ABSTRACT
COVID-19 plays a massive role in the health care sector. A coronavirus is a group of viruses that causes mild, moderate, and severe respiratory infections in humans. In recent times monoclonal antibodies have been used to treat and overcome the infection spread in mild to moderate infections of SARS-COV-2. It is a new neutralizing dual-action monoclonal antibody with an activity that plays a role against severe acute respiratory disorder coronavirus 2, known as SARS-CoV-2. Monoclonal antibodies are engineered molecules in the laboratory which acts as substitute antibody. Monoclonal antibodies can enhance and restore the attack of the immune system on pathogens. It is under development by GlaxoSmithKline, and Vir Biotechnology, Inc. Proteins developed by the laboratory have similar characteristics to natural antibodies produced from the human immune system. This sotrovimab is designed to avoid the entry of pathogens and viral attachment and neutralize the previously infected cells. It has been shown that there is a reduction level in hospitalization or death in age factor between 18-45 with mild-to-moderate COVID-19 by 79% who are at high risk of progressing to serious illness in COMET-ICE (COVID-19 Monoclonal antibody Efficacy Trial – Intent to Care Early) trial. Some of the adverse effects of the sotrovimab were rash and diarrhea (1%). Hypersensitivity reactions, including anaphylaxis, have been reported at at least rate. In this article, we have explained in detail the Sotrovimab overview, history, Clinical data, Special conditions, and Approval data of the European Union
INTRODUCTION
2019 Novel Coronavirus caused a continuous outbreak of lower respiratory tract disease, which was originally called the Novel Coronavirus Pneumonia (NCP) by the Chinese government. Subsequently, the World Health Organization recommended that the disease be named COVID-19. At the same time, the International Committee on Taxonomy of Viruses changed the name of 2019nCoV to SARS-CoV-2.1 The virus that caused the respiratory illness called SARS-CoV-2 is a member of a large virus family called Coronavirus. SARS-CoV-2 first infected humans in 2019. The virus is believed to be spread from person to person through droplets. It can also be spread by touching a surface with the virus and then touching a person’s mouth, nose, or eyes, but this is less common. Research is underway to treat COVID-19 and prevent SARS-CoV-2 infection, also known as severe acute respiratory syndrome coronavirus.2 Although there are several vaccines that have gained a foothold in the fight against COVID-19, therapies, especially antibody drugs, have gone through a significantly more difficult period. The forerunner Eli Lilly has recently been forced to reconsider his strategy, plagued by access issues and by emerging variants. GlaxoSmithKline and Vir Biotechnology are implementing this knowledge to their only agent Sotrovimab [Table 1]. Supported by Phase 3 data and laboratory results, sotrovimab can still play an important role in the pandemic, and these results have shown effectiveness against many virus variants, including latent delta variants.3 Sotrovimab aims to bind to the spike protein of SARS-CoV-24 [Table 9].4 This type of antibody was selected for its advantages in inactivating the virus in-vitro, killing infected cells, providing an increased level of resistance barrier, and reaching high concentrations in the lungs [Figure 1]. It has been authorized for emergency use by the USFDA for the treatment of adult and pediatric patients (age 12 years and older, weighing at least 40 kg) with mild to moderate COVID-19 with positive test results and who are at high risk of developing severe COVID-19 (including hospitalization or demise).5 Additionally, the complete COMET [Table 2] clinical development programme for VIR-7831 are [Figure 2]: On 28 July 2021, Vir Biotech and GSK made an announcement that they had signed an agreement with the European Commission for the supply of 220,000 single-dose monoclonal antibodies Sotrovimab. This Mab is used to treat patients with mild to moderate SARS-CoV-2 who are not in need of oxygen supplementation and meanwhile developing high risk. It can treat adults and adolescents (12 years or older with a weight of about 40kg). The Committee for Medicinal Products for Human Use of the European Medicine Agency, pursuant to Article 5 (3) of Regulation 726/2004, gave a positive opinion for Sotrovimab. This drug will be included in a set of EC therapy candidates as part of the COVID-19 treatment plan. The application for sotrovimab is continuously checked by the authorities by the EMA [Table 3]. In June, results of the COMET-ICE phase 3 study showed a 79% reduction in hospitalization and death cases. At the end of day 29, the drug was compared with a placebo, and the primary one reached the endpoint.

Figure 1.
Sotrovimab Antibody History
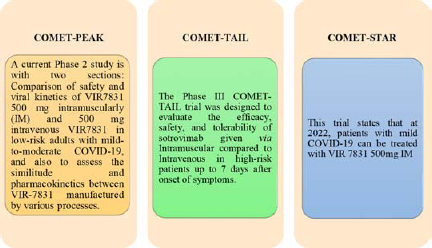
Figure 2.
COMET Trials.
| Generic Name | Sotrovimab |
|---|---|
| Drug bank Accession Number | DB16355 |
| PubChem SID | 434370441 |
| Type | Biotech |
| Groups | Investigational |
| Biologic Classification | Protein Based Therapies-mAb |
| Protein Average Weight | 149000.0 Da |
| Associated Conditions | Mild to Moderate COVID-19 |
| Pharmacodynamics | Neutralizes the spike protein of COVID-19 |
| Absorption | Using the non-comparative analysis method, the mean maximum concentration after one hour of IV sotrovimab infusion was 137 µg/mL, and the mean concentration on day 29 was 34 µg/mL. |
| Volume of Distribution | It is an Fc-enhanced human immunoglobulin G (IgG) and thus has the potential for placental transfer. |
| Half-life | The half-life is longer than that of unmodified Immunoglobulin G due to the variability of LS .6–10 |
| Clinical trial number | Clinical trial design | Website |
|---|---|---|
| NCT04545060 | COMET-ICE | ClinicalTrials.gov |
| NCT04779879 | COMET-PEAK | |
| NCT04501978 | ACTIV-3-TICO | |
| NCT04634409 | BLAZE-4 |
| Drug/Compound | Xevudy (Sotrovimab) |
|---|---|
| API | Sotrovimab/VIR-7831 |
| Formulation | Concentrate solution for infusion |
| ROA | Intravenous infusion |
| Dose | 500 mg (62.5 mg/mL in 8ml of sotrovimab is present in each vial) |
TARGET POPULATION
It is used to treat elders and teens (12 years or older, weighing 40 kg) with COVID-19 who do not require supplemental oxygen and are at risk of developing severe COVID-19.
Risk factors include, but are not limited to:
• Vulnerable population (Geriatrics)
•Obese
•Congestive Heart Failure, including hypertension
•Asthma
•Type 1 and Type 2 diabetes
•Chronic Kidney Disease, including those on dialysis
•Cirrhosis
•Immunosuppression, as assessed by a physician.
Examples: Bone marrow or organ transplantation, immunodeficiency, HIV (if poorly controlled or have signs of AIDS), cancer treatment, sickle cell anemia, thalassemia, and long-term use of drugs that weaken the immune system.
CONDITIONS OF USE
Posology
Dosing recommendations: A single diluted 500 mg intravenous infusion is the recommended dose for the elderly and teens (over 12 years old and weighing at least 40 kg) [Table 4].
| For IV use | • Before administration, dilute the formulation• Sotrovimab has to be administered as a single IV infusion within a period of 30 min.• The patient should be observed for a minimum of one hour throughout and once the completion of infusion.• It should not be administered as a bolus or intravenous bolus injection.• Preparation for administration of the dosage requires sterile technique by qualified healthcare personnel. |
| Preparation for Dilution | • Remove the sotrovimab vial from the refrigerator (2°C to 8°C).• Cool the vial to room temperature for approximately 15 min, protected from light.• Perform Visual inspection on the vial to make sure that it is free of particles and that the vial is not visibly damaged.• If the vial cannot be used, discard it and start over with a new vial.• Before use, shake the vial gently to avoid air bubbles.• Do not shake the vial vigorously. |
| Dilution Instructions | • Draw 8 ml from the infusion bag containing 50 ml or 100 ml of NaCl (0.9%) injection and discard it.• Now inject 8 ml of sotrovimab into the infusion bag through the septum• The vial can only be used for one patient, and discard any unused parts that remain in the vial as this product does not contain a preservative and it is for single-use only.• Gently shake the infusion bag back and forth 3 to 5 times before the start of infusion to avoid the formation of bubbles.• Use the diluted solution immediately unless it is not possible to administer it immediately.• The diluted solution can be stored for up to 4 hr at room temperature (20°C to 25°C) and up to 24 hr in the refrigerator (2°C to 8°C). |
| Administration Instructions | • Connect the infusion set to the infusion bag using a standard measuring tube.• The solution should be administered intravenously using a 0.2 μm inline filter. Fill the infusion set with 9 mg/mL (0.9%) sodium chloride.• It is given intravenously for 30 min at roomtemperature |
Treatment duration and monitoring: Single dose.
The patient should be monitored for a minimum of one hour throughout and once the completion of infusion.
Specific populations:
- Pediatric Use: For patients 12 years of age or older and weighing more than 40 kg, it is not recommended to adjust the dose. There are no data on children under 12 weighing less than 40 kg.
- Geriatric use: The pharmacokinetics in patients 65 years old and above has not been calculated, and also it is considered that the dose adjustment is not necessary.
- Renal impairment: The active substance has not been studied in renal impairment patients, and it does not believe that the dose needs to be adjusted. 11,12
- Hepatic impairment: It is unclear whether patients with hepatic insufficiency need to adjust the dose. Formal studies have not yet been conducted [Table 5].
| Hypersensitivity | In a study of inpatients, allergic reactions were reported after infusion of sotrovimab. If there are any signs and symptoms of a clinically significant hypersensitivity reaction, the administration has to be stopped immediately, and appropriate treatment has to be started. |
| Infusion-related reactions (IRRs) | The COMET-ICE study and the entire ongoing clinical project reported the IRR of Sotrovimab. All IRRs in the COMET-ICE study were mild to moderate. In the event of an IRR, slow or stop the infusion and consider appropriate supportive therapy. |
| Traceability | Record the name and the batch number of the administered drug so as to enhance the traceability of biomedical products. |
Contraindications
- Allergic reaction to the Active Pharmaceutical Ingredient or to any of the excipients
- History of Anaphylactoid to monoclonal antibodies.
Interactions [Table 6]: Drug interaction studies have not been performed with sotrovimab. Sotrovimab is not excreted or metabolized by the kidneys or by cytochrome P450 (CYP) enzymes; therefore, it is not possible to interact with drugs excreted by the kidneys or concomitant drugs of CYP enzyme substrates, inducers, or inhibitors. Co-administration of sotrovimab and the COVID19 vaccine has not been studied.
| Pregnancy | • There are insufficient or limited data on the use of sotrovimab in pregnant women. Animal reproduction toxicity studies have not been performed.• In the Matrix protein cross-linking assay, target binding was not detected due to the abundance of human embryonic proteins.• Sotrovimab is a human IgG, and it can be transferred from the decidua basalis to the villous chorion.• The potential therapeutic benefit or risk of sotrovimab for placental transfer to the developing fetus is unclear.• Sotrovimab should only be used in a physiological condition if the expected benefits to the mother justify the potential risk to the fetus. |
| Lactation | There is no sufficient data on the excretion of sotrovimab in human breast milk; thereby, the risk to newborns/infants cannot be ruled out. It is well known that human IgG is secreted into human breast milk. Therefore, the decision to discontinue breastfeeding or treatment with sotrovimab should be made, taking into account the benefits of breastfeeding for the baby and the benefits of treatment for the woman in mind. |
| Fertility | Nil |
Incompatibilities: This medicinal product should not be mixed or used at the same time as other medicinal products from the same line of business.
Overdose: There is no particular treatment for sotrovimab overdose. If the event occurs, the patient should be treated in a supportive manner, and appropriate monitoring should be carried out if necessary.
Storage conditions: Refrigerate the sealed vials in the original package at 2°C – 8°C (36 ° F – 46 ° F). Do not freeze or shake. Protect from light [Table 7].
| Sealed vials | Diluted solution for administration |
|---|---|
| One year | The diluted solution has to be infused immediately unless it is impossible under the circumstances. If possible, it can be stored for up to 4 hr at room temperature (20°C to 25°C) or up to 24 hr at refrigeration (2°C to 8°C). |
OTHER INFORMATION
Undesirable effects
•Safety profile summary The safety of the active ingredient was determined by a randomized interim analysis placebo-controlled study of 868 out-of-hospital COVID19 patients (COMETICE) [Table 10]. All patients received a single500mg intravenous infusion of sotrovimab or equivalent placebo. Table 1 shows below ≥1% (in any group) of the adverse events reported by COMET- ICE. Two patients experienced treatment interruptions, all due to extravasation at the infusion site; each completed the infusion. The IRRs, which include hypersensitivity reactions, were mild and moderate. The only event in the Sotrovimab group with a frequency of ≥1% was diarrhea (<1% in the placebo group).
During the trial, any hospitalization, which includes those due to the development of COVID-19, were considered a Serious Adverse Event. SAE was reported in 7 in 430 (2%) in the sotrovimab group, where two subjects reported diverticulitis and had a history of diverticulitis and obesity. 26 in 438 (6%) in the placebo group reported serious adverse events in which two or more subjects reported pneumonia and/or dehydration from COVID19. The following unique reports in the Sotrovimab group included:
- Non-small cell lung cancer,
- Cases of intestinal occlusion,
- High blood sugar and diabetes.
Unique reports in the placebo group included:
- Decreased blood volume (hypovolemia),
- Acute respiratory distress syndrome,
- Dyspnea,
- Hypercapnia,
- Pulmonary embolism,
- Respiratory distress,
- Obstructive pancreatitis,
- Acute renal failure.
Researchers believe that there’s a Serious Adverse Event which will be regarding the study drug and it could be a COVID-19 respiratory illness event that occurred within the placebo group.
Reporting of suspected adverse reactions
It is important to report any suspicious side effects once the drug has been approved. This allows you to continuously monitor the balance between drug benefits and risks. Suspected side effects should be reported through the national reporting system listed by healthcare professionals [Table 8].
| Conditions | Sotrovimab 500 mg (n=430) | Placebo (n=438) |
|---|---|---|
| COVID-19 pneumonia | 4 | 14 |
| Headache | 3 | 9 |
| Pneumonia | 0 | 7 |
| Dehydration | 0 | 5 |
| Dyspnea | 2 | 5 |
| Nausea | 4 | 5 |
| Diarrhea | 6 | 3 |
| MOA | Sotrovimab (recombinant human IgG1) binds to the highly conserved epitope of the receptor-binding region (RBD) of the mutant protein (S) of SARS-CoV-2 with high affinity (Kd = 0.21 nM) but does not compete for human angiotensin-converting binding to enzyme receptor 2. There include long-lasting M428L and N434S (LS-transformed) amino acid substitutions of sotrovimab. |
| Antiviral activity | Sotrovimab has proven its antiviral activity, i.e., it neutralized SARS-CoV-2 virus in vitro (EC50 100.1 ng/ml) and in vivo(≥5 mg/kg in SARS-CoV-2 infected hamsters) and also successfully deactivated the pseudotyped virus containingSARS-CoV-2 Spike. |
| Randomly selected participants | 46% are men |
| The median age of the randomized general population | 53 years |
| Total Participants | 65 years or elder – 22% above 70 years old – 11%Whites – 87%Black or African American – 7% Asian – 7% |
| Race | Hispanic or Latino (63%) |
Summary of Clinical Features
• Clinical efficacy
COMET-ICE is a randomized, double-blind, placebo-controlled phase II / III study evaluating sotrovimab as a treatment for COVID19 in outpatients at high risk for disease complications. Enrolled patients are 18 years or older and have at least one of the following comorbidities: Diabetes, Obesity (BMI>30), Chronic renal disease, congestive cardiac failure, COPD, or moderate to severe asthma over 55 years and older. The trial includes a few criteria to participation:
Patients with symptoms ≤ 5 days,
Ambient air oxygen saturation has to be ≥ 94% and SARS-CoV-2 infection.
The patients are administered with single dose IV infusion 500mg of sotrovimab (N = 291) or placebo (n = 292) for 1 hr. Patients who require supplemental oxygen or hospitalization were excluded from the trial The participants (58%) received sotrovimab or placebo within three days of the onset of COVID19 symptoms, and 42% received treatment within 4-5 days. The baseline disease and demographic characteristics were well balanced between the treatment groups.13
CONCLUSION
Currently, Phase 3 study is going on in COMET-ICE Trial. When the study is complete, as expected, sotrovimab will be launched in the first half of 2022. It has been proven that Sotrovimab has been shown to be effective in reducing risk compared to placebo in approximately 85% of patients admitted in the early stages of COVID-19 or who died in high-risk outpatients, supported by interim results from phase 3 COMET-ICE. This collection of data helps to analyze the harmonized scientific opinion at the EU level to support national decision-making on the possible use of the antibody prior to the MA. The clinical impact of these in vitro variant data was not yet known, including the variant from India, Brazil, California, New York, South Africa, and the UK.
Cite this article
Sridevi MG, Shoba E, Sathyalakshmi R, Saba M. Current approach on COVID-19 for Emergency Use Authorization in Europe: Sotrovimab. J Young Pharm. 2022;14(4):372-6.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020;10(1):40 [PubMed] | [CrossRef] | [Google Scholar]
- SARS-CoV-2. National Cancer Institute, National Institutes of Health. [[cited Dec 25 2021]];Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/sars-cov-2 [PubMed] | [CrossRef] | [Google Scholar]
- [[cited on Nov 15 2021]];GSK and Vir, navigating early antibody pitfalls, tout delta variant-busting data for latecomer sotrovimab, Fierce Pharma. 2021;06 Available from: https://www.fiercepharma.com/pharma/gsk-and-vir-tune-their-sotrovimab-pitch-heels-delta-busting-variant-data [PubMed] | [CrossRef] | [Google Scholar]
- [Array];Sotrovimab for Injection (500 mg/8 mL) [Home Page on the Internet]. Gsk. Available from: https://www.sotrovimab.com/ [PubMed] | [CrossRef] | [Google Scholar]
- [Array];Sotrovimab. (Xevudy) antibody [Home page on the Internet]. Precision Vax LLC. Available from: https://www.precisionvaccinations.com/vaccines/sotrovimab-vir-7831-antibody [PubMed] | [CrossRef] | [Google Scholar]
- [Array];Sotrovimab. [Home page on the Internet]. Drug Bank Online. Available from: https://go.drugbank.com/drugs/DB16355 [PubMed] | [CrossRef] | [Google Scholar]
- [[cited Jul 4 2022]];PubChem [internet]. (Us) Bethesda: National Library of Medicine. PubChem Substance Record for SID 434370441, 1MTK0BPN8V, Source: ChemIDplus. National Center for Biotechnology Information. 2004 Available from: https://pubchem.ncbi.nlm.nih.gov/substance/434370441 [PubMed] | [CrossRef] | [Google Scholar]
- Ning L, Abagna HB, Jiang Q, Liu S, Huang J. Development and application of therapeutic antibodies against COVID-19. Int J Biol Sci. 2021;17(6):1486-96. [PubMed] | [CrossRef] | [Google Scholar]
- Tuccori M, Ferraro S, Convertino I, Cappello E, Valdiserra G, Blandizzi C, et al. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline. InMAbs. 2020 Taylor and Francis [PubMed] | [CrossRef] | [Google Scholar]
- Owji H, Negahdaripour M, Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. Int Immunopharmacol. 2020;88:106924 [PubMed] | [CrossRef] | [Google Scholar]
- [[cited on Mar 30 2022]];Coronavirus disease. Center for Disease Control and Prevention. [updated 2022 Mar 24 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) [ homepage on the Internet]Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html#print [PubMed] | [CrossRef] | [Google Scholar]
- [[cited on Mar 22 2022]];Provisional COVID-19 deaths by race and Hispanic origin, and age. [ homepage on the Internet]. Center for Disease Control and Prevention. 2022 updatedAvailable from: https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-by-Race-and-Hispanic-O/ks3g-spdg [PubMed] | [CrossRef] | [Google Scholar]
- European Medicine Agency. [[cited 12/9/2022]];Conditions of use, conditions for distribution and patients targeted and conditions for safety monitoring addressed to the member states for remdesivir available for compassionate use. European Medicine Agency. Available from: https://www.ema.europa.eu/en/documents/referral/sotrovimab-also-known-vir-7831-gsk4182136-covid19-article-53-procedure-conditions-use-conditions_en.pdf [PubMed] | [CrossRef] | [Google Scholar]
