ABSTRACT
Medicinal plants are a valuable source of supplementary remedies for the treatment of various ailments. Lawsonia inermis (belongs to family Lythraceae) or commonly known as Henna is a glabrous and branching small tree that is indigenous to the subtropical regions of Asia and North Africa. It has traditionally been used as a dandruff-fighting and antifungal agent when applied to the hair, hands, and feet. The staining properties of Henna are derived from the lawsone content, which is found primarily in the leaves. The purpose of this review is to conduct a literature search and critically review the relevant published articles on the phytochemical and pharmacological activities of L. inermis. The phytochemical screening reported that naphthoquinone derivatives, Henna essential oil, flavonoids, tannins, phenols, quinones, alkaloids, glycosides and saponins can be isolated from various parts of the Henna tree. The selection of appropriate solvents is critical in phytochemical screening, as different solvents resulted in different extraction yields. The pharmacological activities found in Henna are ameliorative activity, alleviating wound healing process, antifungal, antioxidant, antibacterial, hepatoprotective, nootropic, anti-ulcer, anti-inflammatory and anti-cancer activity. In conclusion, phytochemical screening is critical for identification of plants constituents, and Henna possesses wide range of pharmacological properties. As a result, further studies on Henna’s phytochemical and pharmacological qualities should be done to fully exploit its benefits.
INTRODUCTION
Plants have long been employed as a source of medicine throughout human history. The knowledge of the different medicinal values of plants has been passed down through the generations via observation and experimentation. However, from time to time, people started to be interested in knowing where the plant properties originate from and the scientific explanation for how those properties are capable of producing therapeutic effects.1 This is the beginning of phytochemistry which can be portrayed as a study of phytochemicals which involved chemicals that can be derived from plants. It deals with the structure and biological properties of secondary metabolites that responsible to give a therapeutic effect. The study of phytochemicals started in the 19th century and gained notoriety in pharmacognosy when people start to extract the active metabolites from plants such as quinine from Cinchona bark (1820), codeine from the Cannabis, morphine from the unripe seed of opium (Papaver somniferum) etc.2 Phytochemistry and pharmacology are closely related, as compounds that can be isolated in plants have pharmacological propeties. Lawsonia inermis or commonly known as Henna belongs to the family of Lythraceae. Table 1 shows the taxonomic tree of Henna. Some of the phytochemicals constituents that can be derived from this plant are flavonoids, tannins and naphthoquinone derivatives.
| Domain | Eukaryota |
|---|---|
| Kingdom | Plantae |
| Phylum | Spermatophyta |
| Subphylum | Angiospermae |
| Class | Dicotyledonae |
| Order | Myrtales |
| Family | Lythraceae |
| Genus | Lawsonia |
| Species | Lawsonia inermis |
The purpose of the present review is to perform a literature search and critically evaluate relevant publications on the phytochemical and pharmacological activities of L. inermis. The relevant information and publications were obtained from the US National Library of Medicine (PubMed), Google Scholar, National Center for Biotechnology (NCBI) and Science Direct. Only articles published starting from 2015 and written in English were reviewed. Figure 1 represents the flow chart for the selection process of the articles in this review. This review focused on the phytochemical and pharmacological findings of Henna. It is significant to provide a succinct analysis and correlation between phytochemical constituents found in the L. inermis and their pharmacological effects.
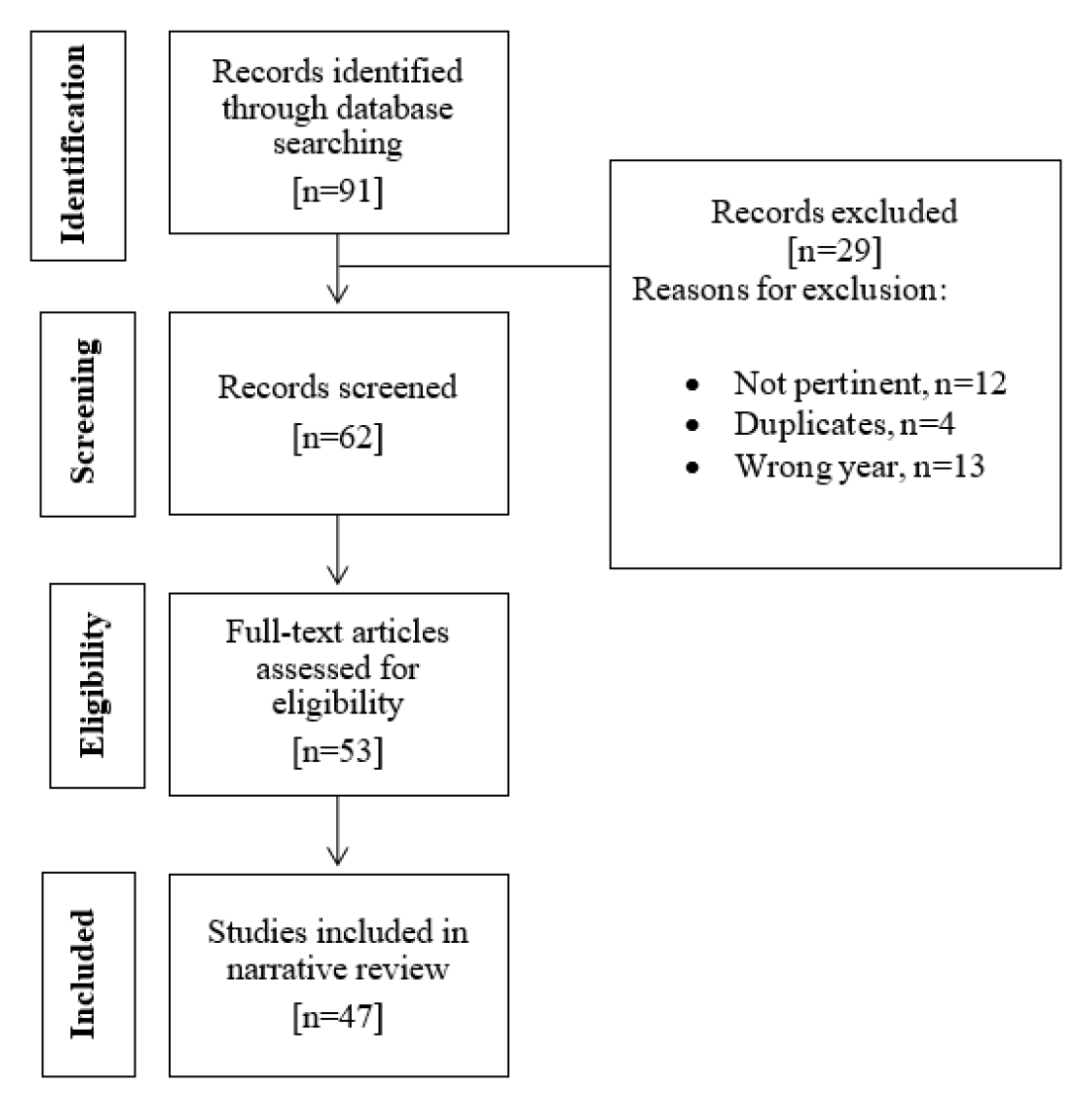
Figure 1.
Flow chart for the selection process of literature for narrative review.
Lawsonia inermis
Botanical Description and Background
The Henna tree can be described as a glabrous, branched small shrubbery tree and normally grows to a height of 2 to 6 meters.3 It has a greyish-brown bark and branches that are quadrangular in shape. The quadrangular young branches are initially green colour but become red as they grow. The flower of Henna can be described as having a 2 mm calyx tube, 3mm spreading lobes. The shape of the petals is orbicular or obovate. The fruit has a spherical shape with a 4mm diameter.4
Another scientific name of L. inermis includes Lawsonia alba Lam., Lawsonia speciosa L. and Lawsonia spinosa L.4 Henna has different vernacular names in India. For example, it is knowns as Mehndi in Hindi, Rakigarbha in Sanskrit and Muruthani in Tamil.5 Not only in India, but Henna also has different common names across the country. For instance, Henna is called krâpéén in Cambodia, inai in Indonesia and Malaysia, Henna strauch in Germany and alcanna vera in Italy.4
It is indigenous to subtropical regions of Asia and North Africa and can be found in India and Persia.6 Henna is suitable to be cultivated in tropical arid zones and tropical savannah at latitudes between 15° and 25° N° and S°. It can yield the greatest proportion of dye at temperatures ranging from 35°C to 45°C.7 It can survive in various environmental settings, from alkaline to acidic soil with scarce and heavy annual rainfall. The ideal temperature of soil for germination is between 25°C to 30°C.8
Henna is associated with Indian culture as it is an essential tradition during a wedding in which the bride will apply Henna to create a unique design on her hands, arm, and feet.9 Malaysian also practice this tradition where they have a special tradition called ‘Malam Berinai.’ The bridegroom will put Henna to their hands, and usually, the bride will apply Henna with a unique design, whereas the groom will apply Henna in the middle, ring, and little fingers.10 Traditionally, people will make a paste from the leaves of the Henna tree by crushing the leaves and mix them with water and essential oil. In the present period, people can easily find products of Henna in many beauty shops. Henna has a natural cooling effect on the skin, providing a pleasant, tingling sensation.9
The staining properties of Henna come from the compound named 2-hydroxy-1,4-naphthoquinone or also known as lawsone which gives the red-orange colour.11 Lawsone can primarily be found in the leaf petioles, and Henna leaves appear to contain up to 5% by weight of the compound.12 Apart from its staining properties, Henna can be employed in different conditions. According to Emin and Mehmet,13 the aqueous extract derived from the leaves of L. inermis could be used as a corrosion inhibitor for steel, zinc, and carbon steel. In addition, research conducted by Abdollah et al.14 revealed that a traditional medicine product containing 25% of natural Henna oil and 1% of hydrocortisone effectively treated diaper dermatitis among infants and toddlers.
In every plant, the secondary metabolites are responsible for exerting pharmacological activities. Secondary metabolites refer to the medicinal importance of a plant depending on the chemicals derived from a particular plant. Alkaloids, flavonoids, coumarins and tannins are all examples of secondary metabolites found in L. inermis These metabolites are closely connected to pharmacological activities that contribute to the provision of therapeutic effects.15 Some pharmacological effects present in Henna are antibacterial activity, wound healing activity, and antioxidant properties.
The phytochemical constituents of L. inermis
Naphthoquinone derivatives
Lawsone (Figure 2)48 (2-hydroxy-1,4-naphthoquinone) can be found in the leaves of the Henna tree. Lawsone is a compound that responsible for the dyeing properties of Henna where lawsone and its glycosides will adhere to the hair or skin proteins and produce red or orange colour stain.16 The concentration of lawsone from Henna leaves was determined in four different cities in Morocco (Tazzarine, Foum Zguid, Zaroga and Errachidia). The High-Performance Liquid Chromatography-Electrospray Ionisation-Qtrap-Mass Spectrometry (HPLC-ESI-Qtrap-MS) was used for quantification analysis. According to this analysis, Henna from Foum Zguid contained the highest content of lawsone (0.6%) while Henna from Errachidia showed the lowest concentration of lawsone.17 1,4-naphthoquinone,2-amino (21.2%) and 1,4-naphthoquinone,3-hydroxy (11.1%) were the major compounds extracted from the hydroethanolic extract of L. inermis leaves.18
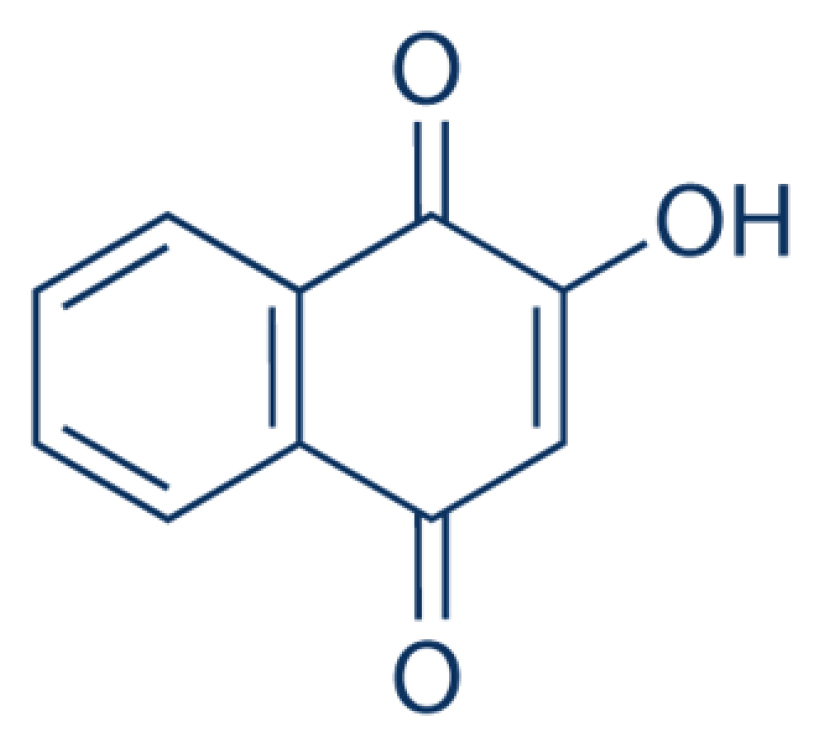
Figure 2.
Lawsone.
Mariana et al.19 conducted a study by using leaves of Henna collected from the garden of Mario Melo Avenue, Recife Pernambuco, Brazil. The lawsone content of the leaves was extracted using the Soxhlet technique with ethanol as the solvent. The crude sawdust was subjected to column chromatography in silica gel, eluted with ethanol-dichloromethane at a ratio of 0.2:9.8. In this study, the formation of a red-orange solid was physically analysed. This study reported that the physical and spectroscopic data of the analysed compound were consistent with those reported in the literaimture.20 The supplementary material includes spectral analysis and physical-chemical results. These results revealed that the purified product was free of contamination, indicating that the red-orange solid was a pure preparation of lawsone.
Oda et al.16 conducted a study on the lawsone content in different parts of the Henna tree. The methanolic extracts of L. inermis flowers, branches and leaves were measured for lawsone content by using HPLC with Tandem Mass Spectrometry (MS-MS). The results of this study showed the lawsone contents in the Henna flowers, branches and leaf extracts were 116.9, 5.4 and 486.2 μ g/g respectively. The leaves of Henna contained the highest concentration of lawsone, followed by the flowers and branches.
The flowers and branches of Henna can be utilised as medicines due to their mild colouring impact and low levels of lawsone. Other naphthoquinones derivatives such as lawsoinermone and lawsoniaside (1,2,4-trihydroxynaphthalene2-O – β-D-glucopyranoside) can also be obtained from leaves of Henna.21–23
Essential Oil
Henna Essential Oil (HeEO) was extracted from the leaves and small flowers of L. inermis by hydrodistillation. HeEO is a compound that can be used to make perfume.16 In this study, the identification of compounds in the sample was done by Gas Chromatography-Mass Spectrometry (GC/MS) method. GC/MS analysis revealed at least 30 different components can be found in Henna leaves. Monoterpene hydrocarbons were the main class of constituents with 81.40%. The high percentage of monoterpene hydrocarbons marked the essential oil produced.24
Flavonoids
A new flavone derivative, 7-hydroxy-3,5-dimethoxy- 6,8-dimethylflavone, was found in the aerial parts of L. inermis. The structures of this new compound were determined by spectroscopic and Mass Spectrometry (MS).25 A high amount of apigetrin and apigenin 5-glucoside were found in leaf extract of L. inermis at 1180.9 mg/ 100 g DW and 596.3 mg/100 g DW, respectively.26 The phytochemical screening on methanolic extracts of Henna leaves showed the presence of flavonoids.27 The methanolic extract of Henna leaves that cultivated in three different sites in Morocco showed the highest flavonoid compounds in Henna cultivated in Alnif at 13.52 g/100 g followed by 8.04 g/100 g Henna from Tafraoute Sidi Ali. The lowest flavonoids compound was found in Henna from Tazzarine at 5.68 g/100 g.28
A sample of Henna leaves was collected in Elele, Ikwerre L.G.A., Rivers State. The Henna leaves were extracted using two different solvents (N-butanol leaf extract and ethylacetate leaf extract). Flavonoids were identified using the ammonium test and an aluminium chloride solution. A yellow colour layer in the ammonical layer indicates the presence of flavonoids in the ammonium test. For aluminium chloride solution, a yellow colour layer in ammonium chloride later can be observed if flavonoids are present. The findings of this study revealed that both extracts contained a high concentration of flavonoids.29 Flavonoids can also be found in the Henna leaves obtained in Duzau village of Babura local government area of Jigawa State, Nigeria. Usman and Rabiu30 discovered the presence of flavonoids in the aqueous, ethanol, and petroleum ether extracts. Danzarami et al.31 conducted phytochemical screening on Henna leaves collected from Zaria Local Government area of Kaduna State, Nigeria. Methanol, ethylacetate and water were used as the solvents. Flavonoids can be found in methanol and ethylacetate extracts but not in water extract. The ethanolic extract of Henna leaves showed the presence of flavonoids. In this study, the presence of flavonoids was evaluated by dissolving the crude extract of L. inermis in 2mL of 10% lead acetate. The formation of yellowish-green colour indicates the presence of flavonoids.32
Tannins
The main constituent in Henna includes tannic acid. In a study conducted by Tauheed et al.,27 they found that the crude methanol extracts were positive for the presence of tannins from the leaves of Henna. Another study conducted by Dahake and Kamble33 found that ethanolic extract of Henna leaves contained tannins. Potassium hydroxide test was used to detect the presence of tannins. This test showed a positive result which indicates the presence of tannins in Henna leaves. A study conducted by Khan et al.32 reported that tannins can be found in Henna leaves collected in Pakistan. In this study, the presence of tannins was evaluated by mixing 2 mL of the crude extract with 0.1% of Ferric chloride. A brownish-green colour will appear if tannins are present.
A sample of Henna leaves was collected in Yemen. The crude extract of Henna leaves was extracted using two different solvents. The first solvent is an aqueous solvent that yields aqueous extract whereas methanol was used as an organic solvent (methanolic extract). For the aqueous extract, 30 g of powdered Henna leaves were suspended in 300 mL of sterile water for two days. The methanolic extraction was carried out by soaking the 30 g of powdered Henna leaves in 300 mL of 70% methanol for 48 hr. The presence of tannins was detected using Ferric chloride tests. The result showed that tannins were present in both methanolic and aqueous extracts of Henna leaves.34
The presence of tannins was identified using the lead acetate test, the Brayer’s test, and the ferric chloride test. For the lead acetate test, if tannins were present in the extracts, a white precipitate will form, whereas, for the Brayer’s test, a dark blue colour will appear. For the ferric chloride test, if tannins are present, greenish-black precipitation can be seen. The presence of tannins was detected in N-butanol leaf extract and ethyl acetate extract of Henna leaves collected in Rivers State, Nigeria but only in a low concentration.29 In contrast, Danzarami et al.31 reported the absence of tannins in ethylacetate extracts but the presence of tannins can be seen in methanol and water extract.
Phenols
The content of phenols in this species was previously analysed by High-Pressure Liquid Chromatography (HPLC) analysis. The result showed high amounts of gallic acid at 81 mg/100 g DW(26). Soukaina et al.28 conducted a study to evaluate the Henna leaves in three different sites in Morocco which are Alnif, Tafraoute Sidi Ali and Tazzarine. The collected Henna leaves were extracted using methanol. The Folin-Ciocalteu reagent was used to examine the presence of phenolic compounds. The results of this study reported that Henna leaves cultivated in Tazzarine showed the highest phenolic compounds with 31.9 g/100 g of sample. The phenolic compounds present in Henna cultivated in Alnif and Tafraoute Sidi Ali is 25.6 g/100 g and 27.1 g/100 g respectively.28 Danzarami et al. 31 conducted phytochemical screening of Henna leaves collected in Nigeria. The extraction of Henna was carried out by soaking 50 g of Henna leaves in 500 mL of methanol, ethyl acetate and distilled water for three days. This study reported that phenols can be found in methanol and water extracts.
Quinones
Leaves of Henna in Yemen were extracted using two different solvents: aqueous solvents and organic solvents (methanol). The presence of Henna was detected by using sodium hydroxide. The study reported that the presence of quinones was found in the aqueous extract but not in the methanolic extract.34
Alkaloids
Mayer’s test was used to detect the presence of alkaloids in Henna leaves that were collected in Yemen. Yellow precipitate indicates the presence of alkaloids in Mayer’s test.35 This study reported the presence of alkaloids were found in both methanolic and aqueous extract.34 Henna leaves cultivated in Nigeria showed a moderate concentration of alkaloids in N-butanol leaf extract and ethylacetate leaf extract.29 Danzarami et al.31 also conducted phytochemical screening of Henna leaves in Nigeria but in a different region. The results of this study revealed that alkaloids can be found in ethylacetate and water extracts. Khan et al.32 conducted phytochemical screening of Henna leave cultivated in Pakistan. The ethanolic extract of L. inermis showed the presence of alkaloids. In this study, the presence of alkaloids was confirmed by mixing 2 mL of the crude extract with 2 mL of Wagner’s reagent. The presence of alkaloids is indicated by the formation of a brownish precipitate.
Glycosides
Ali et al.34 conducted research for the phytochemical screening of Henna leaves that harvested in Yemen. Aqueous extract and methanolic extract were yielded in this study. Fehling’s solution was carried out for glycosides identification. This study reported that glycosides were found in both aqueous and methanolic extract. Chuku et al.29 also carried out phytochemical screening in Henna leaves that were collected in Elele, Ikwerre L.G.A., Rivers State. This study also employed two different solvents: N-butanol (N-butanol leaf extract) and ethylacetate (ethylacetate leaf extract). The presence of glycosides will produce brick-red precipitate in Fehling’s solution. According to this study, glycosides were found in moderate amounts in both N-butanol leaf extract and ethylacetate leaf extract.
Saponins
Chuku et al. 29 conducted a study on phytochemical screening L. inermis. The leaves of Henna were collected in Rivers State, Nigeria. N-butanol and ethylacetate extracts were used in this study. Three different tests were used to determine the presence of saponins: the Frothing test, the emulsion test, and the haemolytic test. If saponins are detected in the frothing test, a stable froth will form, whereas the formation of an emulsion can be seen in the emulsion test. Lastly, the presence of saponins in the hemolytic test can be confirmed if hemolysis of red blood cells occurs. The results of this study reported that saponins are slightly present in the Henna leaves. In addition, Ali et al.34 reported that saponins can be found in aqueous extract but not in methanolic extract. Usman and Rabiu30 conducted a phytochemical screening of Henna leaves collected in Duzau village of Babura local government area of Jigawa State, Nigeria. The plant was extracted using the percolation method. Three different solvents were used in this study (water, ethanol and petroleum ether). 30 g of Henna powder was soaked in 150 mL of solvents for seven days. The emulsion test was used to determine the presence of saponins. The results of this study reported that saponins can be found in aqueous extract, ethanol extract and petroleum ether extract.
The pharmacological activities of L. inermis
L. inermis have been proven to be pharmacologically active in a number of bioactivity studies. Table 2 summarizes the pharmacological activities identified in L. inermis.
| Bioactivity studied | Extract of L. inermis | Major findings | References |
|---|---|---|---|
| Ameliorative | Methanol extract of leaves. | Rats infected with trypanosomiasis were able to improve erythrocyte membrane integrity and maintain a high erythrocyte count. | 27 |
| Wound healing | Hydroethanolic extract of leaves. | Improved the process of wound healing by enhancing collagen deposition production and facilitating epithelization. | 18 |
| L. inermis ointment. | Improved the healing process of episiotomy wounds in primiparous women due to antibacterial and anti-inflammatory properties. | 38 | |
| Aqueous extract of Henna leaves. | G/OST-Henna improved the wound healing process by significant increase in fibroblast activities and proliferation rate. | 39 | |
| Antifungal | Acetone extract of leaves. | Antifungal effects towards Geotrichum spp., Trichophyton spp., and Curvularia spp. The highest antifungal activity was against Curvularia spp. where only a low concentration of acetone extract was needed to kill the fungus. | 41 |
| Ethanol and methanol extract. | Inhibited the growth of C. albicans. This extract can be further used to fight oral cavity infections caused by C. albicans. | 42 | |
| Henna leaves extract. | Achieved antifungal effects against P ochrochloron, A. ochraceus, C. albicans, A. niger, A. flavus and P. funiculosum. | 26 | |
| Antioxidant | Methanolic extract of leaves. | Strong antioxidant effects due to cell cycle inhibition, promote apoptosis and diminished oxidative stress. | 26 |
| Extraction of HeEO. | A distinctive antioxidant activity where it was able to scavenge DPPH activity at 42% due to synergistic activity of terpenes and phenols. | 24 | |
| Hydroethanolic extract of leaves. | Higher antioxidant properties compared to butyl hydroxytoluene. The antioxidant properties were able to alleviate the wound healing process. | 18 | |
| Antibacterial | Ethanolic extract of leaves. | Possessed antibacterial activity for both gram-negative and gram-positive bacteria. | 33 |
| Methanolic and aqueous extract of leaves. | Antibacterial activity against E. coli, S. aureus and P. aeruginosa. Methanolic extract showed higher antibacterial activity compared to aqueous extract. | 34 | |
| Hepatoprotective | Lawsone content in Henna leaves. | Decreased in serum ALT, ASP, ALP and LDH levels and reduced abnormalities in histopathological observations. | 43 |
| Nootropic | L. inermis extract of ethanol (LiEt) and chloroform (LiChf). | Reversed memory loss at low dose due to inhibiting negative feedback of neurotransmitter release. | 44 |
| Anti-ulcer | Henna leaves and stem mixed with distilled water. | As additive therapy as it prevents the progression of decubitus ulcer. | 47 |
| Anti-inflammatory | N-butanol extract and ethylacetate extract of Henna leaves. | Significantly reduced the level of oedema on carrageenan-induced oedema on chick paw. | 29 |
| Anti-cancer | Ethanolic extract of Henna leaves. | Reduced the viability of the tested cancer cells, increased the ROS level and lead to apoptosis. | 46 |
Ameliorative activity
The ameliorative effect can be defined as improving or making a better condition from a certain problem. A crude methanol extract that contains alkaloids, triterpenes and steroids, carbohydrates, tannins, cardiac glycosides, saponin glycosides and flavonoids showed a positive ameliorative effect against trypanosomiasis. A total of 25 adult Wistar rats of both sexes were used in this study. The rats were injected individually with 106 Trypanosoma congolense per mL of blood and randomly divided into five groups. Each group consist of five rats. The first three groups consist of rats treated with different doses of methanolic extract of Henna. Group I received 125 mg/kg whereas groups II and III received 250 and 500 mg/kg of extract respectively. Rats in group IV were given 3.5 mg/kg of diminazene aceturate while 2 mL/kg of physiological buffered saline was given to rats in group V. The antitrypanosomal effect of Henna was evaluated by observing several parameters which are a day-to-day level of parasitaemia, weekly observation of Packed Cell Volume (PCV), Erythrocyte Osmotic Fragility (EOF) and the Concentration of Malondialdehyde (MDA) concentration on day 21. The results showed that rats treated with 250 mg/kg of the extract have significantly reduced levels of parasitaemia. The PCV showed a higher level in groups I, II and III than group IV and V but significantly higher in group II. The values of EOF and MDA showed the lowest value in group II when compared with groups IV and V. Therefore, the result of this study showed that in trypanosome-infected rats, the leaves of L. inermis were able to improve erythrocyte membrane integrity and maintain high erythrocyte count. Consequently, it will help in reducing the chances to get anaemia and thus intensify the productivity of trypanosome-ridden animals.27
Wound healing activity
The wound healing process is divided into four stages which are the hemostasis phase, inflammatory phase, proliferative phase and maturation phase.36 Carbohydrates are vital in the wound healing process because they are involved in the synthesising by-products such as glycoproteins and enzymes, which are essential in the healing process. The enzymes such as hexokinase and citrate synthase have a substantial effect on the wound healing process.18,37 A new formulation of ointment from hydroethanolic extract leaves of L. inermis was able to help in the process of wound healing by gene expression of glucose transporter-1 (Glut-1) and insulin-like growth factor I (IGF-1) in Wistar rats. In this study, the Wistar rats were divided into four groups (A-D). Group A consist of the control group whereas Group B, C and D consist of Wistar rats that treated with 1.5, 3 and 6 g (w/w) of L. inermis respectively. An excisional wound with size of 314 mm2 was surgically created and the ointment were applied once daily on the wounded area. The results of this study reported that the wounded area was significantly smaller for rats that were treated with L. inermis. Among the three treated groups, the wound area is the smallest in group D (treated with 6 g (w/w) of L. inermis). The administration of L. inermis enhanced the collagen deposition production by proliferation, differentiation and migration of fibrocytes. In addition, L. inermis appears to facilitate epithelization by provoking carbohydrates, especially glucose, reducing the distance between the wound edges and promoting re-epithelialization to minimize the time required to close the wound. Therefore, this newly formulated topical ointment can improve the healing process.18
Sanaz et al.38 reported that L. inermis ointment able to provide wound healing effect on episiotomy wound for a woman who has previously been pregnant and given birth. In this study, Persian oak and Henna were used to assess the wound healing activity. Both plants are thought to have anti-inflammatory and antibacterial properties that assist in the healing process. This double-blind trial was conducted on 160 women that underwent episiotomy. Episiotomy is a common process during childbirth where an incision was made to widen the opening of the vaginal. The primiparous women were divided into four groups. Group 1 is the control group whereas Group 2 is the placebo group. Group 3 and 4 consists of primiparous women that applied topical Henna and topical Persian oak ointment respectively. The healing score for episiotomy wound was evaluated using Redness, Edema, Ecchymosis, Discharge, and Approximation (REEDA) scale before the intervention and on days 7th, 10th and 14th after the ointment was applied. A higher score reflects poor wound healing, while greater wound healing is indicated by a lower score. The Visual Analogue Scale (VAS) was used to measure the intensity of pain. The result of this clinical trial reported that on the 10th and 14th day, the REEDA score for groups 3 and 4 was significantly lower compared to groups 1 and 2. Henna ointment help in alleviating the healing process of episiotomy wound by reducing the diameter of the cutaneous wound due to antibacterial and anti-inflammatory properties present in Henna. The pain intensity was reduced significantly in the Henna-treated group which indicates Henna can be used as an additive therapy to reduce pains and improve episiotomy.
Hadisi et al.39 developed a new bioactive Gelatin-Oxidized Starch (G/OST) nanofibers comprised of L. inermis for the treatment of second-degree burn wounds. Nanofibers as a wound dressing are becoming increasingly popular because of their structural similarity to the natural Extracellular Matrix (ECM). Nanofibers have a larger surface area and are able to absorb more wound exudates than conventional dressings.39,40 This study reported the addition of Henna lead to a significant increase in fibroblast activities with the culture time. In addition, G/OST that contained Henna showed better cellular proliferation compared to G/ OST mats without Henna. The proliferation rate will increase as the content of Henna increases due to increased collagen secretion. Moreover, the burn wound that was treated with G/ OST containing Henna greatly reduced the inflammatory zone. These results of the study could be correlated with the presence of bioactive components in Henna such as flavonoids which can facilitate fibroblast proliferation and the secretion of collagen. The immunohistochemical images of the burn wound sites treated with G/OST-Henna have been reported to have a decrease in the inflammatory response and rapid increases in macrophage numbers. In short, the newly developed G/OST Henna can be a promising wound dressing for the treatment of burn wounds.
Antifungal activity
The acetone extract of Henna leaves showed antifungal effects against Geotrichum spp., Trichophyton spp., and Curvularia spp. Among these three, the highest antifungal activity that can be observed is against Curvularia spp. The Minimum Fungicidal Concentration (MFC) for Curvularia spp. showed the lowest value of 50 μg/mL, which means only a low concentration of acetone extract is needed to kill the fungi. There is no antifungal activity against Alternaria spp. and Fusarium spp. From five tested fungi, Curvularia spp. showed the greatest inhibitory zone. The antifungal activities from acetone extract of Henna leaves are due to various bioactive phytochemicals such as flavonoids, terpenes and terpenoids and aliphatic compounds. However, chloroform extract from leaves of Henna did not show any antifungal activity.41
The ethanol and methanol extract of L. inermis showed efficacy in inhibiting the growth of Candida albicans. In this research, fifteen herbal plants extract were used to observe the inhibitory activity against C. albicans. Among the herbal extract, L. inermis is one of the plants that give the best inhibitory effect. The methanolic extract of L. inermis gives the best antifungal activity. The calculated Minimum Inhibitory Concentration (MIC) is 2.8 mg/ mL. This finding is beneficial as the extract from Henna can be further used to treat oral cavity infections caused by C. albicans.42 The extract of Henna leaves also been used to determine the antifungal effects against Penicillium ochrochloron, Aspergillus ochraceus, C. albicans, Aspergillus niger, Aspergillus flavus and Penicillium funiculosum using the microdilution method. A strong antifungal effect was shown by extracts of L. inermis against all fungi. The presence of apigenin 5-glucoside is said to be responsible for the strong antifungal effects. The mechanism of action for antifungal effects is reduced intra and extracellular reactive oxidative species.26
Antioxidant activity
Methanolic extracts of L. inermis showed strong antioxidant effects due to the apigetrin and apigenin 5- glucoside. Apigenin has in vitro and in vivo antioxidant effects by inhibiting the cell cycle, promoting apoptosis, and diminishing oxidative stress.26
A study conducted by Elaguel et al.24 reported that HeEO possessed antioxidant properties. The antioxidant activity of the Henna extract is evaluated by its potential to suppress stable free radical 2,2-Diphenyl-1-picrylhydrazyl (DPPH). A distinctive antioxidant activity was observed in HeEO, including DPPH at 42%. This study found that HeEO extract possessed better antioxidant properties compared to phenol extracts. The differences in chemical structures between these two compounds explained the findings. The high-level antioxidant activity believed to be due to the isoprene structure of HeEO like hexahydropseudoionone or hexahydridarnesyl acetone. HeEO is able to scavenge the DPPH activity completely due to the synergistic activity of terpenes and phenolic compounds.
In the newly formulated ointment of L. inermis, the biochemical analysisforDPPHinhibitionassayreportedthatthehydroethanolic extract manifested a significantly higher antioxidant activity than butylated hydroxytoluene. The antioxidant properties exhibited by leaves of Henna were able to enhance the process of wound healing by alleviating the inflammatory phase. The wound healing process was accelerated because antioxidants able to decrease MDA, decrease the oxidation of RNA and DNA and reduce the ratio of lipid peroxidation.18
Antibacterial activity
In a study conducted by Dahake and Kamble,33 they investigated the antibacterial properties of L. inermis against gram-negative and gram-positive. The leaves of Henna were extracted using the Soxhlet extraction method and ethanol as the solvents. The gram-negative bacteria in this study include Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Vibrio cholerae, Shigella flexneri, Klebsiella pneumoniae, Vibrio cholerae and Enterobacter aerogenes whereas gram-positive bacteria include Bacillus subtilis, Bacillus megaterium, Bacillus fusifirmis, Staphylococcus aureus, Streptococcus faecalis, Streptococcus pyogenes and Streptococcus pneumonia. The bacteria were tested for antimicrobial activity by the agar well diffusion method. From this study, they found that ethanolic extract of Henna leaves was able to fight against both gram-negative and gram-positive bacteria. For gram-negative bacteria, L. inermis showed the highest antimicrobial activity on Vibrio cholerae with zone of inhibition of 24.5 mm. The zone of inhibition for gram-positive bacteria varies from 20 mm-26 mm in diameter. S. faecalis recorded the highest zone of inhibition for gram-positive bacteria at 26.3 mm.
In addition, Ali et al.34 conducted a study for the antibacterial activity of Henna leaves that were collected in Yemen. In this study, they used two different extracts of L. inermis which are aqueous extract and methanolic extract. The tested bacteria were E. coli, S. aureus and P. aeruginosa. The Agar diffusion test was used to assess the antibacterial activity of both extracts. The antibacterial activity was assessed by using three different concentrations of extracts (50 μL, 100 μL and 150 μL). The results reported the methanolic extract of L. inermis were more effective against the three tested bacteria compared to aqueous extract. Among the three tested bacteria, both extracts showed the highest antibacterial activity against S. aureus and the lowest antibacterial activity against E. coli. However, the methanolic extract was reported to have higher antibacterial activity compared to aqueous extract because it has a greater zone of inhibition compared to aqueous extract. The variation of antimicrobial compounds isolated from the methanolic extract can explain the greater antibacterial activity of methanolic extract as compared to aqueous extract. A large number of free hydroxyl groups are present in the methanolic extract, allowing them to be combined into the cell wall of bacteria with carbohydrates and proteins and attached to inactivate enzyme sites.
Hepatoprotective activity
The drug-induced liver injury is an unfavorable effect on a patient. A study conducted by Darvin et al.43 observed the hepatoprotective effect of lawsone content in Henna on rifampicin-isoniazid-induced hepatotoxicity in in vitro and in vivo models. Forty-two male Wistar rats were used in this study. The rats were divided into seven groups to assess the hepatoprotective effect of lawsone. Group V and VI consist of rats intoxicated with rifampicin and isoniazid and treated with 50 mg/kg and 100 mg/kg of lawsone respectively. The results showed that for both group V and VI, serum ALT, ASP, ALP and LDH levels decreased significantly. In addition, rats treated with lawsone showed increased serum albumin, significantly improved the ratio of albumin and globulin, and a significantly decreased serum bilirubin. The histopathological observations of the liver showed reduce abnormalities from the treatment of lawsone. Therefore, all these positive observations indicate that treatment of lawsone can improve the recovery process in hepatic function. The hepatoprotective action of lawsone is said to be due to it is able to elicit the antioxidative response.
Nootropic activity
Nootropic can be defined as substances that able to enhance memory and improve cognitive functions. A study conducted by Mir et al.44 reported that extract of L. inermis ethanol (LiEt) and L. inermis chloroform (LiChf) were able to act as cognitive enhancer agents. In this study, 210 Swiss albino mice were used. They were divided into 21 groups and each group consist of 10 mice. Two methods were used to assess the memory-enhancing properties which are ‘induction of amnesia’ and ‘without inducing amnesia’. 1 mg/kg of diazepam were administered intraperitoneally to induce amnesia as this antianxiety agent are known to have amnestic properties. There were 10 groups for ‘without inducing amnesia’. Group II were used as control. Group III-VI consist of mice that were treated with LiEt at 25,50,100,200 mg/kg respectively. Group VII-X were given LiChf using the same dose level with LiEt. In contrast, ‘induction of amnesia’ groups consists of 11 groups. In this group, diazepam was administered intraperitoneally on alternate days. 3 groups served as control. Groups IV-VII consist of mice that treated with LiEt whereas Group VII-XI were mice that treated with LiChf. The dose level used in these groups is the same as ‘without inducing amnesia’ groups. The LiEt and LiChf were given orally for 15 consecutive days. The results reported that at 25 mg/kg, both extracts able to enhance memory. For ‘induction of amnesia’ groups which were given diazepam and both extracts, reported reversal of amnesia for all tested doses. The highly significant nootropic properties can be seen at the lowest doses of extracts (25 mg/kg). The effect at low doses could be due to attenuation of acetylcholine auto receptors on presynaptic neurons, which would inhibit negative feedback of neurotransmitter release, whereas, at high doses, it could be due to stimulation of post-synaptic receptor stimulation, which would rapidly destroy acetylcholine and thus reduce Henna’s nootropic potential. In short, LiEt and LiChf displayed nootropic properties and able to reverse the loss of memory.
Anti-ulcer activity
Rafiei et al.45 reported that the extract from Henna leaves and stem were able to prevent the development of pressure ulcer grade one in Intensive Care Unit (ICU) patients. Pressure ulcer or also known as bed sores is a third costly disorder after cancer and cardiovascular disease as it slows the process of recovery and increases the rate of hospitalization. This clinical trial was conducted on 72 ICU patients that have pressure ulcer grade one. They were divided into two groups: control and intervention groups. A mixture of Henna leaves and stem and distilled water was applied topically in the intervention group. The Pressure Ulcer Scale for Healing (PUSH) was used to evaluate ulcerative activity. The pressure ulcer area was inspected on the 1st, 4th and 7th days. The lowest score (0) indicates that the ulcer is healed whereas the highest score (17) showed that the ulcer is progressing. The results of this trial reported that the average PUSH score is low in the intervention group. The PUSH score showed a significant difference between the first 3 days and on the 4th and 7th day which indicate the ulcer is healing. Since the intervention group’s average PUSH score and average area of pressure ulcers at the end of the trial were higher than the control groups, it can be concluded that the intervention slowed down ulcer progression. Henna’s anti-inflammatory properties are thought to aid in preventing the pressure ulcer from progressing to a more severe stage.
Anti-inflammatory activity
Chuku et al.29 conducted a study on the anti-inflammatory activity of L. inermis. In this study, twenty white cockerels were used. They used carrageenan in order to stimulate inflammation and oedema in the 7-day-old chicks. Three different concentrations of N-butanol leaf extract suspension and ethylacetate extract suspension were administered to the chicks (50, 100 and 200 mg/ kg). The results of this study reported that both extracts showed a significant decrease in the level of oedema. Both extracts showed the highest anti-inflammatory activity at 50 mg/kg.
Anticancer activity
Ishteyaque et al.46 found that the ethanolic extract of L. inermis has anti-cancer activity. These properties were investigated on human lung carcinoma (A549), Hepatocellular carcinoma (HepG2) and colorectal cancer (DLD1) by assessing the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, measuring the Reactive Oxygen Species (ROS) and mitochondrial membrane potential of the cancer cell lines. Firstly, the MTT assay revealed a substantial decrease in cell growth and cytotoxicity in a concentration-dependent manner. The IC50 values for A549, HepG2 and DLD1 were 490 μg/mL, 610 μg/mL and 480 μg/mL respectively, indicating that they were effective against the cells. The cancer cells treated with L. inermis extracts showed a decrease in viability. DLD1 exhibited the greatest reduction in viability, at 52%. The viability of A549 and HepG2 was reduced up to 40% and 45% respectively. At 300 μg/mL of L. inermis extracts, the ROS levels in A549, HepG2 and DLD1 were 110%, 84% and 101% respectively. A high level of ROS production can lead to apoptosis. The alteration in mitochondrial membrane potential also can be seen in the treated cells. This finding suggests that the loss of mitochondrial potential is due to Henna extract able to induce ROS. According to this study, the cytotoxic, anti-cancerous activity and antiproliferative properties possess by Henna is due to the presence of lawsone.46
CONCLUSION
Phytochemical screening is crucial for determining the constituents of plants. A suitable reagent and test should be used in order to identify the desired constituents and each test should be conducted meticulously to avoid errors. It is crucial to ascertain whether the various phytochemicals act synergistically, additively, or antagonistically with one another. The pharmacological activity of Henna was found to be derived from secondary metabolites. As a result, they play a vital role in providing therapeutic effects. Henna has a wide range of pharmacological properties. Therefore, to better understand and appreciate the benefits of Henna, further extensive research on its phytochemical and pharmacological properties should be conducted.
Cite this article
Supian FNA, Osman NI. Phytochemical and Pharmacological Activities of Natural Dye Plant, Lawsonia inermis L. (Henna). J Young Pharm. 2023;15(2):201-11.
ACKNOWLEDGEMENT
The authors acknowledge the support provided by the Faculty of Pharmacy, UiTM Puncak Alam and Geran Penyelidikan Khas (GPK) UiTM [600-RMC/GPK 5/3 (268/2020)] in completing this review work.
ABBREVIATIONS
| A549 | Human lung carcinoma |
|---|---|
| DLD1 | Colorectal cancer |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ECM | Extracellular matrix |
| EOF | Erythrocyte osmotic fragility |
| GC/MS | Gas Chromatography-Mass Spectrometry |
| G/OST | Gelatin-oxidized starch |
| GLUT-1 | Glucose transporter-1 |
| HeEO | Henna essential oil |
| HepG2 | Hepatocellular carcinoma |
| HPLC | High Performance Liquid Chromatography |
| HPLC-ESI-Qtrap-MS | High-Performance Liquid Chromatography-Electrospray Ionisation-Qtrap-Mass Spectrometry |
| ICU | Intensive Care Unit |
| IGF-1 | Insulin-like growth factor I |
| LiChf | L. inermis chloroform |
| LiEt | L. inermis ethanol |
| MDA | Malondialdehyde |
| MFC | Minimum Fungicidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| MS | Mass Spectrometry |
| MS-MS | Tandem Mass Spectrometry |
| MTT | 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide |
| NCBI | National Center for Biotechnology |
| PCV | Packed cell volume |
| PUBMED | US National Library of Medicine |
| PUSH | Pressure Ulcer Scale for Healing |
| REEDA | Redness, Edema, Ecchymosis, Discharge, and Approximation |
| ROS | Reactive Oxygen Species |
| VAS | Visual analogue scale |
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- Mendoza N, Silva EME. Introduction to phytochemicals: secondary metabolites from plants with active principles for pharmacological importance. Phytochem – source antioxidants role dis prev. 2018:25 [Google Scholar]
- Alamgir A. Therapeutic use of medicinal plants and their extracts. 2018;2:1-4. [Google Scholar]
- Badoni Semwal R, Semwal DK, Combrinck S, Cartwright-Jones C, Viljoen A. Lawsonia inermis L. (Henna): ethnobotanical, phytochemical and pharmacological aspects. J Ethnopharmacol. 2014;155(1):80-103. [PubMed] | [CrossRef] | [Google Scholar]
- [[cited Jun 3 2021]];JR-S Lawsonia inermis (Egyptian privet) [Internet]. 2017 Available from: [PubMed] | [CrossRef] | [Google Scholar]
- Makhija IK. [[cited Jun 3 2021]];Lawsonia inermis – From traditional use to scientific assessment | PharmaTutor [internet]. Pharmatutor. 2011 Available from: [PubMed] | [CrossRef] | [Google Scholar]
- Malekzadeh F. Antimicrobial activity of Lawsonia inermis L. Appl Microbiol. 1968;16(4):663-4. [PubMed] | [CrossRef] | [Google Scholar]
- Kannahi M, Nadu T. Antimicrobial activity of Lawsonia inermis leaf extracts against some human pathogens. Int J Curr Microbiol Appl Sci. 2013;2(5):342-9. [PubMed] | [CrossRef] | [Google Scholar]
- Baessler L. [[cited Nov 12 2020]];Henna tree information–where does henna come. 2018 In: [internet] Available from: [PubMed] | [CrossRef] | [Google Scholar]
- Stalnaker H. [[cited Nov 29 2020]];The healing benefits of Henna—the Lotus Room Ayurveda Nashville [internet]. 2017 Available from: [PubMed] | [CrossRef] | [Google Scholar]
- Sumatera N, Erwany L, Nasution I, Sibarani R, Takari M. Local wisdom in malam Berinai tradition in Malay society, Tanjungbalai, north Sumatera, Indonesia. J Arts Humanit. 2016;5(5):68-77. [PubMed] | [CrossRef] | [Google Scholar]
- Bhuiyan MAR, Islam A, Ali A, Islam MN. Color and chemical constitution of natural dye Henna (Lawsonia inermis L.) and its application in the coloration of textiles. J Clean Prod. 2017;167:14-22. [CrossRef] | [Google Scholar]
- Petkewich R. [[cited Nov 12 2020]];Chemical and Engineering News: what’s that stuff?. Henna. 2006 Available from: [CrossRef] | [Google Scholar]
- Wagini NH, Bello A, Safana A, Abubakar S, Usman HB, Yusuf M, et al. Phytochemical screening and antibacterial properties of Henna (Lawsonia inermis) roots extracts. Katsina J Nat Appl Sci. 2015;4(2):151-60. [CrossRef] | [Google Scholar]
- Keshavarz A, Zeinaloo AA, Mahram M, Mohammadi N, Sadeghpour O, Maleki MR, et al. Efficacy of traditional medicine product Henna and hydrocortisone on diaper dermatitis in infants. Iran Red Crescent Med J. 2016;18(5):e24809 [PubMed] | [CrossRef] | [Google Scholar]
- Hussein AR, A el-Anssary A. Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herb Med. 2019;1(3) [PubMed] | [CrossRef] | [Google Scholar]
- Oda Y, Nakashima S, Kondo E, Nakamura S, Yano M, Kubota C, et al. Comparison of lawsone contents among Lawsonia inermis plant parts and neurite outgrowth accelerators from branches. J Nat Med. 2018;72(4):890-6. [PubMed] | [CrossRef] | [Google Scholar]
- Alem FZ, Gita SA, Cougnaud L, Affnar C, Nounah I, Youssef B, et al. Lawsone quantification in Lawsonia inermis L. by HPLC-MS: how does the temperature and pluviometry affect lawsone concentration?. Ind Crops Prod. 2020;158:112960 [CrossRef] | [Google Scholar]
- Daemi A, Farahpour MR, Oryan A, Karimzadeh S, Tajer E. Topical administration of hydroethanolic extract of Lawsonia inermis (Henna) accelerates excisional wound healing process by reducing tissue inflammation and amplifying glucose uptake. Kaohsiung J Med Sci. 2019;35(1):24-32. [PubMed] | [CrossRef] | [Google Scholar]
- Xavier MR, Santos MMS, Queiroz MG, De Lima Silva MS, Goes AJS, De Morais MA, et al. Lawsone, a 2-hydroxy-1,4-naphthoquinone from Lawsonia inermis (Henna), produces mitochondrial dysfunctions and triggers mitophagy in Saccharomyces cerevisiae. Mol Biol Rep. 2020;47(2):1173-85. [PubMed] | [CrossRef] | [Google Scholar]
- Communication IC, Mahkam M, Nabati M, Kafshboran HR. Isolation, identification and characterization of lawsone from Henna leaves powder with Soxhlet technique. Iran Chem Commun. 2014;2:34-8. [PubMed] | [CrossRef] | [Google Scholar]
- Al-snafi AE. A review on Lawsonia inermis: A potential medicinal plant. Int J Curr Pharm Res. 2019;11(5):1-13. [PubMed] | [CrossRef] | [Google Scholar]
- Yang CS, Chen JJ, Huang HC, Huang GJ, Wang SY, Sung PJ, et al. New benzenoid derivatives and other constituents from Lawsonia inermis with inhibitory activity against NO production. Molecules. 2017;22(6):926 [PubMed] | [CrossRef] | [Google Scholar]
- Dhouafli Z, Leri M, Bucciantini M, Stefani M, Gadhoumi H, Mahjoub B, et al. 1,2,4-trihydroxynaphthalene-2-O-β-D-glucopyranoside delays amyloid-β42 aggregation and reduces amyloid cytotoxicity. BioFactors. 2018;44(3):272-80. [CrossRef] | [Google Scholar]
- Elaguel A, Kallel I, Gargouri B, Ben Amor I, Hadrich B, Ben Messaoud E, et al. Lawsonia inermis essential oil: extraction optimization by RSM, antioxidant activity, lipid peroxydation and antiproliferative effects. Lipids Health Dis. 2019;18(1):196 [PubMed] | [CrossRef] | [Google Scholar]
- Yang CS, Chen JJ, Huang HC, Huang GJ, Wang SY, Chao LK, et al. New flavone and eudesmane derivatives from Lawsonia inermis and their inhibitory activity against NO production. Phytochem Lett. 2017;21(Jun):123-7. [CrossRef] | [Google Scholar]
- Elansary HO, Szopa A, Kubica P, Ekiert HA, Al-Mana FA, Al-Yafrsi MA, et al. Antioxidant and biological activities of Acacia saligna and Lawsonia inermis natural populations. Plants (Basel). 2020;9(7):1-17. [PubMed] | [CrossRef] | [Google Scholar]
- Tauheed AM, Shittu SH, Suleiman MM, Habibu B, Kawu MU, Kobo PI, et al. In vivo ameliorative effects of methanol leaf extract of Lawsonia inermis Linn. on experimental Trypanosoma congolense infection in Wistar rats. Int J Vet Sci Med. 2016;4(2):33-40. [PubMed] | [CrossRef] | [Google Scholar]
- El Massoudi S, Benidir M, Chabir R, Benjelloun M, El Ghadraoui L, Errachidi F, et al. Morphological, biochemical, and climatological analysis of three Moroccan Henna verities. Scientific World Journal. 2019;2019:1418456 [PubMed] | [CrossRef] | [Google Scholar]
- Chuku LC, Chinaka NC, Damilola D. Phytochemical screening and anti-inflammatory properties of henna leaves (Lawsonia inermis). Eur J Med Plants. 2020;31(18):23-8. [CrossRef] | [Google Scholar]
- Usman RA, Rabiu U. Antimicrobial activity of Lawsonia inermis (Henna) Extracts. Bayero J Pure App Sci. 2018;11(1):166-71. [CrossRef] | [Google Scholar]
- Danzarami D, Umar M, Akafyi DE, Oko JO, Yusuf I, Okeh Q, et al. Phytochemical screening and chromatographic analysis of Henna (Lowsonia inermis) plant obtained from Zaria, Kaduna. Niger Inst Leather. Sci Technol Samaru, Zaria, Kaduna, Niger. 2016;1(5):1-4. [CrossRef] | [Google Scholar]
- Khan BA, Khan A, Khan MK, Braga VA. Preparation and properties of High sheared poly(vinyl alcohol)/chitosan blended hydrogels films with Lawsonia inermis extract as wound dressing. J Drug Deliv Sci Technol. 2021;61:102227 [CrossRef] | [Google Scholar]
- Dahake PR, Kamble SI. Study on antimicrobial potential and preliminary phytochemical screening of Lawsonia inermis Linn. Int J Pharm Sci Res. 2015;6(8):3344-50. [CrossRef] | [Google Scholar]
- Ali KS, Al-hood FA, Obad K, Alshakka M. Phytochemical screening and antibacterial activity of Yemeni henna phytochemical screening and antibacterial activity of Yemeni Henna (Lawsonia inermis) against some bacterial pathogens. IOSR JPBS. 2016;11(2):24-7. [CrossRef] | [Google Scholar]
- Parbuntari H, Prestica Y, Gunawan R, Nurman MN, Adella F. Preliminary Phytochemical Screening (Qualitative Analysis) of cacao Leaves (Theobroma cacao L.). EKSAKTA. 2018;19(2):40-5. [CrossRef] | [Google Scholar]
- Maynard J. Shield Healthcare. [[cited Jun 21 2021]];How wounds heal: the 4 main phases of wound healing; 2015. Shield Healthcare. Available from: [CrossRef] | [Google Scholar]
- Demling RH. Nutrition, anabolism, and the wound healing process: an overview. EPlasty. 2009;9:e9 [PubMed] | [Google Scholar]
- Zibanejad S, Miraj S, Rafieian Kopaei MM. Healing effect of Quercus persica and Lawsonia inermis ointment on episiotomy wounds in primiparous women. J Res Med Sci. 2020;25:11 [PubMed] | [CrossRef] | [Google Scholar]
- Hadisi Z, Nourmohammadi J, Nassiri SM. The antibacterial and anti-inflammatory investigation of Lawsonia inermis-gelatin-starch nano-fibrous dressing in burn wound. Int J Biol Macromol. 2018;107(B):2008-19. [PubMed] | [CrossRef] | [Google Scholar]
- Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463(2):127-36. [PubMed] | [CrossRef] | [Google Scholar]
- Abirami S, Edwin Raj B, Soundarya T, Kannan M, Sugapriya D, Al-Dayan N, et al. Exploring antifungal activities of acetone extract of selected Indian medicinal plants against human dermal fungal pathogens. Saudi J Biol Sci. 2021;28(4):2180-7. [PubMed] | [CrossRef] | [Google Scholar]
- Samadi FM, Suhail S, Sonam M, Sharma N, Singh S, Gupta S, et al. Antifungal efficacy of herbs. J Oral Biol Craniofac Res. 2019;9(1):28-32. [PubMed] | [CrossRef] | [Google Scholar]
- Darvin SS, Esakkimuthu S, Toppo E, Balakrishna K, Paulraj MG, Pandikumar P, et al. Hepatoprotective effect of lawsone on rifampicin-isoniazid induced hepatotoxicity in in vitro and in vivo models. Environ Toxicol Pharmacol. 2018;61:87-94. [PubMed] | [CrossRef] | [Google Scholar]
- Mir NT, Saleem U, Anwar F, Ahmad B, Ullah I, Hira S, et al. Lawsonia inermis markedly improves cognitive functions in animal models and modulate oxidative stress markers in the brain. Medicina (Kaunas). 2019;55(5):192 [PubMed] | [CrossRef] | [Google Scholar]
- Rafiei Z, Mazaheri M, Eghbali-Babadi M, Yazdannik A. The effect of Henna (Lawsonia inermis) on preventing the development of pressure ulcer Grade One in Intensive Care Unit patients. Int J Prev Med. 2019;10:26 [PubMed] | [CrossRef] | [Google Scholar]
- Ishteyaque S, Mishra A, Mohapatra S, Singh A, Bhatta RS, Tadigoppula N, et al. In vitro: Cytotoxicity, Apoptosis and ameliorative potential of Lawsonia inermis extract in human Lung, Colon and liver cancer cell line. Cancer Investig. 2020;38(8-9):476-85. [PubMed] | [CrossRef] | [Google Scholar]
- Hekmatpou D, Ahmadian F, Eghbali M, Farsaei S. Henna (Lawsonia inermis) as an inexpensive method to prevent decubitus ulcers in critical care units: A randomized clinical trial. J Evid Based Integr Med. 2018;23:2515690X18772807 [PubMed] | [CrossRef] | [Google Scholar]
- Lawsone [Internet]. [[cited 2021Sep1]];ChemSpider. Royal Society of Chemistry. Available from: [PubMed] | [CrossRef] | [Google Scholar]
