ABSTRACT
Fungal infection associated with eye annually affects more than one ten lakh people globally. It can affect vision and sometimes causes complete blindness. Drug delivery through ocular route has always been a challenge, as in the eye numerous barriers are located in conjunctiva, cornea, iris-ciliary body and retina which prevents drug dose from reaching the site resulting into low bioavailability of drug. Treatment through conventional dosage form often possess disadvantage of low retention time of drug as they are flows out from the corneal cavity by tear flow and nasal drainage. To overcome these challenges, the need of the hour is to fabricate a novel drug delivery system that would overcome the barrier channels of eye and could results into enhanced drug absorption at its site of action. Till date numerous technologies viz. Nanoparticles, Nanostructured Lipid Carriers (NLC), Nano micelles, Microneedles, Liposomes etc have been developed. These technologies can overcome the barriers located in the eye and improve bioavailability of drugs. The present review highlights about nanostructured lipid carriers and their potential use in ocular delivery. The aim is to study nanostructured lipid carriers as nanocarriers to increase the permeability, solubility, bioavailability and retention time of active pharmaceutics agents through ocular administration. High-pressure homogenization, emulsification-solvent evaporation and micro-emulsion are some of the methods by which they can be prepared. So, it is concluded that nanostructured lipid carriers represent a potential nanocarrier for the reliable delivery of drugs to the ocular tissues with increased solubility, bioavailability and residence time.
INRODUCTION
Fungal infection is generally characterized by progressive onsets of species of fungi and causes severe health problems in immune-restricted individuals. It is greatly occurred in patients having hematologic, allogeneic, prolonged leukopenia and autologous grafts disorders. Fungal infections generally curve whole body’s system and lead serious lethality to body’s cellular system.1 Keratitis is defined as inflammation of the cornea; it is mainly divided according to its causes into infectious and non-infectious. However, it occurs usually after a micro-organism invading the corneal abrasion which may lead to severe visual disability if left untreated. Therefore, appropriate treatment of corneal is critical in order to regain normal physiological conditions of the cornea. It commonly causes blindness or ocular morbidity, especially in developing nations.2 For the reason that fungi cannot enter the cornea or Intact Corneal Epithelium (ICE) by blood vessels, major factors contribute for fungal keratitis are chronic keratitis, post-traumatic infections, corneal surgery, prolonged contact lens wear, and immunocompromised disease.3 The common fungi causing ocular infections are yeast-like fungi (such as Candida albicans) and filamentous fungi (such as Aspergillus species).4 The prevalence of ophthalmic fungal infections has increased significantly in last few decades due to increasing incidence of acquired immunosuppression resulting from long-term use of broad-spectrum antibiotics, immunosuppressants and AIDS. The etiology of ocular fungal infection is interconnected to the epidemiology of disease. The term visceral or innermost endophthalmitis refers a blood-borne infection in the eye by pathogens. Fungal keratitis is the third scientific manifestation of ophthalmic fungal infection.5–7 Currently clinical drugs include polyenes like amphotericin B and azole compounds like fluconazole (Table 1). Stability, low permeability, and solubility of these agents limit its use in treatment of fungal keratitis.8 Therefore, drug treatment of FK is still difficult, and the development of a potent, broad-spectrum, antifungal novel drug delivery approach is urgently required.
| Antifungal agents | Side effects | Spectrum of Activity | Dose |
|---|---|---|---|
| Polyenes | |||
| Amphotericin B | Nephrotoxicity, electrolyte imbalance, Hypokalaemia and hypocalcaemia. | Broad spectrum; active against, Aspergillus species and moniliasis. | Topical: 1.5-5mg/mL Intravitreal: 5-0.1 ug/mL |
| Natamycin | Conjunctival chemosis, hyperaemia. | Broad spectrum | Topical: 50mg/mL |
| Azoles | |||
| Ketoconazole | Broad spectrum | ||
| Fluconazole | Active in case of gram +ve and gram-ve microbe. | Topical: 2mg/mL | |
| Itraconazole | Temporary blurred vision, ocular discomfort, dry eye, taste prevention. | Broad spectrum | Topical: 10mg/mL Intravitreal: 0.005mg/0.05mL |
| Voriconazole | Visual disturbances, Drug interaction is common, hepatic reactions. | Broad spectrum | Topical: 1mg/mL Intravitreal: 0.05-0.2mg/0.2mL |
| Posaconazole | Topical: 100mg/mL | ||
| Econazole | Topical: 20mg/mL | ||
| Miconazole | Mild burning and stinging sensations, Ocular irritation. | Broad spectrum | Topical: 10mg/mL Intravitreal: 0.025-0.05mg/0.1mL |
| Echinocandins | |||
| Capsofungin | Topical: 1.5-5mg/mL Intravitreal: 0.1mg/0.1mL | ||
| Micafungin | Topical: 10mg/10mL | ||
Human eye is an extremely delicate organ that is susceptible to varied infections. The eye infections causing agents can range from various micro-organisms including fungi, viruses, bacteria to parasites. Treatment may include antibacterial agents, antifungal agents, antiseptic agents, antiviral agents, and anthelminthic agents, depending on the type, cause and reason of infection.9 Efficient drug delivery to eye still remains a challenge for researchers and scientist. For the reason that the eye creates some physiological barriers that serve as an obstacle and limits ophthalmic drug delivery to the required site. Experts are focusing on new domains of study to improve delivery systems to specifically deliver drugs to target sites.10,11 The advancement into area of delivery of drug to the eye has given rise to new techniques for best treatment of eye diseases by novel therapies. These new delivery systems are expected to provide sustained activity through the method which has significantly less invasive nature or class, more efficient and safer delivery methods. Various new ophthalmic drug delivery systems are now a days in the practice of interventional studies (clinical trials). They formulated to have a prolonged duration of action, thereby achieving sustained release of drugs. Likewise, many are earlier accessible in market. Most of them were prepared to obstruct the issues linked to the posterior section that commonly alter the eye over a long interval of time. Recently, some novel drug delivery systems viz. nanoparticles, nano-emulsions, nanosuspensions, liposomes, nanofibers, micelles etc. have been developed to enhance local ocular drug delivery Figure 1.12,13 These delivery systems are needed for ocular drug delivery because of the many drawbacks of conventional ophthalmic dosage forms. The main problem that conventional treatment suffers is the low ocular bioavailability of drugs, that causes most of drug to be gets wasted, a small amount of which literally attains the target area of interest. The rationale for this stuck is the specific structure of the eye and the behaviour of its tissue. Due to these reasons, ocular delivery always remains continues to be a vital challenge. If take the case of eye drops even as a traditional means of drug delivery, the requirement for novel drug delivery systems for ophthalmic treatments can be emphasized by explaining the drawbacks correlated along conventional/traditional eye drops. The posterior section of the eye has not received eye drop treatment. Eye drops are known to “only” cure disorders associated with the anterior segment. At this point, most of drug is waste from the target site due to ejection of droplets from eye, quick tear movement, blinking, and triggering tears. Therefore, only a small amount of, less than 5% drug, reaches the intraocular tissue.13,14
Figure 1:
Delivery of Nanocarriers to Eye.
In general, NDDS for ocular drug delivery are fabricated for two objectives.10,15,16
- To develop the sustained and controlled release drug.
- Improve drug’s corneal perforation.
NANOCARRIERS FOR OCULAR DELIVERY
Liposomes
These are lipid vesicles/cyst with one or more phospholipid bilayers entrapping water inside, consequently the aqueous layer amphiphiles self-assemble to create single or numerous concentric bilayers.17 Dimension of liposome typically varies from 0.08 to 10 μM, and depend on the dimension and phospholipid bilayer, liposome is divided into 3 groups; small unilamellar vesicles (10-100 nm), large unilamellar vesicles (100-300 nm) and multilamellar vesicles (containing two or more bilayers). The good biocompatibility, capability to enclose both hydrophilic and lipophilic drugs and cell film-like structure, make liposomes an absolute delivery system for ocular applications. Liposomes have shown excellent efficacy for drug delivery to each anterior and posterior section of eye in several research studies.18 Novel drug delivery system likes liposomes are very adaptable for to delivery of certain drug molecules.19
Benefits across conventional drug delivery system
- Small-scale dimension.
- Controlled release.
- Enhanced ophthalmic bioavailability and absorption.
- Biocompatible, biodegradable or non-immunogenic.
Class of drugs that can be encapsulate
Each hydrophilic and hydrophobic drug molecule can be encapsulated in liposomes.
Niosomes
It is a two-layer drug transporter system developed by the self-consortium of non-ionic surface-active agents and cholesterol in an aqueous portion that can encapsulate both hydrophilic and hydrophobic molecules. These are chemically stable for topical ocular administration, have minimum toxicity due to their non-ionic character, do not require special precautions or conditions for handling of surfactants, and have unique structural properties; is preferred over other vesicular systems. It is also used in customize forms e.g., Discosomes (10– 14 μM) in ophthalmology.20
Nano micelles
Nano micelles are the globally used transporting system for formulation of therapeutic molecules in transparent aqueous solutions. These are composed of amphiphilic components. These components are surface-active or polymeric in characters. There is now great interest in developing nano micelle formulation method for ocular delivery of drug. Micelle formulations enhance the BA of drug in ocular cells. Nano micelles remain in the systemic blood circulation longer and deposited on affected tissues.21
Microemulsion
Microemulsion is a novel ocular delivery system that disperses oil and water along with a surfactant. microemulsion has the benefits of high thermodynamic reliability, enhanced solubility, and enhanced corneal permeability. Parameters affecting the durability of microemulsion systems are the choice of organic, aqueous phase, surfactant and cosurfactant system.22 Tears are alluring in ophthalmic delivery systems because it makes more viscous and fluid after application, increasing retention in the eye while maintain therapeutic efficacy. Agents used for ocular administration are chloramphenicol, timolol and sirolimus developed into different micro-emulsion with enhanced durability, BA and solubility.23
Hydrogels
These are networks of cross-linked polymer chains that are compatible with living tissues in the intraocular space.24 Chemical action takes place through covalent bonds between polymer chains, whereas physical action happens due to hydrophobic, Vander-Waals and hydrogen force. These bonds provide durability to the sustained-release agents and keep up the concentration of the drug. For essential (natural) polymer, polysaccharides for e.g., dextran, hyaluronic acid or chitosan seemed to be broadly utilized for hydrogel development. Chitosan (cationic nanocarrier) interacts with anionic polymer to develop hydrogels. These synthetic polymers poly ethylene glycol, poly vinyl alcohol and PLGA are generally used to fabricate hydrogels.25
Nanoparticles
Colloidal particles that range between 10 to 1000nm are known as nanoparticles. They are widely used in electronic, optical, biomedical, scientific research and pharmaceutical fields. In the fields of pharmaceutics these colloidal dispersions are widely used as delivery carrier system. According to the drug delivery system, nanoparticles can be formulated using neutral and synthetic polymers, lipids.
Advantages of nanoparticles drug delivery systems are:
- High stability,
- High carrier capacity,
- Can incorporate both lipophilic and hydrophilic substances,
- Can deliver the drug from various routes i.e., oral, ocular, topical etc.
Although there are many more advantages of nanoparticle as drug delivery system. But when the polymer and organic solvents are used, they can affect toxicity and biocompatibility. Therefore, the lipid nanoparticles have gained importance in recent years. They are similar to polymeric nanoparticles in their ability related to incorporation of drugs, but are formulated with lipids without the use of organic solvents and these are biocompatible also. Therefore, the chances of toxicity can be significantly decreased using lipid nanoparticles.26
Lipid Nanoparticles (LNPs)
LNPs attracted a lot of attentions in the prior 1990s just after the 1st generation Solid Lipid Nanoparticles (SLNs) were developed.25 SLNs attracted a great deal of attentiveness globally, various research batches began operations on them early on in their discovery, and to this day SLNs have proven to be superior to present traditional/conventional transporter such as liposomes, emulsions and polymeric NPs.27,38 Solid lipid nanoparticles are in submicron range 50 to 1,000 nm, or proper choice of lipid and surfactant influences long-time storage stability, particle size, drug loading, and release behaviour.29,30 Potential SLN-associated issues like drug loading ability limitation, drug release changes, and drug emission in storage are ignored or reduced with the use of new generation Nano Structed Lipid Carriers (NLCs).31
NANOSTRUCTED LIPID CARRIERS
NLC is the 2nd generation of LNPs consists of a dual complex of solid or liquid lipids, and the mean size ranges from 10 to 500 nm.32 Preferably, solid-lipid is combined with liquid-lipid within a proportion of 70:30 to 99.9:0.1. They include specific nanostructures, resulting in improved drug loading and tighter binding of the drug inside, extending shelf life. NLCs can be administered to patients orally, topically, intravenously and ocularly.33 It also supports us to decrease the dose, toxic effects and deliver the drug to target site. NLCs have extensive application into health zone as these are alike to the body lipids.

Benefits of NLCs
- Improved physical durability,
- Easy manufacturing and scale-up,
- Improved solubility in aqueous media,
- High encapsulation of hydrophobic and hydrophilic agents,
- Dominated particle dimension (size),
- Efficacious transporter system especially for hydrophobic drugs,
- Prolonged drug release.
The tiny dimension of lipid particle makes sure nearby exposure along with the stratum corneum, allowing delivery of drug to mucous membranes or skin.
To overcome the limitations of various routes are first-pass metabolism, low bioavailability and low solubility NLCs have been fabricated.34
Limitations of NLCs
Even with the substantial capabilities of NLC in selected/targeted drug delivery, it possesses some drawbacks such as:
- Cytotoxic results related to intercellular substance type and concentration,
- Irritating effects of various surfactants,35
- Applications and efficiencies in the instance of peptide and protein drugs and gene delivery systems need further exploitation.
Types of NLCs
NLCs are divided into 3 types depending on distinction in their lipid and oil conformation:
Imperfect NLCs
The production of these NLCs need blending structurally different lipids such as fatty acids and glycerides, resulting in defects in the crystal sequence, which shows high drug pay load.
Amorphous NLCs
In this type of NLC, the lipids are mixed in this way that it cannot solidify, producing a structureless amorphous kind matrix. Certain lipids like Hydroxyctacosanyl hydroxy stearate and isopropyl myristate are blended with solid lipids. According to result, NLC in an amorphous form slightly than regular, preventing elimination of the drug by β-modification throughout its storage.
Multiple NLC
In general, the solid complex of various O/F/W types of NLC includes dispersed nanoscale liquid oil sections, and these nanoscale compartments increase drug solubility that enhances the drug loading capacity.36,37 Preferably, such lipid particles are suitable for consolidation of hydrophobic active agents or hydrophilic active agents can only be comprised at short concentrations. These complexes could be dissolved and prepared to produce lipid and drug complex NPs, and they have a drug loading of 30 to 50% for hydrophilic substances.
Components of NLCs
Various types of solid lipid, liquid lipid (synthetic or natural oils), surface-active agent, cosurfactant and surface modifier utilized in development of nanostructured lipid carriers are summarized in Table 2.
| Solid lipids | Stearic acid, compritol 888ATO, GMS etc. |
| Liquid lipids | Oleic acid, olive oil, castor oil, turmeric oil, corn oil, paraffin oil etc. |
| Emulsifiers | Span 20, span 40, span 80, tween 20, tween 80, poloxamer 188, poloxamer 407, trehalose, soya lecithin etc. |
| Co-surfactants | Cremophor EL, solutol HS 15, polaxamine 908 etc. |
Methods used for the fabrication of NLCs
Depending upon the energy desired these methods are divide in 3 major groups Figure 2.
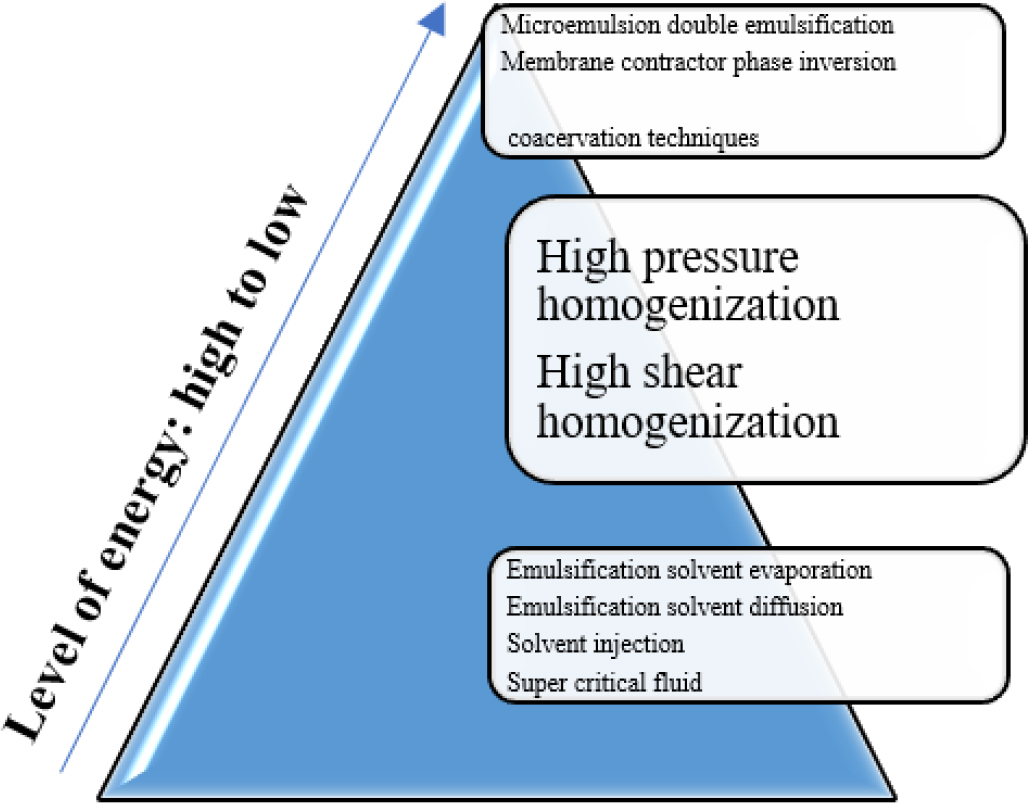
Figure 2:
Methods of NLC Preparation.
High energy required approaches
HPH (High Pressure Homogenization)
This technique persists one of the most favourable technique due to its short manufacturing time, ease of scale-up and no need for solvents compared to other techniques.38 These techniques can be divided into hot high pressure homogenization protocols and cold high-pressure homogenization conventions.39
High Shear Homogenization
This method dissolves or disperses a lipophilic drug in a molten complex of liquid and solid lipid. The heat utilized have to be 10°C greater than the melting point of solid lipid to form recrystallization more tough. An aqueous surface-active agent’s solution at the uniform degree is flowed into the lipid portion or a pre-micro emulsion is prepared below action of high-rate homogenizer.
Low energy required approaches
Micro-emulsion
Here, molten lipid is blended with a water-soluble aqueous phase carrying surfactants or cosurfactants to formulate a water/oil and oil/water emulsion, depending on every ratio implemented. The transparent, heat stable micro-emulsions are later prepared that is again dissolved in the cold hydrophilic portion to more reduce the particle size and produce NLC. This technique is simple, economical, suitable and reproducible for heat labile agents. Also, no special equipment or energy is required to manufacture Nanostructured Lipid Carriers (NLCs).40,41
Double Emulsification Technique
This technique is depended upon the solvent emulsification evaporation method and is mainly utilized to produce LNP filled with water soluble drugs. The molecule and stabilizer are contained in the interior inorganic phase of the double (o/w/o) emulsion. Because of the enhanced particle size than SLN, these preparations are called lipospheres.
Phase-Inversion
Here, the drug, lipids, water, and surface-active agents are thoroughly combined and warmed until more than the phase inversion temperature of surface-active agents. In the time of heating procedure, the surface-active agent is dried out, changing its HLB and subsequently changing its similarity for each portion. Upon rapid cooling (such as in an ice), the surface-active agents become hydrophilic once more, let NLCs to form small particles.42,43 The benefits of this process are less energy consumption and ignorance of organic solvents. Even so, the NLC formed are less stable and may require multiple temperature cycles.
Very less or no energy required approaches
Emulsification solvent evaporation technique
In this technique, the lipid or drug are dispersed into a water insoluble organic solvents (such as dichloromethane or chloroform) or homogenized in an aqueous portion including a surface-active agent prior the solvent is evaporated. The pre-emulsion formed is then processed through sonication. The dispersion is then chilled to room temperature to acquire an aqueous NLC dispersion. It is corelated along with a quick or easy development procedure. On other side, it requires the utilization of organic solvent and the resulting suspensions thin as well as require again dispersion or ultrafiltration.44
Emulsification-Solvent-Diffusion Technique
It is alike to the solvent emulsification-evaporation process, in which lipids are dispersed in a slightly hydrophilic organic solvent like benzoyl alcohol. The oil/water emulsion is mixed in water by continuous homogenizing and diffusion of the organic solvent solidifies the dispersed medium to formulate NLCs. A lipid crystallizes just as solvent disperses into the liquid portion.
Characterization and evaluation of the NLC
Evaluation of nano systems is necessary for assessing quality of prepared nanostructures and, inevitably, forin vivo administrations. Characterizations of NLC are difficult due to their small size, the rigid (complex) nature of the lipid involved, and the dynamic nature of the instruments.42,45 Measurements of particle size, zeta potential, surface charge, entrapment efficiency, drug content and in vitro release is essential for assessing quality and stability of NLC.
Particle Size, Size Distribution, and Zeta Potential
Average dimension of particle and size-distribution were important criterion which impact the tangible property, durability, and in vivo effects of NLC. Its size may compute using distinct approaches. DLS (Dynamic Light Scattering) is a technique often employed to calculate the dimension of nanostructures. Dynamic light scattering is depended on Brownian motion of particle in a vehicle in that potency of luminesce dispersed by the particle is computed. Evaluation should be carried out in triplicate (to verify accuracy) for each sample after appropriate dilution is strained in deionized buffer and water. Poly Dispersity Index (PDI) describes the size-distribution of the NLC or is depend upon the back dispersed power potency of particles. A low poly dispersity index value (≤0.3) indicates uniformity of technique or well-reasoned particles size-distribution, while a high poly dispersity index value (> 0.5) indicates a wide size distribution.
Zeta potential weighs the potential difference between a particle. This gives important details about the accumulation propensity of NPs and their physical durability. In general, NLC with an absolute and negate outer-surface charge are tangibly durable because of electrodynamic aversion in linking neighbouring imposed particles, reducing liability of the particles to aggregate. NLC with zeta potentials more than +30 mV and less than ±30 mV is predicted to have sufficient physical durability.
Morphology of NLCs
Various microscopy process is frequently used to find out the morphology of nanocarriers, involving AFM (atomic force microscopy), TEM (transmission electron microscopy), SEM (scanning electron microscopy). TEM is often utilized to study the morphology of NLC. In this process, droplets of adulterated NLC suspension are settled on the carbon covered copper matrix, tanned (e.g., phosphotungstic acid), or dried below ambient conditions before imaging.
Determination of Encapsulation Efficiency
Encapsulation proficiency calculate the percent of drug encapsulated in NLC and consequently reflects the proficiency of nano system to encapsulate drug. Due to the character of NLC, high encapsulation efficiency of hydrophobic drugs was noticed owing to uniform fusion of a drug into lipid or creation of an inflexible solid nub afterward refrigerating which retard drain of encapsulated drugs. An encapsulation proficiency may be computed by the first equation, at this point quantity of drug into NLC was determined by analyser at an appropriate λmax after adding a sufficient amount of organic solvent to rupture the NLC and release the encapsulated drug.46,47
Incidental measurements of entrapment proficiency can further be performed in accordance with second equation, here, quantity of the liberated drug in the filtrate following separation of entrapped NLC suspensions are computed by analyser on proportional λmax.
In vitro release studies
In vitro liberation of drugs from NLC is generally examined by employing dialysis techniques.48–50 Drug loaded NLC Immerse in the dissolution medium and place in a constant temperature homogenizer at 37°C. At fixed time gaps, sample is taken and replaced with a uniform volume of new release vehicle to keep sink states. Drug concentrations could be determined by analyser at a suitable λmax or utilizing a justified HPLC (high-performance liquid chromatography) technique opposed to level of well-known concentration. Tests should be analysed in triplicate or average quantity of drug liberation should be graphed opposed to time. Especially, an uncharged liberated drug mixture must be utilized as control under the alike practical states. Liberation details may be analysed for investigation of kinetics of the drug liberation by NLC.
Differential Scanning Calorimetry (DSC)
It is used to regulate the crystallinity and polymorphism of mass solids, drugs or NPs through measuring the glass-transition or the melting-temperature on the particular enthalpy.51 Around 10 mg of lipid, drug, and freeze dried NLCs were put in a pinhole aluminium pan with base seal and lid and heated. A vacant aluminium pan is utilized like a reference. DSC plots were gained at a continuous level heating speed of 5°C/ min. in unmixed dry nitrogen over temperature range 20°C to 80°C. The inspection was replicated 3 times and the results are represented as the average of 3 measurements. Ultimately, the enthalpy was computed by employing the Mettler star software’s.52,53
Wide-angle X-ray Diffraction (XRD)
Geometrical dispersion of emission from crystal planes in a scatter of nanoparticles could estimate by X-ray diffraction to identify crystallinity. An X-ray diffractometer (Philips, Hamburg, Germany) along with radiographic copper anode (γ = 1.5406 Å) utilized to get the crystallinity of freeze-dried NLCs. A powder sample of lipid, drug, and freeze dried NLC approximately 10 mm long was put on an X-ray plate and a voltage of 45 kV and a current of 40 mA were applied on room temperature with a scan rate of 5°C/min. or 2θ scan order scale. An X-ray diffraction pattern was gained in the order 20°C to 80°C.54,55
Drug Release
Controlled liberation of drugs from NLC may lead to increased half-life and delayed synthetic strike in the systemic blood circulation. The nature release of drug from NLC depends on the manufacturing temperature, the formulation of the emulsifier, or the oil included in the lipid complex.56 The amount of drug on nanoparticle shell and particle external surface is liberated in bursts, while the drug include in the particle core is liberated over a longer period of time. Delayed drug release can be described by taking into consideration both the drug distribution in the middle of lipid complex, water and obstacle as the role of interfacial film.57,58 A dialysis technique or use of Franz cells are measurement means for in vitro drug liberation from the nanoparticles. Elucidation of in vitro drug release profile could take into account the special circumstances of in vivo situation. Synthetical humiliation of LNPs cans be affected to some level by particle formation.
APPLICATIONS OF NLC IN OCULAR DELIVERY
Designing a novel delivery approach that can proficiently target the infected ocular tissue, providing high drug levels, and keep sustained with no or minimum side effects. One of the encouraging approaches currently is the use of lipid-nanoparticle carriers categorized by a submicron-meter dimension. Due to their properties and various advantages, solid lipid nanoparticles and nanostructure lipid carriers are promising systems for ocular drug delivery.59
Advantages of NLCs as ocular drug delivery system
- High encapsulation efficiency,
- High ocular permeation,
- Appropriate pharmacokinetic properties,
- Sustained and controlled release of drug,
- Enhancing drug corneal permeability,
- Enhancing drug retention time,
- Improving ocular bioavailability and distribution,
- Preventing ocular toxicity,
- Good stability and biocompatibility.60
Ocular drug delivery has many drawbacks and remains challenging due to specific physiological and anatomical features of the eyes. Eyes are a very complex and delicate organ which have numerous barriers. Novel drug delivery systems such as SLNs and NLCs can overcome these barriers and enhance ocular bioavailability. Lipid nanoparticles are capable of passing blood ocular barrier, obtain sustained and controlled drug release, protect drugs from enzymes degradations and improve the drug deposition and residence time in eyes.60 Ophthalmic SLNs and NLCs are characterized by improved local resistance and reduced regulatory requirements through the utilization of physiologically compatible lipid. More advantages involve its capability to encapsulate hydrophobic drugs, shelter of unstable components, and modification of release behaviour.61 SLN has been utilized for ophthalmic delivery for the previous several decennary. Currently, numerous studies by using NLC as an ophthalmic delivery system have become familiar. NLC has been utilized for ophthalmic delivery of certain substances such as amphotericin B or ciprofloxacin. NLCs loaded with triamcinolone acetonide improved ocular BA, enhanced drug residence time on the ocular space, and decrease precorneal leakage due to sustained release of drug through the delivery system.9,62,63 It is a fact that SLN and NLC increase the contact time between the drug and the eye surface, enhancing corneal penetration. NLCs have a wide variety of pharmaceutical applications, including hypertension, diabetes, Parkinsonism, epilepsy, hyperlipidaemia, cancer, alopecia, hormone deficiency, topical inflammation, ocular, hepatic, and fungal diseases.64
Effective management of ophthalmic disease is still a challenging task due to the many ocular conditions located in front of and behind the eye. A number of ocular delivery methods, such as topical, intraocular, periocular or in combination with ocular devices, are used to deliver the drug to its targeting site. To reduce duration and drug use, side effect, and to improve ocular retention time, drug penetration, and ocular bioavailability nanotechnologies were used. This technology increased the effectiveness of drug and showed good biocompatibility, indicating that will be widely used in the management of eye infections.65
Recent Studies on NLCs
Some attributes of SLNs and NLCs make them interesting delivery system for eyes. These carriers are often applied topically to the eye. Cavalli et al. evolved a SLN system of tobramycin for topical delivery to eye. In vivo testing has shown sustained release of drug over a period of 6 hr in contrast to short duration from uniform dose of eye drops.66
In study of Attama et al. they have prepared lipid nanoparticles of diclofenac sodium in combination with phospholipids with hot high pressure homogenization technology. By coating the surface of nanoparticles with phospholipids, the permeability of diclofenac sodium through the cornea is improved, demonstrating the functionality of this structure for ocular use.67
Araujo et al., Developed triamcinolone acetonide-loaded NLC from high pressure homogenization technique using Precirol ATO5 (solid lipid) and squalene (liquid lipid) respectively, and Lutrol F68 (surfactant) for ocular applications. The analysis proved that drug was mostly entrapped into the NLC, which is characterized by an amorphous matrix. In addition, the in vivo Draize test showed any symptoms of eye toxicity.68
In a recent ophthalmic study of Zhang et al., genistein-loaded NLCs were prepared using a melt emulsification technique. The prepared genistein loaded Eudragit modified NLC showed a longer precorneal clearance compared to bare NLC and increased AUC by 1.22 times. The modification of eudragit also improve the corneal permeability. Draize and cytotoxicity tests confirmed that NLC produced no toxicity to ocular corneal cells.69
E Gonzalez-Mira et al. prepared flurbiprofen loaded NLCs by high pressure homogenization method and taken the optimum quantities of lipids to obtain desired particle size, drug loading and entrapment efficiency in ocular tissues. The finally optimized Flurbiprofen loaded NLCs did not shown any irritation, so become a promising efficient drug delivery system tent to provide controlled release. Additionally long-term stability studies of NLCs were also performed due to their thermodynamic nature.70
Ocular infections can damage the eye surface with possible visual impairments and blindness. Ciprofloxacin (CIP) ocular solution is prescribed as first-line therapy in ocular bacterial infections. Natamycin (NT) ocular suspension is used for antifungal therapy. Nanostructured Lipid Carriers (NLCs) have been widely examined for ocular penetration enhancement and distribution to deeper ocular tissues. The objective of the current study was to prepare NLCs loaded with a combination of CIP and NT (CIP-NT-NLCs) and loaded them in an in situ gelling system (CIP-NT-NLCs-IG). The results suggest that this dual nanoparticulate based in situ gelling drug delivery system can serve as a promising carrier for treatment of topical ocular infections.71
OTHER APPLICATIONS
NLCs in cancer chemotherapy
Over the past two to three years, many chemotherapeutic drugs have been encapsulated or incorporated into NLCs, and there in vivo and in vitro effects have been evaluated. The results of these studies have been shown to reduce side effects, increase potency, improve drug stability, and improve pharmacokinetics, making them tools for clinical use. Some of the problems commonly encountered with antibodies, such as tissue toxicity, poor quality and stability, can be partially overcome by using NLCs for delivery.72–74
NLC in delivery of protein and peptide
Lipid nanoparticles and lipid microparticles such as NLC and SLN are other carriers for the treatment of proteins, peptides and antigens.75 Many studies in this field have shown that they can bind both hydrophilic and lipophilic proteins under certain conditions. It can be used by various routes such as parenteral, oral, nasal and pulmonary.76 These transporters stabilize the protein, prevent proteolytic degradation, and provide sustained release of the encapsulated drug. Peptides such as cyclosporine A, insulin, calcitonin, and somatostatin are included in lipid products and are currently under evaluation.
NLC in CNS targeting
The small size of NLC (less than 50 nm) can be useful for pharmaceutical applications. Small size tends to significantly reduce health caused by reticuloendothelial diseases. NLCs can also be used for medicinal purposes. NLC can improve the ability of drugs to enter the blood-brain barrier and is a promising drug targeting system for the treatment of organ diseases. The advantages of using NLCs over polymeric nanoparticles are: lower cytotoxicity, higher drug loading and better efficiency.75
NLC in cosmetics and dermatologic preparations
LNPs are considered the next drug delivery system after liposomes. The treatment of dermatological diseases appears to be more effective as there is less risk of side effects, but the stratum corneum inhibits the penetration of xenobiotics into the skin. Microparticle delivery systems can improve dermal penetration.77,78 Because of the abundance of epidermal lipids present in the permeability barrier, it seems promising that lipid transporters will attach themselves to the skin and allow lipid exchange between the stratum corneum and the carrier from the outer layer. In addition to liposomes, Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) have also been extensively studied. After evaporation of water from the lipid nano dispersion applied to the skin surface, the lipid particles form a sticky layer that closes the skin.
CONCLUSION
As aforementioned, pervasiveness of ophthalmic fungal infection has enhanced recently. Yet, traditional topical delivery system normally performs poorly in treating these infections because of problems associated with delivery of drug to the eye. Therefore, these drug delivery systems need frequent dosing, lengthy courses of treatment, which are not safe, and not readily accepted by patients. Recently, researchers’ attention has turned to the development of NDDS. In this current pharmaceutical world of analysis and evaluation, various modern nanoscale delivery systems are being investigated both in the industrial or laboratory scale significantly. Targeting drugs to the eyes using ocular route is even now an inconvenient task due to the formulation limitations and the composite physiological, anatomical and various obstacles that exist in ophthalmic cells. Lipid nanoparticles revealed exceptional ophthalmic permeation nature and penetration-improving abilities. NLC is the modern and new-generation of lipid NP formulations, with versatility in drug loading, transformation of its release profiles, and enhanced pharmaceutical action. That specific properties of NLC are assigned to their distinctive formation, which is the combination of solid lipid or liquid lipids. A few specific benefits of these nanocarrier are diminished drug payload, improved patient compliance, decreased drug toxicity, inexpensive high-scale production, biodegradability and biocompatibility of their components, easy to manufacture, or chemical durability of the pharmaceutical agents found them more optimistic or close to great all rounded nano sized carriers into their generations.
References
- Badiee P, Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis and clinical management. Indian J Med Res. 2014;139(2):195-204. [PubMed] | [Google Scholar]
- Wu H, Ong ZY, Liu S, Li Y, Wiradharma N, Yang YY, et al. Synthetic β-sheet forming peptide amphiphiles for treatment of fungal keratitis. Biomaterials. 2015;43:44-9. [PubMed] | [CrossRef] | [Google Scholar]
- Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943-8. [PubMed] | [CrossRef] | [Google Scholar]
- Kakkar S, Kaur IP. A novel nanovesicular carrier system to deliver drug topically. Pharm Dev Technol. 2013;18(3):673-85. [PubMed] | [CrossRef] | [Google Scholar]
- Thomas PA. Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 2003;16(4):730-97. [PubMed] | [CrossRef] | [Google Scholar]
- Klotz SA, Penn CC, Negvesky GJ, Butrus SI. Fungal and parasitic infections of the eye. Clin Microbiol Rev. 2000;13(4):662-85. [PubMed] | [CrossRef] | [Google Scholar]
- Garg P. Fungal, mycobacterial, and Nocardia infections and the eye: an update. Eye (Lond). 2012;26(2):245-51. [PubMed] | [CrossRef] | [Google Scholar]
- Kalkanci A, Sengul Ozdek. Ocular fungal infections. Curr Eye Res.. 2011;36(3):179-89. [PubMed] | [CrossRef] | [Google Scholar]
- Bremond-Gignac D, Chiambaretta F, Milazzo S. A European perspective on topical ophthalmic antibiotics: current and evolving options. Ophthalmol Eye Dis. 2011;3:29-43. [PubMed] | [CrossRef] | [Google Scholar]
- Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today.. 2008;13(3-4):144-51. [PubMed] | [CrossRef] | [Google Scholar]
- Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26(5):1197-216. [PubMed] | [CrossRef] | [Google Scholar]
- Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems: a shift to the posterior segment. Drug Discov Today. 2008;13(3-4):135-43. [PubMed] | [CrossRef] | [Google Scholar]
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348-60. [PubMed] | [CrossRef] | [Google Scholar]
- Gan L, Wang J, Jiang M, Bartlett H, Ouyang D, Eperjesi F, et al. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov Today. 2013;18(5-6):290-7. [PubMed] | [CrossRef] | [Google Scholar]
- Yasamineh S, Yasamineh P, Ghafouri Kalajahi H, Gholizadeh O, Yekanipour Z, Afkhami H, et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int J Pharm. 2022;624:121878 [PubMed] | [CrossRef] | [Google Scholar]
- Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269(1):1-14. [PubMed] | [CrossRef] | [Google Scholar]
- Chauhan SB, Gupta V. Recent advances in liposome. Res J Pharm Technol. 2020;13(4):2053-8. [CrossRef] | [Google Scholar]
- Nikam S, Ghule A, Inde A, Jambhulkar A. Advancement in ocular drug delivery system to overcome ocular barrier. 2013;2:78-83. [CrossRef] | [Google Scholar]
- Sankar C, Muthukumar S, Arulkumaran G, Shalini S, Sundaraganapathy R, samuel Sj, et al. Formulation and characterization of liposomes containing clindamycin and green tea for anti-acne. Res J Pharm Technol. 2019;12(12):5977-84. [CrossRef] | [Google Scholar]
- Kumar P, Verma N. An overview on niosomes: as an auspesious drug delivery system on the bases of application. Res J Pharm Technol. 2021;14(5):2896-902. [CrossRef] | [Google Scholar]
- Li CC, Abrahamson M, Kapoor Y, Chauhan A. Timolol transport from microemulsions trapped in HEMA gels. J Colloid Interface Sci. 2007;315(1):297-306. [PubMed] | [CrossRef] | [Google Scholar]
- Yadav S, Kawtikwar PS, Sakarkar DM, Gholse YN, Ghajbhiye SD. Microemulsion: a review. Res J Pharm Technol. 2009;2(3):441-8. [PubMed] | [CrossRef] | [Google Scholar]
- Kirchhof S, Goepferich AM, Brandl FP. Hydrogels in ophthalmic applications. Eur J Pharm Biopharm. 2015;95(B):227-38. [PubMed] | [CrossRef] | [Google Scholar]
- Bian F, Shin CS, Wang C, Pflugfelder SC, Acharya G, De Paiva CS, et al. Dexamethasone drug eluting nanowafers control inflammation in alkali-burned corneas associated with dry eye. Invest Ophthalmol Vis Sci. 2016;57(7):3222-30. [PubMed] | [CrossRef] | [Google Scholar]
- Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;7:587997 [PubMed] | [CrossRef] | [Google Scholar]
- Grana A, Limpach A, Chauhan H. Formulation considerations and applications of solid lipid nanoparticles. [PubMed] | [CrossRef] | [Google Scholar]
- Souto EB, Wissing SA, Barbosa CM, Müller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm.. 2004;278(1):71-7. [PubMed] | [CrossRef] | [Google Scholar]
- Sakellari GI, Zafeiri I, Batchelor H, Spyropoulos F. Formulation design, production and characterisation of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for the encapsulation of a model hydrophobic active. Food Hydrocoll Health. 2021;1:None [PubMed] | [CrossRef] | [Google Scholar]
- Souto EB, Müller RH. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. J Microencapsul.. 2006;23(4):377-88. [PubMed] | [CrossRef] | [Google Scholar]
- Nguyen TT, Nguyen TTD, Tran NM, Van Vo G. Lipid-based nanocarriers via nose-to-brain pathway for central nervous system disorders. Neurochem Res.. 2022;47(3):552-73. [PubMed] | [CrossRef] | [Google Scholar]
- Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305-13. [PubMed] | [CrossRef] | [Google Scholar]
- Musielak E, Feliczak-Guzik A, Nowak I. Synthesis and potential applications of lipid nanoparticles in medicine. Materials (Basel). 2022;15(2):682 [PubMed] | [CrossRef] | [Google Scholar]
- Liu J, Hu W, Chen H, Ni Q, Xu H, Yang X, et al. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328(2):191-5. [PubMed] | [CrossRef] | [Google Scholar]
- Araújo J, Gonzalez E, Egea MA, Garcia ML, Souto EB. Nanomedicines for ocular NSAIDs: safety on drug delivery. Nanomedicine. 2009;5(4):394-401. [PubMed] | [CrossRef] | [Google Scholar]
- Schäfer-Korting M, Mehnert W, Korting HC. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev. 2007;59(6):427-43. [PubMed] | [CrossRef] | [Google Scholar]
- Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598-613. [PubMed] | [CrossRef] | [Google Scholar]
- Fan Y, Liu JH, Lu HT, Zhang Q. Electrochemical behaviour and voltammetric determination of paracetamol on nafion/TiO2-graphene modified glassy carbon electrode. Colloids Surf B Biointerfaces. 2011;85(2):289-92. [PubMed] | [CrossRef] | [Google Scholar]
- Singh A, Neupane YR, Mangla B, Kohli K. Nanostructured lipid carriers for oral bioavailability enhancement of exemestane: formulation design, , and studies. J Pharm Sci.. 2019;108(10):3382-95. [PubMed] | [CrossRef] | [Google Scholar]
- Sadiah S, Anwar E, Djufri M, Cahyaningsih U. Preparation and characteristics of Nanostructured Lipid Carrier (NLC) loaded red ginger extract using high pressure homogenizer method. J Pharm Sci Res. 2017;9(10):1889-93. [PubMed] | [CrossRef] | [Google Scholar]
- Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. 2020;12(3):288 [PubMed] | [CrossRef] | [Google Scholar]
- Fang CL, Al-Suwayeh SA, Fang JY. Nanostructured Lipid Carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 2013;7(1):41-55. [PubMed] | [CrossRef] | [Google Scholar]
- Khosa A, Krishna KV, Dubey SK, Saha RN. Lipid nanocarriers for enhanced delivery of temozolomide to the brain. In: Drug delivery systems. 2020:285-98. [PubMed] | [CrossRef] | [Google Scholar]
- Gao S, McClements DJ. Formation and stability of solid lipid nanoparticles fabricated using phase inversion temperature method. Colloids Surf A Physicochem Eng Aspects. 2016;499:79-87. [CrossRef] | [Google Scholar]
- Duong VA, Nguyen TT, Maeng HJ. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules. 2020;25(20):4781 [PubMed] | [CrossRef] | [Google Scholar]
- Subramaniam B, Siddik ZH, Nagoor NH. Optimization of nanostructured lipid carriers: understanding the types, designs, and parameters in the process of formulations. J Nanopart Res. 2020;22:1-29. [PubMed] | [CrossRef] | [Google Scholar]
- Fathi HA, Allam A, Elsabahy M, Fetih G, El-Badry M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf B Biointerfaces.. 2018;162:236-45. [PubMed] | [CrossRef] | [Google Scholar]
- Soni NK, Sonali LJ, Singh A, Mangla B, Neupane YR, Kohli K, et al. Nanostructured lipid carrier potentiated oral delivery of raloxifene for breast cancer treatment. Nanotechnology. 2020;31(47):475101 [PubMed] | [CrossRef] | [Google Scholar]
- Zhu Y, Liang X, Lu C, Kong Y, Tang X, Zhang Y, et al. Nanostructured lipid carriers as oral delivery systems for improving oral bioavailability of nintedanib by promoting intestinal absorption. Int J Pharm. 2020;586:119569 [PubMed] | [CrossRef] | [Google Scholar]
- Makoni PA, Khamanga SM, Walker RB. Muco-adhesive clarithromycin-loaded nanostructured lipid carriers for ocular delivery: formulation, characterization, cytotoxicity and stability. J Drug Deliv Sci Technol. 2021;61:102171 [CrossRef] | [Google Scholar]
- Tripathi D, Sonar PK, Parashar P, Chaudhary SK, Upadhyay S, Saraf SK, et al. Augmented brain delivery of cinnarizine through nanostructured lipid carriers loaded gel: and pharmacokinetic evaluation. BioNanoScience.. 2021;11(1):159-71. [CrossRef] | [Google Scholar]
- Vitorino C, Almeida J, Gonçalves LM, Almeida AJ, Sousa JJ, Pais AA, et al. Co-encapsulating nanostructured lipid carriers for transdermal application: from experimental design to the molecular detail. J Control Release. 2013;167(3):301-14. [PubMed] | [CrossRef] | [Google Scholar]
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71(4):349-58. [PubMed] | [CrossRef] | [Google Scholar]
- Kasongo KW, Shegokar R, Müller RH, Walker RB. Formulation development and evaluation of didanosine-loaded nanostructured lipid carriers for the potential treatment of AIDS dementia complex. Drug Dev Ind Pharm.. 2011;37(4):396-407. [PubMed] | [CrossRef] | [Google Scholar]
- Montenegro L, Sarpietro MG, Ottimo S, Puglisi G, Castelli F. Differential scanning calorimetry studies on sunscreen loaded solid lipid nanoparticles prepared by the phase inversion temperature method. Int J Pharm. 2011;415(1-2):301-6. [PubMed] | [CrossRef] | [Google Scholar]
- Wissing SA, Müller RH, Manthei L, Mayer C. Structural characterization of Q10-loaded solid lipid nanoparticles by NMR spectroscopy. Pharm Res. 2004;21(3):400-5. [PubMed] | [CrossRef] | [Google Scholar]
- Lee CH, Moturi V, Lee Y. Thixotropic property in pharmaceutical formulations. J Control Release. 2009;136(2):88-98. [PubMed] | [CrossRef] | [Google Scholar]
- Elmowafy M, Al-Sanea MM. Nanostructured lipid carriers (NLCs) as drug delivery platform: advances in formulation and delivery strategies. Saudi Pharm J.. 2021;29(9):999-1012. [PubMed] | [CrossRef] | [Google Scholar]
- Su Z, Niu J, Xiao Y, Ping Q, Sun M, Huang A, et al. Effect of octreotide–polyethylene glycol (100) monostearate modification on the pharmacokinetics and cellular uptake of nanostructured lipid carrier loaded with hydroxycamptothecine. Mol Pharm. 2011;8(5):1641-51. [PubMed] | [CrossRef] | [Google Scholar]
- Okur NU, Gokce EH. Ophthalmic applications of SLN and NLC. Curr Pharm Des. 2017;23(43):6676-83. [PubMed] | [CrossRef] | [Google Scholar]
- Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci.. 2018;13(4):288-303. [PubMed] | [CrossRef] | [Google Scholar]
- Karunakar G, Patel NP, Kamal SS. Nano structured lipid carrier-based drug delivery system. J Chem Pharm Res. 2016;8(2):627-43. [PubMed] | [CrossRef] | [Google Scholar]
- Araújo J, Nikolic S, Egea MA, Souto EB, Garcia ML. Nanostructured lipid carriers for triamcinolone acetonide delivery to the posterior segment of the eye. Colloids Surf B Biointerfaces. 2011;88(1):150-7. [PubMed] | [CrossRef] | [Google Scholar]
- Yaziksiz-Iscan Y, Wissing SA, Müller RH, Hekimoglu S. Different production methods for Solid Lipid Nanoparticles (SLN) containing the insect repellent DEET. In: The 4 World Meeting Pharmacy, Biopharmacy and Pharmaceutical Technology, Florenz. 2002 [PubMed] | [CrossRef] | [Google Scholar]
- Sánchez-López E, Espina M, Doktorovova S, Souto EB, García ML. Lipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye–Part II-Ocular drug-loaded lipid nanoparticles. Eur J Pharm Biopharm. 2017;110:58-69. [CrossRef] | [Google Scholar]
- Ahmed S, Amin MM, Sayed S. Ocular drug delivery: a comprehensive review. AAPS PharmSciTech. 2023;24(2):66 [PubMed] | [CrossRef] | [Google Scholar]
- Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid Lipid Nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238(1-2):241-5. [PubMed] | [CrossRef] | [Google Scholar]
- Attama AA, Reichl S, Müller-Goymann CC. Diclofenac sodium delivery to the eye: evaluation of novel solid lipid nanoparticle formulation using human cornea construct. Int J Pharm.. 2008;355(1-2):307-13. [PubMed] | [CrossRef] | [Google Scholar]
- Araújo J, Gonzalez-Mira E, Egea MA, Garcia ML, Souto EB. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int J Pharm. 2010;393(1-2):167-75. [PubMed] | [CrossRef] | [Google Scholar]
- Zhang W, Li X, Ye T, Chen F, Yu S, Chen J, et al. Nanostructured lipid carrier surface modified with Eudragit RS 100 and its potential ophthalmic functions. Int J Nanomedicine. 2014;9:4305-15. [PubMed] | [CrossRef] | [Google Scholar]
- Gonzalez-Mira E, Egea MA, Souto EB, Calpena AC, García ML. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology.. 2011;22(4):045101 [PubMed] | [CrossRef] | [Google Scholar]
- Youssef AAA, Dudhipala N, Majumdar S. Dual drug loaded lipid nanocarrier formulations for topical ocular applications. Int J Nanomedicine. 2022;17:2283-99. [PubMed] | [CrossRef] | [Google Scholar]
- Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev.. 2012;64:83-101. [PubMed] | [CrossRef] | [Google Scholar]
- Satyanarayana T. Asian Journa Pharmaceu. Asian Journal of Medical and. 2015;319:323 [PubMed] | [CrossRef] | [Google Scholar]
- Müller RH, Mäder K, Gohla S. Solid Lipid Nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161-77. [PubMed] | [CrossRef] | [Google Scholar]
- Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59(6):478-90. [PubMed] | [CrossRef] | [Google Scholar]
- Basu B, Garala K, Bhalodia R, Joshi B, Mehta K. Solid lipid nanoparticles: A promising tool for drug delivery system. J Pharm Res. 2010;3(1):84-92. [PubMed] | [CrossRef] | [Google Scholar]
- Reddy LH, Murthy RS. Etoposide-loaded nanoparticles made from glyceride lipids: formulation, characterization, drug release, and stability evaluation. AAPS PharmSciTech. 2005;6(2):E158-66. [PubMed] | [CrossRef] | [Google Scholar]
- Üner M, Yener G. Importance of Solid Lipid Nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2(3):289-300. [PubMed] | [Google Scholar]
