10,11 Ertugliflozin (20 mg/kg b.wt)12 and Sinapic acid (20 mg/kg b.wt)13 suspensions were made with 0.1% Sodium CMC as the suspending agent. Oral gavage was used to administer both drugs to the respective groups.
Pharmacokinetic interaction study in normal and diabetes induced rats
Three groups (six rats per group; n=6) were isolated.
Group-1: Treated with ertugliflozin (20mg/kg).
Group-2: Ertugliflozin (20mg/kg) was administered to pre-treated animals with sinapic acid(20mg/kg) (single dose interaction study).
Group-3: Received sinapic acid (20mg/kg) for 7 days consecutively, on the eighth day rats received sinapic acid (20 mg/kg) plus Ertugliflozin (20 mg/kg) (study of multiple dose interactions).
Blood samples were drawn from the retro-orbital plexus at 0 hr, 0.5 hr, 1 hr, 2 hr, 4 hr, 6 hr, 8 hr, 12 hr, and 24 hr intervals using heparinized capillaries into a microcentrifuge tube consisting of anticoagulant (sodium citrate).14 After completion of Centrifugation, plasma samples were separated and stored at -20°C until HPLC sample analysis.
Pharmacodynamic Interaction study in Diabetic rats
Diabetic albino Wistar rats were selected randomly and seperated into 4 groups of 6 animals in each.
Group I: Treated with ertugliflozin for 7 days.
Group II: Treated with sinapic acid for 7 days.
Group III: Treated with sinapic acid, after 1 hr, Ertugliflozin was administered (single dose interaction study).
Group IV: Pre-treated with sinapic acid for 7 days, on the 8th day after 1 hr administration of sinapic acid, ertugliflozin was given.
Blood samples were drawn from the retro-orbital plexus utilising heparinized capillaries into a micro centrifugation tube consisting of anticoagulant (sodium citrate) at predetermined time intervals.14 Centrifugation separated plasma stored at -20°C until further study. Blood glucose levels in these samples were determined using the GOD/POD method.15
Data and statistical analysis
Data is presented as mean ± Standard Deviation (SD). A one-way ANOVA using Dunnett’s test applied to determine the significance. *p<0.05, **p<0.01 denoted as statistically significant in comparison to ertugliflozin alone. Kinetica software (version 5.0, Thermo Fisher Scientific, Inc., USA) used for plasma concentration–time data analysis.
RESULTS
Pharmacokinetic interaction study in both normal and diabetic rats
Figures 1 and 2 depicts plasma concentration-time profiles of ertugliflozin in normal and diabetic rats, accordingly Table 1 and Table 2 displayed the mean kinetic characterestics for ertugliflozin alone and co-administration with Sinapic acid in normal as well as diabetic rats respectively. In pharmacokinetic profile conducted in normal rats showed an increase in the Cmax 54.3% in MDI, 20.7% in SDI, AUC0-n 44.21% in MDI, 20.7% in SDI, AUCtotal was 31.3% in MDI, 12.96% in SDI, MRT was 44.19% in MDI, 21.01% in SDI, CL was reduced to 21.14% in MDI,7.92% in SDI after co-administration of sinapic acid with ertugliflozin when compared to the ertugliflozin alone. An increased Cmax 58.91% in MDI, 29.80% in SDI, AUC0-n 50.23% in MDI, 22.13% in SDI, AUCtotal was 44.9% in MDI, 25.2% in SDI, MRT was 53.69% in MDI, 23.31% in SDI, CL was reduced to 46.7% in MDI, 8.83% in SDI were obtained in sinapic acid and ertugliflozin treated group when compared to the ertugliflozin alone in diabetic rats. Notable consequences observed in combined sinapic acid and ertugliflozin therapy when compared to the Ertugliflozin alone therapy in MDI studies.
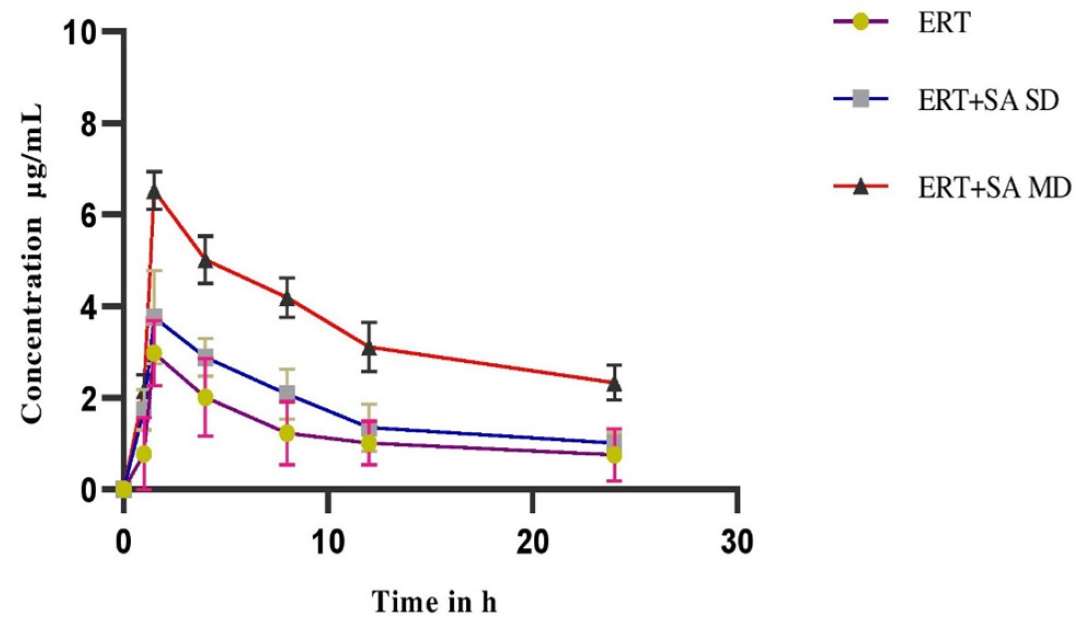
Figure 1:
Plasma Concentration-time profile of Ertugliflozin before and after pretreatment with Sinapic acid in normal rats expressed as (mean ± SD) (SD-Single Dose, MD-Multiple Dose).
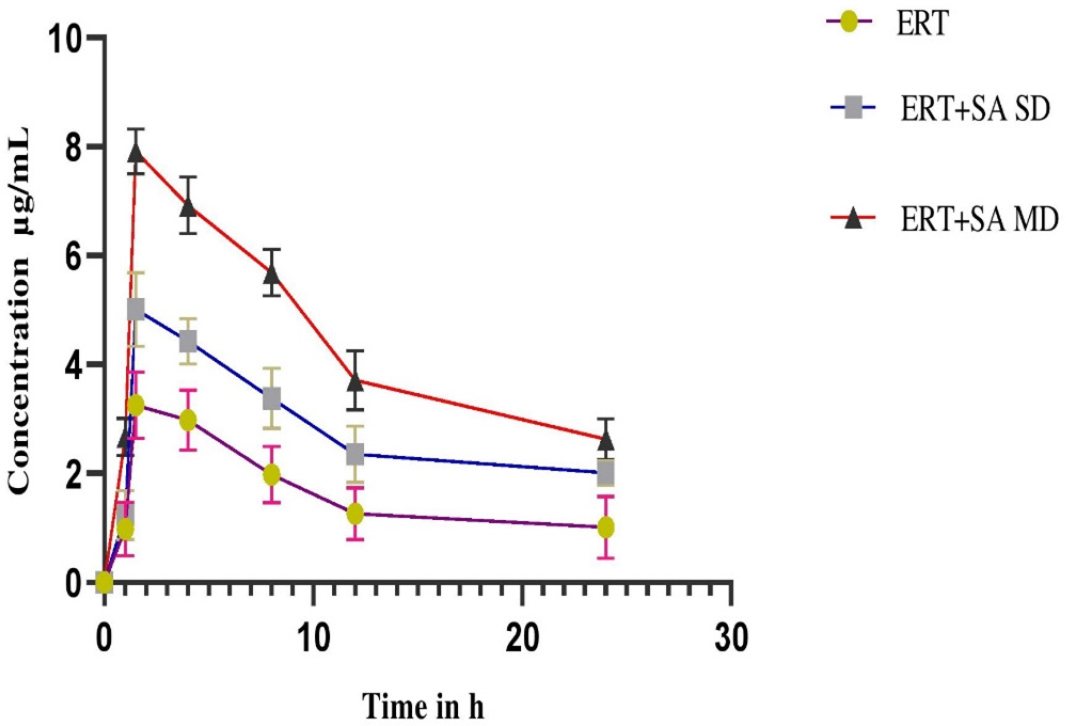
Figure 2:
Plasma Concentration-time profile of Ertugliflozin before and after pretreatment with Sinapic acid in Diabetic rats expressed as (mean ± SD) (SD-Single Dose, MD-Multiple Dose).
| PK parameters | ERT | ERT+SA (SDI) | ERT+SA (MDI) |
|---|---|---|---|
| Cmax (μg/mL) | 2.98±0.78 | 3.76± 1.02ns | 6.53±2.07** |
| Tmax (h) | 1.5 | 1.5 | 1.5 |
| AUC0-n (μg.h/mL) | 92.78± 1.32 | 117.13±2.06* | 166.32±5.11* |
| AUCtotal (μg.h/mL) | 121.82±2.22 | 139.84±3.04* | 184.23±1.88* |
| t1/2 (h) | 9.0 1±0.71 | 10.65±0.99 | 14.42±1.02* |
| MRT (h) | 10.12±2.21 | 13.89±3.74 | 21.89±3.91* |
| Vd (mL/kg) | 1106±20.18 | 1192±19.12* | 1234±24.67* |
| CL (mL/h/kg) | 178.12±4.61 | 162.25±4.99 | 147.03±3. 19** |
| PK parameters | ERT | ERT+SA (SDI) | ERT+SA (MDI) |
|---|---|---|---|
| Cmax (μg/mL) | 3.25±1.24 | 4.63±1.81 | 7.91±1.44** |
| Tmax (h) | 1.5 | 1.5 | 1.5 |
| AUC0-n (μg.h/mL) | 105.78±7. 12 | 135.09±10.51 * | 211.32±8.43* |
| AUCtotal (μg.h/mL) | 136.13±5.34 | 182.84±6.77* | 247.88±6.12* |
| t1/2 (h) | 9.86±1.17 | 12.65±1.23 | 15.81± 1.46* |
| MRT (h) | 10.99±2.31 | 14.34±2.01 | 23.69±2.2 1* |
| Vd (mL/kg) | 1231±24.12 | 1258±18.12 | 1291±27.02* |
| CL (mL/h/kg) | 197.12±8.71 | 181.67±5.23* | 134.41±6.41** |
Pharmacodynamic interaction study in diabetic rats
Ertugliflozin significantly showed antihyperglycemic activity at 1.5 hr in diabetic rats. The mean blood glucose levels, percentage blood glucose reduction of experimental groups expressed in Figures 3 and 4. In presence of sinapic acid, ertugliflozin hypoglycemic effect markedly augmented (p<0.01) in multidose diabetic rats.
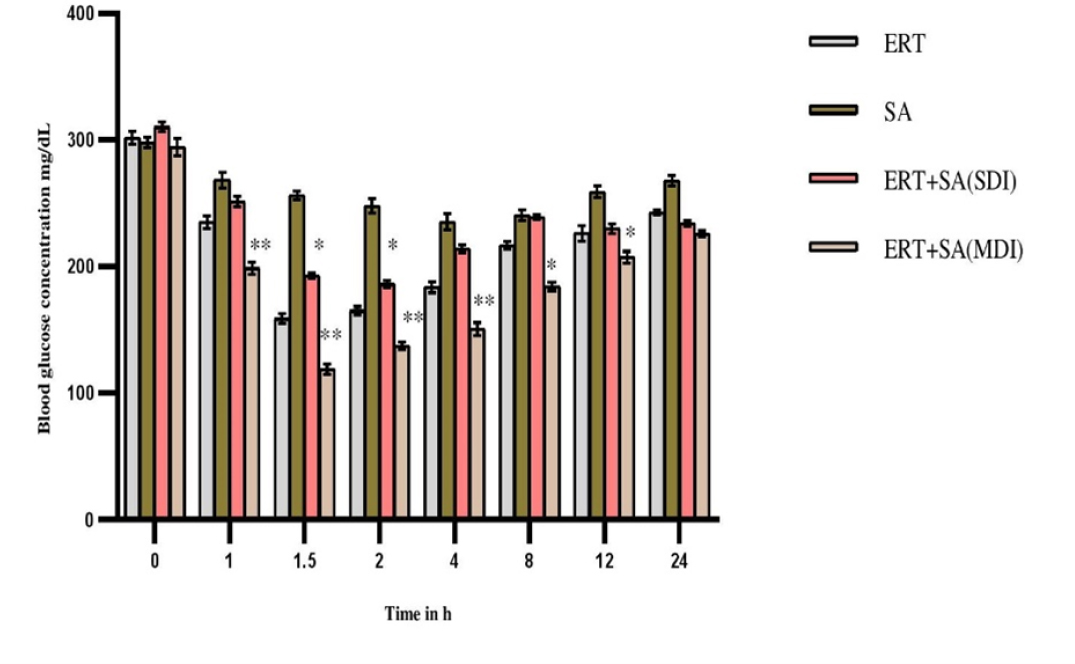
Figure 3:
Pharmacodynamic parameters in diabetic rats-Mean Blood glucose levels (mg/dL).
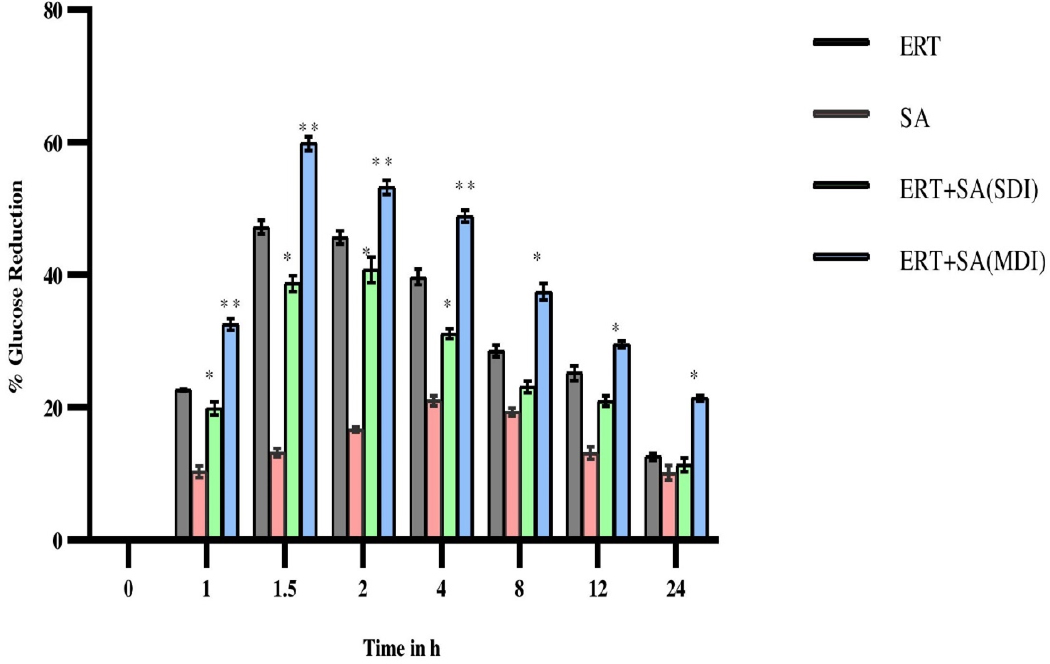
Figure 4:
Percentage of glucose reduction in diabetic rats after oral administration of Ertugliflozin, Sinapic acid and in combination of Ertugliflozin and Sinapic acid (SDI and MDI).
DISCUSSION
Patients typically do not know about possible herb-drug interactions and some patients do not consider herbal supplements to be drugs. Significantly, doctors lack knowledge regarding the concurrent prescription of herbal supplements that may cause mimic, heighten or decrease the pharmacological activity of drugs.13
The effectiveness of sinapic acid has been demonstrated against a number of pathological illnesses, including oxidative stress, inflammation, diabetes, neurodegeneration and anxiety. In addition, sinapic acid is frequently employed as a nutritional supplement owing to its chemo-preventive properties. SGLT2 inhibitors produces glycosuria resulting lower plasma glucose concentrations. Ertugliflozin is a one of the potent agents of SGLT2 inhibitor in type 2 diabetes mellitus (T2DM).16 This study has shown that sinapic acid increased Cmax, AUC0-t, Vd and MRT while decreased the CL of ertugliflozin proving sinapic acid altered the kinetics of ertugliflozin. Ertugliflozin is eliminated mainly via glucuronidation metabolism (UGT1A9 and UGT2B73), minor oxidative metabolism which contributes through CYP2C8 and CYP3A5.17 Researchers investigated that Co-administration of ertugliflozin with rifampin in healthy adults resulted in minor changes in pharmacokinetic parameters of Ertugliflozin and found that Ertugliflozin was safe when given along with rifampin or alone.16 Pre-treatment with sinapic acid at various doses in different studies showed the alterations in pharmacokinetic parameters of carbamazepine, aripiprazole and Ibrutinib and bioavailability of those drugs due to significant inhibition of CYP3A2, the CYP2C11-mediated metabolism of Carbamazepine in the liver and the inhibition of intestinal P-glycoprotein/MDR1, notable inhibition of CYP3A2-facilitated metabolism of ibrutinib in the hepatic and intestine and inhibition of intestinal and liver Pgp/MDR1,inhibition of CYP3A2 and CYP2D6 mediated metabolism of aripiprazole by sinapic acid. These studies corroborated that sinapic acid altered the pharmacokinetic parameters of drugs by inhibiting the CYP enzymes and showed possibility of drug-herb interactions.13,18,19 Based on our results, that following pre-treatment of sinapic acid with ertugliflozin in single and multiple dose studies exhibited modulated kinetic, dynamic parameters of ertugliflozin, this may be due to inhibition of UGT or CYP.
CONCLUSION
The current investigation showed that oral administration of sinapic acid altered the pharmacokinetics of Ertugliflozin through a significant decrease in ertugliflozin-CL, an escalation in its Cmax, AUC and MRT. As a result, it is important to exercise caution while using Ertugliflozin in conjunction with any meal, supplement, or traditional herb that contains sinapic acid. Further studies are required to investigate the underlying mechanism for such possibility.
References
- Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120-9. [PubMed] | [CrossRef] | [Google Scholar]
- Theivasigamani K, Palaniappan S. Assessment. J Young Pharm. 2023;15(1):174-81. [CrossRef] | [Google Scholar]
- Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269-73. [PubMed] | [CrossRef] | [Google Scholar]
- Anjali K, Dilip TR, Shejul YK, Prashant B, Puja G, Sharma P, et al. Progression and management of diabetes in Indian settings with universal access to health care: Protocol and plans for CHIPS cohort study. medRxiv. 2023;9(2):1-5. [PubMed] | [CrossRef] | [Google Scholar]
- Fediuk DJ, Nucci G, Dawra VK, Cutler DL, Amin NB, Terra SG, et al. Overview of the Clinical Pharmacology of ertugliflozin, a novel Sodium-Glucose Cotransporter 2 (SGLT2) inhibitor. Clin Pharmacokinet. 2020;59(8):949-65. [PubMed] | [CrossRef] | [Google Scholar]
- Pandi A, Kalappan VM. Pharmacological and therapeutic applications of sinapic acid-an updated review. Mol Biol Rep. 2021;48(4):3733-45. [PubMed] | [CrossRef] | [Google Scholar]
- Rao PV, Rao AL, Prasad S. Rapid quantitative estimation of metformin and ertugliflozin in rat plasma by liquid chromatography-tandam mass spectroscopy and its application to pharmacokinetic studies. Egypt Pharm J. 2021;20:1-7. [CrossRef] | [Google Scholar]
- Murugesan A, Annapurna Mathrusri M.. Novel simplified, new analytical method for stress degradation study of ertugliflozin an oral anti-diabetic agent by RP-HPLC method. Acta Sci Pharm Sci.. 2021;5(12):3-9. [CrossRef] | [Google Scholar]
- Han DG, Yun H, Yoon IS. A novel high-performance liquid chromatographic method combined with fluorescence detection for determination of ertugliflozin in rat plasma: assessment of pharmacokinetic drug interaction potential of ertugliflozin with mefenamic acid and ketoconazole. J Chromatogr B Analyt Technol Biomed Life Sci.. 2019;1122-1123:49-57. [PubMed] | [CrossRef] | [Google Scholar]
- Sellamuthu PS, Arulselvan P, Muniappan BP, Fakurazi S, Kandasamy M. Mangiferin from prevents oxidative stress and protects pancreatic β-cells in streptozotocin-induced diabetic rats. J Med Food. 2013;16(8):719-27. [PubMed] | [CrossRef] | [Google Scholar]
- Eggadi V, Sheshagiri SBB, Devandla A, Dasi N, Kulundaivelu U. Effect of atorvastatin on pharmacology of sitagliptin in streptozotocin- nicotinamide induced Type-II diabetes in rats. Biol Med. 2015;6:225 [PubMed] | [CrossRef] | [Google Scholar]
- Basalay MV, Arjun S, Davidson SM, Yellon DM. The role of parasympathetic mechanisms in the infarct-limiting effect of SGLT2 inhibitor ertugliflozin. bioRxiv. 2021 [PubMed] | [CrossRef] | [Google Scholar]
- Raish M, Ahmad A, Ansari MA, Alkharfy KM, Ahad A, Al-Jenoobi FI, et al. Effects of sinapic acid on hepatic cytochrome P450 3A2, 2C11, and intestinal P-glycoprotein on the pharmacokinetics of oral carbamazepine in rats: potential food/herb-drug interaction. Epilepsy Res. 2019;153:14-8. [PubMed] | [CrossRef] | [Google Scholar]
- [PubMed] | [CrossRef] | [Google Scholar]
- Jyothi PT, Reddy NY. Influence of diosgenin on pharmacokinetics and pharmacodynamics of repaglinide in rats. Int J Pharm Biol Sci. 2017;7(1):101-8. [PubMed] | [CrossRef] | [Google Scholar]
- Dawra VK, Sahasrabudhe V, Liang Y, Matschke K, Shi H, Hickman A, et al. Effect of rifampin on the pharmacokinetics of ertugliflozin in healthy subjects. Clin Ther. 2018;40(9):1538-47. [PubMed] | [CrossRef] | [Google Scholar]
- Lapham K, Callegari E, Cianfrogna J, Lin J, Niosi M, Orozco CC, et al. Array. Drug Metab Dispos. 2020;48(12):1350-63. [PubMed] | [CrossRef] | [Google Scholar]
- Raish M, Ahmad A, Ansari MA, Alkharfy KM, Ahad A, Khan A, et al. Effect of sinapic acid on aripiprazole pharmacokinetics in rats: possible food drug interaction. J Food Drug Anal. 2019;27(1):332-8. [PubMed] | [CrossRef] | [Google Scholar]
- Iqbal M, Raish M, Ahmad A, Ali EA, Bin Jardan YA, Ansari MA, et al. Cytochrome P450 3A2 and PGP-MDR1-Mediated pharmacokinetic interaction of sinapic acid with ibrutinib in rats: potential food/herb–drug interaction. Processes. 2022;10(6):1066 [CrossRef] | [Google Scholar]
