ABSTRACT
Tetralogy of Fallot is a type of critical congenital heart disease that reduces the amount of oxygen delivered to the body in which adults usually live past the age of 21. Despite the fact that the average life span of a patient with Tetralogy of Fallot who does not have surgery is 12 years, it is believed that approximately 10% of patients live to be 21 years old. Tetralogy of Fallot affects around 3.5% of newborns with congenital cardiac disease, or one in 3600 or 0.28 out of every 1000 live births. Males and females are similarly affected. The majority of patients begin to show symptoms in childhood such as fatigue, shortness of breath and cyanosis needing early intervention. We described a case of a man in his early 20s who postponed the operational procedure despite being identified with Tetralogy of Fallot at the earliest possible stage. A few instances of asymptomatic middle-aged individuals surviving have been documented, and the number is declining as a result of early detection. The example demonstrates the viability of detecting such events in an adult patient despite breakthroughs and growth in medicine.
INTRODUCTION
Tetralogy of Fallot, is the rare congenital heart condition combining four heart defects such as large ventricular septal defect, an aorta that over-rides the left and right ventricles, obstruction of the Right Ventricular Outflow Tract (RVOT) (obstruction may be sub valvular, valvular, supravalvular, or in the pulmonary arterial branches), and right ventricular hypertrophy which causes oxygen poor blood to flow out of the heart and into the rest of the body.1 Niels Stenson originally described the Tetralogy of Fallot in 1671, although William Hunter of St. George’s Hospital Medical School in London eloquently illustrated the anatomical description in 1784, and Etienne-Louis Fallot improved it in 1888.2
The intensity of the right ventricular outflow tract narrowing and architecture of the Pulmonary Artery (PA) affect the incidence of oxygen deprivation in adults with ToF. ToF phenotypes range from mild to severe, with pulmonary atresia and transposition of the great arteries being two illustrations. Different management and treatment tactics may be required for the more severe kinds.3 Following surgical correction, the expectancy for ToF has improved significantly, with longevity rates of up to 25 years. The preferred treatment for ToF currently is surgical repair. On the other hand, delaying a diagnosis or treatment is highly associated with undesirable results.
The eventual manifestation of TOF varies depending on the degree of blood flow restriction to the lungs. The second heart sound in TOF individuals may be single and loud, with an abrupt systolic ejection murmur coming from the blocked sub pulmonary outflow tract. There is typically no audible flow across the atrioventricular node link in tetralogy of Fallot. Patients will be blue and have a weaker heartbeat if there is a significant blockage and restricted antegrade flow along the sub pulmonary outflow tract. Technology developments have made ToF diagnosis simpler. After a lesion is found, an ECG and an imaging test should be performed. The ECG will display right axis deviation and strong right ventricular stresses if there are considerable R waves in the anterior precordial leads and large S waves in the lateral precordial leads. On a conventional Chest X-ray, a cardiac silhouette resembling ‘a boot’ can be visible. The hypoplastic pulmonary outflow tract and right ventricular hypertrophy both contribute to the narrowing of the mediastinal shadow and the upward displacement of the right ventricular apex, respectively. The diagnosis is verified with echocardiography (2d ECHO).4
CASE REPORT
A 25-year-old male patient known case of congenital cardiac abnormality and past history of pulmonary Koch for which he took complete treatment presented with chief complain of chest pain, breathlessness, expectorating cough, bluish discoloration of tips of fingers and toes. Up until a month prior to his presentation, the patient was in good health. However, he began to experience mild to moderate shortness of breath on exertion, which was accompanied by atypical pricking like chest pain and a productive cough that is mucopurulent in nature and that is produced more at night. The patient was born out of non-consanguineous marriage at full term with normal vaginal hospital birth. There is no history of smoking, alcohol or drug intake or any other co-morbid conditions in parents. There is no history of similar illness or any congenital heart disease in family.
Patient was malnourished with height-1.6m, weight-32kg, BMI-12.5 and physical examination revealed central and peripheral cyanosis and clubbing pan-digital, an elevated jugular venous pressure, and a loud systolic ejection murmur that was most audible at the left upper sternal border. While on examination vitals included blood pressure-116/78 mmHg in (Right upper limb), 110/72 mmHg (Left upper limb), 124/80 mmHg in (Bilateral lower limb), Pulse -100/min, Respiratory rate -18/min. No evidence of pallor, icterus and edema. On admission patient’s blood sample was send for analysis and immediate ECG was done apart from that patient was also advised for Chest-X-ray, Echocardiography. Blood report shows increased hemoglobin (23g/dl). However, right ventricular enlargement/stress and right axis deviation were seen on the ECG (Figure 1). In chest X-ray (Figure 2) the heart is shaped like a boot on the chest X-ray, which is a classic symptom of TOF physiology caused by RV hypertrophy. An echocardiography (Figure 3) shows VSD, Overriding aorta, RVOT obstruction. Cardiac catheterization was performed the next day which revealed a large peri membranous VSD with Overriding of aorta and severe RVOT obstruction with muscle bundle.
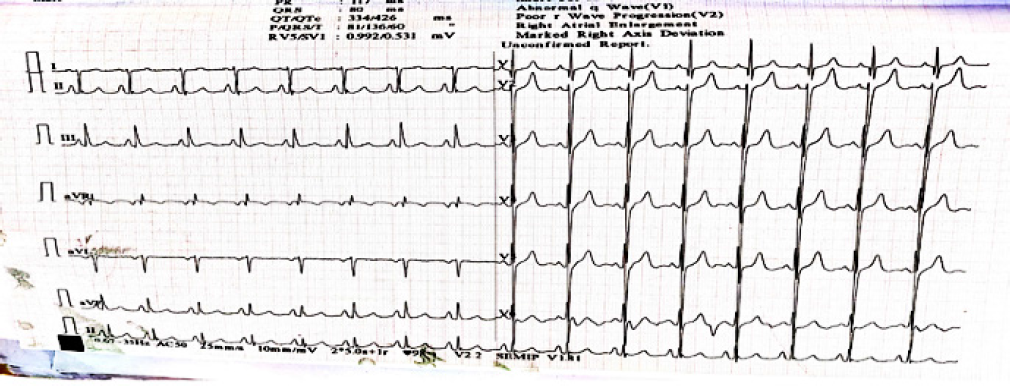
Figure 1.
ECG shows right axis deviation and right ventricular hypertrophy (RVH).
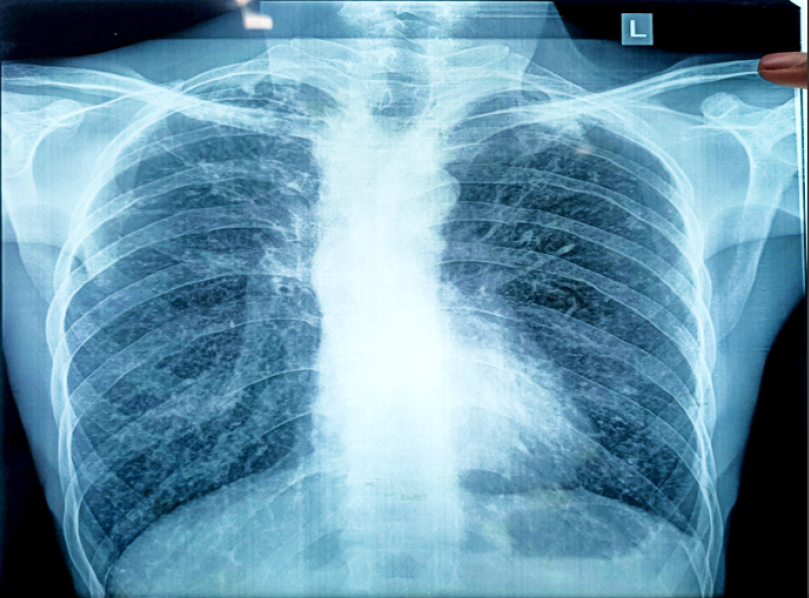
Figure 2.
The chest X-ray shows boot shaped heart which is due to RV hypertrophy which is common sign of TOF physiology.
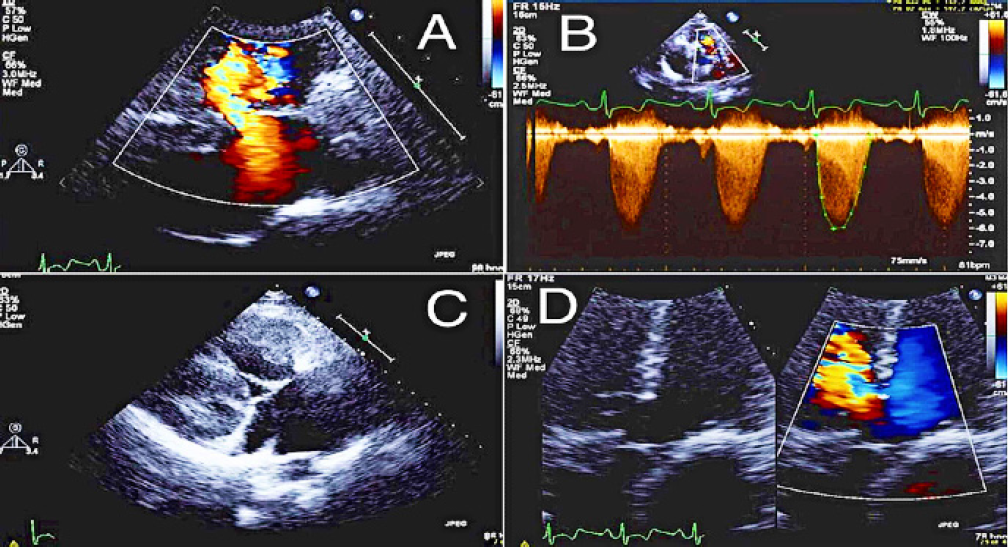
Figure 3.
Patient’s echocardiography showing (A) membranous ventricular septal defect with a right to left shunt, (B) severe pulmonic valve stenosis with a presence of dynamic obstruction, (C) severe right ventricular hypertrophy, and (D) Overriding aorta.
The Cardiac catheterization confirmed the congenital heart abnormality and additional findings of Major Aortopulmonary Collateral Arteries (MAPCA) was found which signified long standing and complicated case of ToF. The patient was advised Coiling of the MAPCAs followed by Open Cardiac repair to correct the structural defects of the heart and thereby protect patient from long term devastating complications. But the patient denied for the same even though knowing negative impact on health and refused for further follow-up.
DISCUSSION
We have discussed a case of adult patient who has delayed ToF correction surgery. Despite the rarity of untreated ToF survival, it has been projected that 10% of those who are afflicted will survive to maturity, with just 5% living to be beyond the age of 40.5–8 The patient also had long-lasting chest discomfort with expectoration and cyanosis/clubbing. A well-developed left ventricle and a substantial bronchial to pulmonary collateral circulation are also related to varying degrees of presenting symptoms brought on by anatomical variations.6 The existence of a small pulmonary artery and the slow onset of sub pulmonary obstruction, left ventricular enlargement, excess cardiac funneling, or systemic to pulmonary shunt may be the cause of people’s extended survival after receiving no medical or pharmacological attention and living to maturity.7 In present case, the cause for postponed surgery was the low socio-economic status of the patient as he was aware of detrimental effects of delayed ToF correction on health but could not afford although the reason for longevity could be due to the gradual progression of narrowing of pulmonary valve.
Cardiac catheterization is crucial in the treatment of ToF in adults because it not only allows for the physical characterization of the pulmonary arteries but also for the detection of unanticipated abnormalities, such as the MAPCA that are seen in 15% of ToF patients. Surgical treatment is made simpler by the use of restorative methods for problems that are comparable.9 Cardiac catheterization in this patient reveals a massive peri membranous VSD with overriding of the aorta and significant RVOT obstruction (Physiology of ToF) and along with MAPCAs in our patient. All these findings support our case. Yet, the gold standard confirmatory test is still 2Dimensional Echo Cardiography (2D ECHO).
During adult ToF repair, difficulty in pumping blood, irregular heartbeat, and sudden cardiac arrest are more likely to occur, which signifies the mortality risk associated with open cardiac repairs. Adult’s quality of life is negatively impacted by long-term cyanosis, which is brought on by persistent left to right shunt, right ventricular malfunction and inadequate pulmonary artery development. Adults have a greater mortality rate than children.10 In our case also, patient was having cyanosis from childhood and it did not decrease as patient did not undergo any definitive treatment.
CONCLUSION
We discussed the case of a patient who refuses to ToF correction surgery several times since he was diagnosed with the condition. Despite medical breakthroughs, those with primary congenital disorders may be untreated until older age, according to the report. When it comes to ToF patients, delaying diagnosis and treatment raises the chance of adverse consequences, as signified by development of MAPCAs in our patient. In this patient the condition of heart was deteriorating with time and despite knowing adverse health effect patient denied for the surgery. There can be many reasons for the same such as fear and risk of surgery, financial issues, lack of knowledge of disease prognosis, etc. We can conclude that any pharmacological treatment would not decrease the symptoms of such patients and therefore patient should correct the condition through surgical intervention as soon as they are diagnosed with Tetralogy of Fallot.
References
- Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;3;342(5):334-42. [Google Scholar]
- Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet. 2009;374(9699):1462-71. [PubMed] | [CrossRef] | [Google Scholar]
- Van der Ven JPG, van den Bosch E, Bogers AJCC, Helbing WA. Current outcomes and treatment of tetralogy of Fallot. F1000 Research. 2019;8 [PubMed] | [CrossRef] | [Google Scholar]
- Bailliard F Anderson RH. Tetralogy of Fallot. Orphanet J Rare Dis. 2009;4(1):2 [PubMed] | [CrossRef] | [Google Scholar]
- Bertranou EG, Blackstone EH, Hazelrig JB, Turner ME, Kirklin JW. Life expectancy without surgery in tetralogy of Fallot. Am J Cardiol. 1978;42(3):458-66. [PubMed] | [CrossRef] | [Google Scholar]
- Thomas SH, Bass P, Pambakian H, Marigold JH. Cyanotic tetralogy of Fallot in a 77 year old man. Postgrad Med J. 1987;63(739):361-2. [PubMed] | [CrossRef] | [Google Scholar]
- Chin J, Bashour T, Kabbani S. Tetralogy of Fallot in the elderly. Clin Cardiol. 1984;7(8):453-6. [PubMed] | [CrossRef] | [Google Scholar]
- Alkashkari W, Al-Husayni F, Almaqati A, AlRahimi J, Albugami S. An adult patient with a tetralogy of Fallot case. Cureus. 2020;12(11):e11658 [PubMed] | [CrossRef] | [Google Scholar]
- Rammohan M, Airan B, Bhan A, Sharma R, Srivastava S, Saxena A, et al. Total correction of tetralogy of Fallot in adults-surgical experience. Int J Cardiol. 1998;63(2):121-8. [CrossRef] | [Google Scholar]
- Higgins CB, Mulder DG. Tetralogy of Fallot in the adult. Am J Cardiol. 1972;29(6):837-46. [PubMed] | [CrossRef] | [Google Scholar]
