ABSTRACT
Background: Type 2 diabetes is a metabolic disorder that requires continuous pharmacological treatment for its management. Due to the complexity of the treatment and increased risk of adverse reactions to the existing treatment, there is a need to demonstrate the efficacy and safety of new medications. Therefore, this study was conducted to identify the efficacy and safety of Hydroxychloroquine in people with type 2 diabetes. Materials and Methods: The Population, Intervention, Comparator, Outcome format was utilised to assess the inclusion criteria of randomized controlled trials. A total of five scientific databases were searched with the designed search terms. All the studies included were assessed for their risk of bias and a meta-analysis was performed by using RevMan. Results: A total of six randomized controlled trials were included in this systematic review. The majority of studies included hydroxychloroquine as an add on treatment for the existing treatment regimen. From the study results, it was found that hydroxychloroquine group was equally effective in lowering blood glucose levels when compared with other treatment groups. Also, it was found that there was a significant reduction in the daily insulin requirement in the hydroxychloroquine treatment group. Despite the reduction in lipid profile in hydroxychloroquine group, there was no significant reduction in body weight observed between hydroxychloroquine and non-hydroxychloroquine groups. Lastly, hydroxychloroquine was well tolerated in people living with type 2 diabetes with less severe adverse effects. Conclusion: Overall, hydroxychloroquine group was found to be safe and equally effective in reducing blood glucose levels when compared with other anti-diabetes medications groups.
INTRODUCTION
Diabetes is the most common and chronic metabolic disorder that occurs when there is a lack of production of insulin or insufficient production of insulin in the body.1 Type 2 diabetes (T2D) is the most common type that accounts for 90% of the world’s diabetes population.2
According to International Diabetes Federation, it is estimated that the number of people living with diabetes worldwide may increase from 537 million in 2021 to 783 million by the year 2045.1 T2D requires continuous pharmacological and non-pharmacological treatment such as exercise, weight reduction and dietary modification for its management.2 Despite the availability of multiple medications and treatment options, there are limitations such as adverse effects of anti-diabetes medications and the increase in the treatment cost due to use of high-cost medications which affected the adherence level to anti-diabetes medications and diabetes management.3–5 Also, literature suggests that T2D is an inflammatory disease due to the activation of interleukins.6 But the effect of current anti-diabetes medications as an anti-inflammatory is unknown.7 Taking into account all these factors it is suggested that there is a need for identifying new medications in the treatment of diabetes.8
Hydroxychloroquine (HCQ) is an anti-inflammatory medication used in the treatment of uncomplicated malaria and autoimmune disorders.9 Recent studies have proven that HCQ has anti-diabetic properties and may also help in reduction of the incidence of diabetes.7,10 However, the mechanism of HCQ by which it alters the blood glucose levels is not clear and the long term effects of use of hydroxychloroquine in people with diabetes are still unknown.11 Moreover, no systematic review was conducted till date to assess the efficacy and safety of HCQ over other anti-diabetes medications. Therefore, this systematic review and meta-analysis was conducted to find the efficacy and safety of HCQ in T2D.
MATERIALS AND METHODS
This systematic review adopted PICO (Population, Intervention, Comparator, Outcome) format for inclusion of studies. The details of PICO for the randomized controlled trials (RCTs) have been presented in Table 1. A comprehensive literature search was made using the search terms in electronic databases CINAHL, Embase, PubMed, ProQuest Central and Scopus. The search was made from inception to till December 2021 and was limited to humans. Two authors (RKP and KU) finalized the search terms based on the existing literature. The search terms included in this study were “Hydroxychloroquine” OR HCQ AND “Diabetes OR Diabetes Mellitus OR Type 2 Diabetes”. To broaden the search, these terms were combined with Boolean operators and MeSH terms. Also, manual search of the references of included studies and review articles was performed to identify the additional studies. Only those studies which identified the effect of HCQ in people living with T2D were included.
| Population | Patients of any gender living with type 2 diabetes with or without comorbidities |
| Intervention | Hydroxychloroquine as a monotherapy or in combination with other oral hypoglycemic medications and/or insulin |
| Comparator | Other oral anti-diabetes medications and/or insulin and/or placebo. |
| Outcome | Changes in the glycemic parameters such as HbA1c, CBG, RBS, FBS, daily insulin requirements and adverse drug reactions |
All the studies obtained after using the search terms were imported into Covidence12 to perform the screening. Two reviewers (RKP and PA) independently conducted the title, abstract and full-text screening of studies. Any conflicts regarding the inclusion of studies and/or inclusion of data were resolved upon consensus between authors (Figure 1
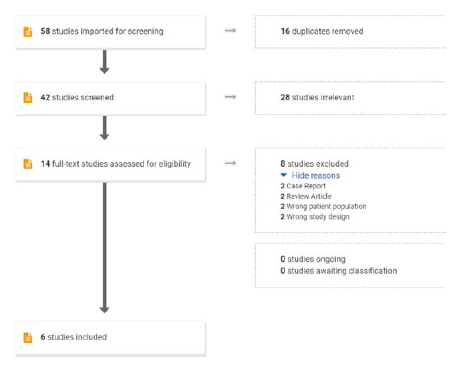
Figure 1.
Flow chart of screening process.
Data Extraction
The data extraction of the included studies was performed by two individual reviewers (RKP and PA). The data extraction was performed in a Microsoft excel sheet which consisted of all details of the studies including the participant characteristics such as age, gender, occupation and education, changes in blood glucose parameters such as HbA1c (Glycated haemoglobin), CBG (Capillary Blood Glucose), RBS (Random Blood Sugar), FBG (Fasting Blood Sugar), daily insulin requirements and safety profile of HCQ which includes adverse drug reactions. After completion of the data extraction, the data was reviewed by KU and MG for maintaining the uniformity of the data.
Risk of Bias (RoB)
RKP and SPB independently performed the Risk of Bias assessment by using Cochrane RoB tool for Randomized Controlled Trials. The tool consists of five domains selection, performance, attrition, reporting and others. The studies were segregated based on their level of risk of bias such as High, Low and Unclear. The Review Manager 5.413 was used to generate RoB summary (Figure 2) and graph (Figure 3).
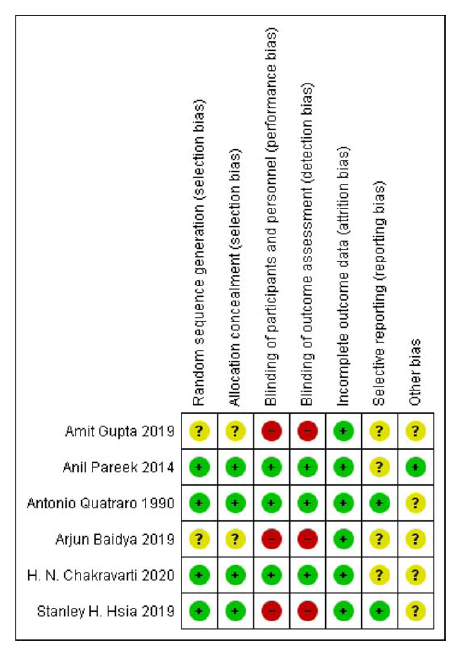
Figure 2.
Risk of bias summary.
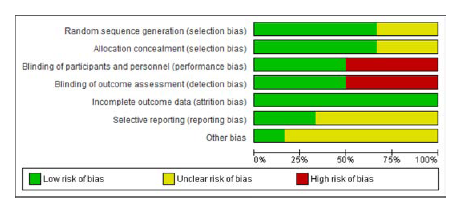
Figure 3.
Risk of bias graph.
Data Analysis
Review Manager Software (RevMan 5.4.1) was used to perform the meta-analysis. The findings were reported by constructing the forest plot. Those studies with summarised domains along with standard deviation are included for meta-analysis. I2 was done to estimate the heterogeneity between the included studies. The fixed-effect model was used for studies without heterogeneity, while the random-effect was incorporated for studies with heterogeneity.
RESULTS
Description of Studies
The literature search results are summarized in Figure 1. After implying the inclusion and exclusion criteria, a total of six RCTs 8,14–18
were included in this study. Among these six RCTs, HCQ was given as an additive drug to patients receiving various combinations of anti-diabetes medications. HCQ was added to a combinational therapy of Sulfonylurea and Metformin in three studies.8,14,15 Combination therapy of DPP IV inhibitor (Vildagliptin) and Metformin in one study,16 combination therapy of Insulin with Metformin and DPP IV inhibitor (Sitagliptin) in one study,17 Insulin and Sulfonylurea (Glibenclamide) monotherapy in one study.18 Again, among these six RCTs, the additive anti-diabetes effect of HCQ was compared against placebo in three studies,14,17,18 against Pioglitazone in two studies8, 15 and against Canagliflozin in one study.16 The chief characteristics of these studies were given in Table 2 and in the supplementary material.
| S. No | Name of the author (Year Published) | Number of Participants | Duration of study | Existing Therapy | Add on Therapy | |
|---|---|---|---|---|---|---|
| Test | Comparator | |||||
| 1 | Amit Gupta (2019) | 87 | 6 Months | Vildagliptin with Metformin | HCQ | Canagliflozin |
| 2 | Arjun Baidya (2019) | 49 | 6 Months | Insulin with Sitagliptin and Metformin | HCQ | Insulin with Metformin and Sitagliptin |
| 3 | Anil Pareek (2014) | 267 | 6 Months | Glimepiride with Metformin | HCQ | Pioglitazone |
| 4 | H N Chakravarthy (2020) | 326 | 3 Months | Glimepiride with Metformin | HCQ | Placebo |
| 5 | Antonio Quatraro (1990) | 48 | 6 Months | Insulin and Glibenclamide | HCQ | Placebo |
| 6 | Stanley H. Hsia (2019) | 22 | 4 Months | Sulfonyl Urea with Metformin | HCQ | Pioglitazone |
Efficacy of HCQ in Combination with Sulfonylurea and Metformin
Three8,14,15 RCTs evaluated the efficacy of HCQ in reducing glycemic parameters when added to a combination of Sulfonylurea and Metformin. Among these, two studies 8,15 were compared against Pioglitazone as a comparator while one study14 was compared against placebo.
HCQ at doses 200mg, 300mg and 400mg OD in combination with Glimepiride-4mg and Metformin-500mg were reported in comparison with placebo, where HCQ resulted in significant improvement in glycemic parameters such as HbA1c, FPG and PPG. The percentage change in HbA1c from baseline to 12 weeks for different doses of HCQ was -1.20% (-1.15 to -1.25) (p<0.001) for 400 mg and -0.91% (-0.86 to -0.96) (P<0.001) and -0.78% (-0.73 to -0.83) (p<0.005) for 300mg and 200mg respectively.14 Similar observations were also seen in FPG and PPG levels, where 400mg dose of HCQ showed greater reduction (Supplementary material).
HCQ at a dose of 400mg OD was compared with different doses of Pioglitazone (15mg and 45mg OD) as an add on therapy to patients who have been receiving Sulfonylurea and Metformin combination.8,15
There was a significant reduction of -0.85% (-0.54 to -1.19) (p<0.0001) in HCQ group and -0.94% (-0.57 to -1.22) (p<0.0001) in Pioglitazone 15mg group.8 In comparison with 45mg Pioglitazone15, both HCQ and Pioglitazone had a significant reduction in HbA1c (-1.2%; -0.3 to -2.1 and -2.8%; -2.6 to -3.0 respectively) but Pioglitazone was found to be superior over HCQ (p< 0.0001). However, a combined analysis of these both studies showed that Pioglitazone was not superior to HCQ in reducing HbA1c values as an add on to the existing Sulfonylurea and Metformin therapy (Figure 4).
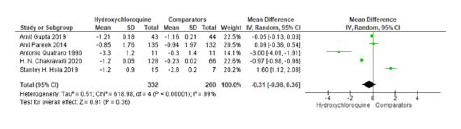
Figure 4.
Meta-analysis of studies included changes in HbA1c between HCQ and Non-HCQ groups.
The decrease in FBG by HCQ 400mg was -0.79 (-1.50 to -0.08) (P=0.008) versus -1.02 (-1.73 to -0.32) (p=0.046) by Pioglitazone 15mg and the decrease in FBG caused by Pioglitazone was not significant compared to HCQ (P=0.648).8 Similar findings were also observed when 45 mg of Pioglitazone was compared with HCQ. FBG decreased from 179 ± 53 mg/ dl to 147 ± 33 mg/dl (p=0.01) in HCQ 400mg group while it decreased from 170 ± 50 mg/dl to 105 ± 21 mg/dl (p<0.0001) in Pioglitazone 45mg group.15 This decrease in FBG between Pioglitazone 45mg and HCQ 400mg was again insignificant (P = 0.11). All these findings in terms of HBA1c and FBG advocate that clinical significance of HCQ in lowering glycemic parameters and is not inferior to Pioglitazone (Figure 5).
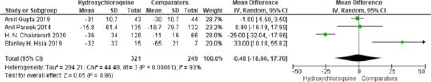
Figure 5.
Meta-analysis of studies included changes in FBG between HCQ and Non-HCQ groups.
Efficacy of HCQ in Combination with Vildagliptin and Metformin
A RCT of HCQ at a dose of 400mg OD in combination with Vildagliptin 100mg and Metformin 2000mg/day was carried out for 24 weeks.16 Canagliflozin at a daily dose of 300mg was added to Vildagliptin and Metformin in another group of patients. Despite significant reduction in mean HbA1c levels 7.11 ± 0.18% (P= 0.001) and 7.44 ± 0.21% (P=0.001) in HCQ and Canagliflozin groups respectively, the difference between groups was not significant (P=0.29).16 Similar findings were observed in FBG levels where it was decreased from 143±12.8 mg/dl to 112±10.7 mg/dl (P=0.001) in HCQ group and 147 ± 13.2 mg/dl to 117 ± 10.7 mg/dl (P=0.001) in Canagliflozin group. These findings advocate the anti-diabetes effect of HCQ at par with Canagliflozin in patients whose blood sugar levels were poorly controlled with Vildagliptin and Metformin.
Efficacy of HCQ in Combination with Insulin and Oral Hypoglycemics
A clinical study reported the clinical efficacy of HCQ in combination with basal insulin and two oral hypoglycemics, Sitagliptin 100mg and Metformin 1000mg.17 It was found that HbA1c was decreased by 1.1% from 8.5 ± 0.5 to 7.4 ± 0.5 (P<0.0001) after 12 weeks and reduced further to 6.9 ± 0.5 % (P<0.0001) after 24 weeks of adding HCQ. There was also a significant reduction in daily basal insulin dose (39.4 ± 8.5 to 29.5 ± 4.5 units/day).
Efficacy of HCQ with Insulin and Glibenclamide
A randomized double-blind efficacy study of HCQ in combination with Insulin reported a significant reduction in HbA1c levels after 6 months.18
HbA1c decreased by -3.3 % (-3.6 to -3) in patients receiving HCQ along with Insulin. HCQ also significantly reduced the daily Insulin requirement by -24 units (-30 to -17.9).
Effect of HCQ on Body Weight
Three studies14,15,17 evaluated the effect of HCQ on the body weight of patients taking anti-diabetes management.
Significant reduction in body weight was observed when HCQ was added to Insulin when given in combination with oral Hypoglycemics (Sitagliptin and Metformin), there was a reduction in mean body weight of sample population from 72.5 ± 6.2 kg to 68.7 ± 7.4 kg (P=0.011).17
However, no significant reduction in body weight was observed when HCQ was added to oral hypoglycemic drug combination of Sulfonylurea and Metformin.14,15 However, in a clinical study,19 where HCQ has compared with Pioglitazone 45mg as an add on to existing Sulfonylurea and Metformin combination, patients who were on Pioglitazone had a significant increase in their bodyweight from 83.7 ± 18.2kg to
87.0 ± 17.9; P=0.02 while patients receiving HCQ had no significant effect on their body weight (85.7 ± 14.3 kg to 85.4 ± 14.5 kg, P= 0.76). This difference between HCQ and Pioglitazone was found to be significant (P=0.004).
Effect of HCQ on Lipid Profile
Two clinical studies8,15 reported the effect of HCQ versus Pioglitazone when added to Sulfonylurea and Metformin combination. When compared to 15mg OD of Pioglitazone, the HCQ 400mg OD group shown reduction in triglycerides (TG), total cholesterol (TC) and low-density lipoprotein (LDL) levels. After 24 weeks of therapy, there was a reduction of -0.37 mmol/L (-0.55 to -0.19; P=0.004) in TC, -0.26 mmol/L (-0.44 to -0.12; P=0.011) in TG and -0.2 mmol/L ( -0.38 to -0.08; P=0.031) in LDL in HCQ group when compared the reduction of 0.03 ( -0.15 to 0.20; P=0.88) in TC, -0.29 ( -0.45 to -0.13; P=0.008) in TG and 0.09 (-0.06 to 0.24; P=0.47) in LDL with Pioglitazone.8 These findings advocate the superiority of HCQ 400mg OD over Pioglitazone 15mg OD in reducing the lipid profile, especially total cholesterol and low-density lipoprotein levels. Similar results were not obtained when 45mg of Pioglitazone was compared with 400mg of HCQ, both added to Sulfonylurea and Metformin combination.15 Here, both Pioglitazone and HCQ significantly reduced total cholesterol levels after 4 months of therapy. Total cholesterol levels decreased from 170.2 ± 32.6 mg/dl to 141.4 ± 27.8 mg/dl (P=0.02) in HCQ group and in pioglitazone group, it reduced from 149.3 ± 30.1 mg/dl to 130.9 ± 26.8 mg/dl (P=0.02). However, the differences between these two groups was not statistically significant (P=0.31).15
HCQ when added to insulin and anti-diabetes medications combination also had a significant effect in lowering TC (215 ± 41mg/dl to 191 ± 37 mg/dl), TG levels (218 ± 143 to 150 ± 97 mg/dl) and LDL levels (131 ± 17 to 114 ± 9 mg/dl).17
Safety of HCQ in Patients with Diabetes
Of all included studies, six studies mentioned adverse effects of HCQ.8,14-18
The most commonly reported adverse effect among these studies was hypoglycemia followed by gastrointestinal system disorders such as diarrhoea. However, the number of hypoglycemia episodes in the HCQ group was less when compared with their comparators. Overall, HCQ was found to be safe and well-tolerated.
DISCUSSION
Diabetes is a chronic disease that requires multiple medications for its management and reduction of long term complications.20 The goal in the treatment of diabetes is maintaining the blood glucose levels within the normal limits and prevention of macro and micro vascular complications.20 This systematic review of six RCTs advocates the efficacy and safety of HCQ in patients with inadequate glycemic control. This is an encouraging finding as it highlights the role of anti-inflammatory agent in the treatment of T2D.
The primary treatment option for the treatment of T2D is Metformin alone or in combination with sulfonylureas such as Glimepiride, Glipizide, Gliclazide or Dipeptidyl peptidase-4 inhibitors such as Sitagliptin, Linagliptin, Vildagliptin. However, recent studies show that nearly 15% of people living with T2D are contraindicated or not tolerated to Metformin based treatment regimen.21 Moreover, despite proven safety nature of insulin, majority of people living with T2D are reluctant to receive insulin thinking of it as a last option in the treatment regimen.22
Recent studies found that despite the reduction of blood glucose levels by increasing insulin sensitivity, biguanides and thiazolidinediones may not improve the beta cell function. Moreover, thiazolidinediones may increase the risk of cardiac problems and fractures.23–25 In addition, diabetes is often characterised by micro and macro vascular complications due to raise in the inflammatory markers such as TNF alpha, Interleukin 1 and 6. Studies found that HCQ lowers these inflammatory markers and also reduced the adipocyte and islet inflammation there by reducing the insulin resistance.14 Hydroxychloroquine was approved by the Central Drugs Standard Control Organisation in 2014 for its use as an adjunct for the treatment of T2D.26 Studies found that HCQ exhibits glucose lowering effect by enhancing the sensitivity of cells to insulin and glucose tolerance by reducing the insulin clearance.27
From the results of this study, it was found that higher doses of HCQ had reduced the glucose levels and body weight when compared with other oral anti-diabetes medications. In a study where HCQ was compared with pioglitazone as an add on to existing Sulfonylurea plus Metformin therapy, HCQ was equivalent to Pioglitazone in reducing the glycemic parameters (HbA1c, FBG) and was superior to Pioglitazone in reducing the lipid profile (TC, TG & LDL) of the patients.15 Similar findings were observed in a study where HCQ significantly lowered total cholesterol (0.18 mmol/L, 95% CI: -0.28 to -0.08), triglycerides (-0.09 mmol/L, 95% CI: -0.15 to -0.04) and LDL levels (-0.21 mmol/L, 95% CI: -0.36 to -0.06).28
From this systematic review, it was found that HCQ was well tolerated by people living with T2D. Also, all the studies in this review found fewer hypoglycemic events when compared with other groups. Recent studies show that people on HCQ had less frequently experienced mild to moderate hypoglycemia.29 It was found that the majority of adverse reactions were related to the gastrointestinal, cutaneous, ophthalmic and cardiac systems.30 All these findings promote the use of HCQ as an effective add on therapy in patients with poor (or) inadequate glycemic control with one or more anti-diabetes medications.
The inclusion of multi-centred studies in this systematic review enhanced the trustworthiness of the data analysis. The majority of RCTs included in this review have added on anti-diabetes medications this we believe is a limitation for not being able to compare the efficacy of the HCQ monotherapy over other oral anti-diabetes medications. Furthermore, due to the current increase in global COVID-19 cases, there are various studies conducted to observe the effect of HCQ in COVID-19 with diabetes. Therefore, we suggest further studies be conducted to observe the efficacy and safety of HCQ in COVID-19 with Diabetes.
CONCLUSION
This study provided insights into the efficacy and safety of HCQ in people living with T2D. It was found that there are fewer adverse effects observed in patients with HCQ when compared with other oral anti-diabetes medications. Overall, it was found that HCQ showed a beneficial effect on people with T2D on various glycemic parameters.
Cite this article
Puvvada RK, Adusumilli P, Maddukuri RK, Samaksha PB, Annam MG, Undela K. Efficacy and Safety of Hydroxychloroquine in the Treatment of Type-2 Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J Young Pharm. 2022;14(4):402-7.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- International Diabetes Federation. [[accessed Apr 08 2022].];IDF diabetes. Atlas Press. 2021 Available from: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf, [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. [[cited Sep 03 2020]];What is diabetes. Available from: https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes [Google Scholar]
- Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354-95. [PubMed] | [CrossRef] | [Google Scholar]
- Xu Y, Yang Z, Lin H, Shen P, Wang H, Zhan S, et al. Long-term patterns of antidiabetic medication use in patients with Type 2 diabetes. Med Sci Monit. 2018;24:8707-15. [PubMed] | [CrossRef] | [Google Scholar]
- Aminde LN, Tindong M, Ngwasiri CA, Aminde JA, Njim T, Fondong AA, et al. Adherence to antidiabetic medication and factors associated with non-adherence among patients with type-2 diabetes mellitus in two regional hospitals in Cameroon. BMC Endocr Disord. 2019;19(1):35 [PubMed] | [CrossRef] | [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98-107. [PubMed] | [CrossRef] | [Google Scholar]
- Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory Agents in the Treatment of Diabetes and Its Vascular Complications. Diabetes Care. 2016;39(Suppl 2):S244-52. [PubMed] | [CrossRef] | [Google Scholar]
- Pareek A, Chandurkar N, Thomas N, Viswanathan V, Deshpande A, Gupta OP, et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: a double blind, randomized comparison with pioglitazone. Curr Med Res Opin. 2014;30(7):1257-66. [PubMed] | [CrossRef] | [Google Scholar]
- Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. 2020;12(8):e12476 [PubMed] | [CrossRef] | [Google Scholar]
- Chen YM, Lin CH, Lan TH, Chen HH, Chang SN, Chen YH, et al. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: a population-based cohort study. Rheumatology (Oxford). 2015;54(7):1244-9. [PubMed] | [CrossRef] | [Google Scholar]
- Wondafrash DZ, Desalegn TZ, Yimer EM, Tsige AG, Adamu BA, Zewdie KA, et al. Potential effect of hydroxychloroquine in diabetes mellitus: A systematic review on preclinical and clinical trial studies. J Diabetes Res. 2020;2020:5214751 [PubMed] | [CrossRef] | [Google Scholar]
- Cochrane. [[cited 10/9/2022]];Review Manager. 2020 Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman [PubMed] | [CrossRef] | [Google Scholar]
- Chakravarti HN, Nag A. Efficacy and safety of hydroxychloroquine as add-on therapy in uncontrolled type 2 diabetes patients who were using two oral antidiabetic drugs. J Endocrinol Invest. 2021;44(3):481-92. [PubMed] | [CrossRef] | [Google Scholar]
- Hsia SH, Duran P, Lee ML, Davidson MB. Randomized controlled trial comparing hydroxychloroquine with pioglitazone as third-line agents in type 2 diabetic patients failing metformin plus a sulfonylurea: A pilot study. J Diabetes. 2020;12(1):91-4. [PubMed] | [CrossRef] | [Google Scholar]
- Gupta A, Ahmed R, Singh SP, Kumar M, Chandra A, Kumar A, et al. Abstract #243 A Real World Observational Comparative Study for the Efficacy and Tolerability of Hydroxychloroquine Vs Canagliflozin in Indian Type 2 Diabetes Patients Having Inadequet Glycemic Control on Vildagliptin Plus Metformin. Endocr Pract. 2019;25:89-90. [CrossRef] | [Google Scholar]
- Arjun B, Ajay C. Efficacy of hydroxychloroquine when added in subjects with inadequately controlled type 2 diabetes mellitus treated with basal insulin and two other oral antidiabetic drugs. Endocr Pract. 2019;25:119-20. [CrossRef] | [Google Scholar]
- Quatraro A, Consoli G, Magno M, Caretta F, Nardozza A, Ceriello A, et al. Hydroxychloroquine in decompensated, treatment-refractory noninsulin-dependent diabetes mellitus. A new job for an old drug? Ann Intern Med. 1990;112(9):678-81. [PubMed] | [CrossRef] | [Google Scholar]
- Hsia SH, Duran P, Davidson MB. A Pilot, Head-to-head comparison of hydroxychloroquine (HCQ) with pioglitazone (PIO) as third-line agents in Type 2 diabetic patients failing metformin and a sulfonylurea (SU). Diabetes. 2018;67((Supplement_1)):1172-P [CrossRef] | [Google Scholar]
- Miccoli R, Penno G, Del Prato S. Multidrug treatment of type 2 diabetes: a challenge for compliance. Diabetes Care. 2011;34(Suppl 2):S231-5. [PubMed] | [CrossRef] | [Google Scholar]
- National Institute of Health and Care Excellence guidelines. [[accessed Apr 08 2022].];Type 2 diabetes in adults: management. 2015 Available from: https://www.nice.org.uk/guidance/ng28/chapter/Recommendations#first-line-drug-treatment, [PubMed] | [CrossRef] | [Google Scholar]
- Stuckey H, Fisher L, Polonsky WH, Hessler D, Snoek FJ, Tang TS, et al. Key factors for overcoming psychological insulin resistance: an examination of patient perspectives through content analysis. BMJ Open Diabetes Res Care. 2019;7(1):e000723 [PubMed] | [CrossRef] | [Google Scholar]
- Kumar A, Prakash AS. Effectiveness and Safety of hydroxychloroquine compared to Teneligliptin in uncontrolled T2DM patients as add-on Therapy. J ASEAN Fed Endocr Soc. 2019;34(1):87-91. [PubMed] | [CrossRef] | [Google Scholar]
- Detert J, Klaus P, Listing J, Höhne-Zimmer V, Braun T, Wassenberg S, et al. Hydroxychloroquine in patients with inflammatory and erosive osteoarthritis of the hands (OA TREAT): study protocol for a randomized controlled trial. Trials. 2014;15(412-) [PubMed] | [CrossRef] | [Google Scholar]
- Hage MP, Al-Badri MR, Azar ST. A favorable effect of hydroxychloroquine on glucose and lipid metabolism beyond its anti-inflammatory role. Ther Adv Endocrinol Metab. 2014;5(4):77-85. [PubMed] | [CrossRef] | [Google Scholar]
- Central Drugs Standard Control Organisation. [[cited 10/9/2022]];New drugs approved by CDSCO. 2014 Available from: https://cdscoonline.gov.in/CDSCO/Drugs [PubMed] | [CrossRef] | [Google Scholar]
- Gerstein HC, Thorpe KE, Taylor DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas—a randomized trial. Diabetes Res Clin Pract. 2002;55(3):209-19. [PubMed] | [CrossRef] | [Google Scholar]
- Simental-Mendía LE, Simental-Mendía M, Sánchez-García A, Linden-Torres E. Effect of hydroxychloroquine on Lipid Levels: A Systematic Review and metaanalysis. Curr Pharm Des. 2021;27(40):4133-9. [PubMed] | [CrossRef] | [Google Scholar]
- Faruqui AR, Xavier D, Kamat SK, Chandy SJ, Medhi B, Tripathi RK, et al. Safety of hydroxychloroquine in healthcare workers for COVID-19 prophylaxis. Indian J Med Res. 2021;153(1 & 2):219-26. [PubMed] | [CrossRef] | [Google Scholar]
- Chen C, Pan K, Wu B, Li X, Chen Z, Xu Q, et al. Safety of hydroxychloroquine in COVID-19 and other diseases: a systematic review and meta-analysis of 53 randomized trials. Eur J Clin Pharmacol. 2021;77(1):13-24. [PubMed] | [CrossRef] | [Google Scholar]
