ABSTRACT
Background: The management of a disease or a health condition often requires more than one drug. Fixed Dose Combinations (FDCs) are pharmaceutical products containing two or more drugs in a single formulation. Fixed dose combinations have the advantage compared to single compound taken separately with respect to efficacy, safety and convenience but irrational combinations result in reduced efficacy and a high risk of adverse drug reactions. Pain is a common health concern affecting the general population and paracetamol is commonly used in the management of pain. This observational study was designed to evaluate the FDCs containing paracetamol in India. Materials and Methods: The brands of paracetamol FDCs marketed in India were selected from different printed and digital databases and analysed with the help of the Government of India websites and a scoring rubric. Results: The total FDC combinations containing paracetamol were 46. The class of drugs which were commonly combined with paracetamol were NSAIDs and opioid analgesics. More than 50% of paracetamol containing FDCs were found to be rational. Only 33% of paracetamol FDCs were approved by the Central Drug Standard Control Organisation (CDSCO) of India. Conclusion: The health care policymakers should take appropriate action on these irrational FDCs available in the market to achieve better safety and efficacy.
INTRODUCTION
The management of any ailment requires one or more drugs. In many cases, two or more drugs can be given as a single formulation in a fixed ratio, called Fixed Dose Combination (FDC).1 Fixed dose combination products are approved if the combination has proven efficacy and safety compared to the single compound taken separately. FDCs have high demand in the Indian pharmaceutical market.2 Fixed drug combinations are preferred if they have proven advantages like synergistic action and increased efficacy (e.g., amphotericin B and flucytosine), improved safety (e.g. levodopa with carbidopa, thiazides with potassium-sparing diuretics), better compliance with reduced pill burden (e.g. anti-tubercular drug combinations; amlodipine/ losartan/rosuvastatin 5/100/20 for patients with hypertension and hyperlipidaemia), and reduced cost of the drug. But FDCs cannot be formulated with a random drug combination, for there should be compatibility in pharmacokinetics, the combination must be safe and cheaper compared to individual drugs.1,3,4,5 Pain is a health concern affecting the general population. The aetiology of pain is diverse. The adequate management of pain improves the quality of life and increases an individual’s productivity. Inadequately managed acute pain adversely affects the quality of life and imposes a significant economic burden.6 Paracetamol, a para-amino phenol derivative, also known as acetaminophen, is not considered as NSAID (Non-Steroidal Anti-inflammatory Drug) medication due its weak anti-inflammatory action, is very commonly used in the management of pain.7 In many instances, analgesic drugs of different classes are combined with paracetamol to achieve improved analgesic action and reduce the side effects from higher doses of individual drugs.6 In India, many combinations of paracetamol are available, as over-the-counter products. But in many cases, the combination may not be necessary for patients and may pose a danger of adverse drug reactions.2 Hence this study was designed to investigate the presence of irrational combinations of paracetamol available in the market.
MATERIALS AND METHODS
This study was an observational study to assess the FDCs of paracetamol in Indian market. The brands of paracetamol FDCs marketed in India were selected from different printed and digital databases and analysed using the Central Drug Standard Control Organisation (CDSCO) website, Drugs Controller General of India (DCGI) list of approved FDCs, WHO-Essential Medicine List (EML), National List of Essential Medicines (NLEM).8–10 The available FDCs were scored using the scoring rubric for rationality as represented in Table 1.11
| Items | Assessment Criteria | Scoring |
|---|---|---|
| Registration Status of APIs | CDSCO drug registration database was checked | Depending on the number of drugs in the FDCs If two, score 0.5 each for each drug listed on the CDSCO drug database, if three score 0.33 each and so on. |
| FDCs listing in DCGI or WHO Essential Medicines List | Manual check of both lists | If used only one Essential Medicine List (DCGI/ WHO model list) score half but if both score 1 |
| Efficacy of the FDC | Search for the online database drugs.com | If there is a monograph for FDC score 1 otherwise score 0 |
| Pharmacodynamics | Standard pharmacology textbooks | If FDC component have similar mechanism of action score 0 but if their mode of action is different score 1 |
| Pharmacokinetics | Check for interactions using Medscape drug interaction checker | If favourable score 1. If unfavourable score -1 and if none score 0 |
| Advantage of reduced dose | Relevant drug monograph on Medscape or Drugs. com | Depending on the number of drugs in FDC, If two score 0.5, for each API that used at lower dose than usual. If three score each reduced dose API 0.33 and so on |
| Advantages of convenience | If the pill count/dose of FDC is less than taking the individual components singly score, if not score 1 |
RESULTS
The total number of formulations of FDCs containing paracetamol in Indian market were 46. A maximum number of formulations of paracetamol FDCs were tablets (65%), followed by oral suspension (11%), film-coated tablets (9%), effervescent tablets (7%), oral disintegrating tablets (4%) , syrups and injections (2% each). The most common class of drugs combined in the form of FDC with paracetamol were NSAIDs and Opioid analgesics (Table 2). A total of 63% of FDC formulations contained 2 active ingredients and 37% of FDCs contained 3 active ingredients. The most common combinations with paracetamol API: Active Pharmaceutical Ingredient; FDC: Fixed Dose Combination; WHO: World Health Organization; NAFDAC: National Agency for Food and Drug Administration were Paracetamol with Aceclofenac and Paracetamol with Tramadol as shown in the Figure 1.
| Classes of Drugs Combined with Paracetamol | Number of Combinations | Number of Formulations |
|---|---|---|
| Paracetamol+NSAIDs | 2 | 16 |
| Paracetamol+Opioid Analgesic | 2 | 10 |
| Paracetamol+antihistamine | 1 | 3 |
| Paracetamol+Sarrattiopeptidase | 1 | 3 |
| Paracetamol+Antioxidant | 1 | 3 |
| Paracetamol+Caffeine | 1 | 1 |
| Paracetamol+NSAID+Muscle relaxant | 1 | 1 |
| Paracetamol+Antiemetic | 1 | 1 |
| Paracetamol+NSAID+PPI | 1 | 2 |
To assess rationality, a scoring scale was adopted from checklists used in a study by Auwal F et al. as represented in Table 1. The checklist contained eight items, each item could be given a maximum of one point, making eight the highest achievable score by any FDC. According to this scoring system, a rational FDC should have a score of 5. When we applied this scale to our study, 26 FDCs were irrational and the remaining 18 were rational (Figure 2). Hence 59% of the FDCs were rational and the remaining were irrational.
When FDCs were checked for listing in the essential drug list of WHO and DCGI it was found that only 4.34 % of paracetamol containing FDCs were listed in both the lists.8,10 Out of the 9 FDCs of paracetamol only 3 combinations (paracetamol with aceclofenac, paracetamol with tramadol, paracetamol with caffein) were listed in CDSCO approved FDC list.9 Table 1: Scoring Rubric to Assess Rationality of Fixed Dose Combinations.11 Drug interactions were only checked for active ingredients. The favourable pharmacokinetic interactions were defined as the one’s where active ingredient had beneficial effects on another by prolonging one of its pharmacokinetic parameters.
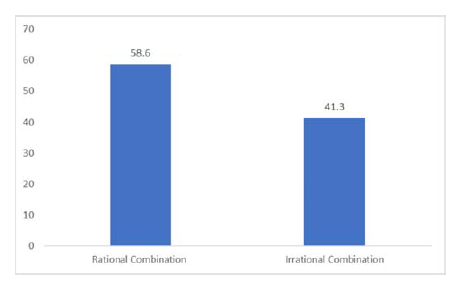
Figure 1.
Percentage of Top 5 Most Extensively Available Paracetamol Fixed Dose Combinations.
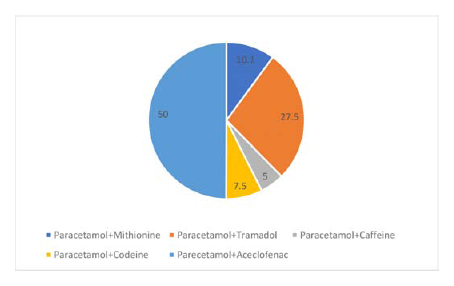
Figure 1.
Classification of Paracetamol Fixed Dose Combinations Based on the Rationality in Percentage
DISCUSSION
Tablets are the common formulation for paracetamol FDCs. Tablets are widely used formulations because of their convenience and stability. Tablets can also be made attractive by improving the texture, taste, and tolerability, thus making them one of the best options for medication delivery.12 Paracetamol was combined with NSAIDs in most of the FDC combinations – aceclofenac and diclofenac and opioids – tramadol, codeine and pentazocine. A few clinical trials have compared the efficacy of paracetamol and NSAIDs given alone and in combination, for pain relief. But there is no strong evidence for this combination in the treatment of common cold, influenza and inflammatory conditions.13
The FDC of tramadol hydrochloride 50 mg with paracetamol 375 mg has proven efficacy in acute postoperative pain after dental, orthopaedic and abdominal surgery, fibromyalgia, low back pain, migraine and as add-on therapy for osteoarthritis and rheumatoid arthritis and severe pain.6
Majority of the FDC combinations contained only 2 active ingredients. This observation is in contrast to the observation by Prajapathi et al., where FDCs of cardiovascular and central nervous system drugs were analysed. They found that the number of active ingredients were more than 3 in majority of FDCs.6 The observed difference might be due to the fact that multiple drugs are used in the management of chronic diseases and FDCs are a convenient way of administering these drugs to patients. In this study only 57% of paracetamol combinations were rational according to the scoring rubric used in this study. The most common reason for a low score in scoring rubric for rationality was the presence of one or more active pharmaceutical ingredients which were not approved by the central regulatory authority.11 In a study conducted by Poudel A et al., to evaluate the availability and rationality of unregistered fixed-dose drug combinations (FDCs), where a toolkit developed by Health Action International Asia Pacific (HAI-AP) was used, the investigators found that almost all the FDCs were irrational. As most of these irrational FDCs are over -the-counter drugs, the patients consuming these combinations will be exposed to medications with unproven safety and efficacy.13
Most of the FDCs were not listed in the essential drug list of WHO and DCGI. Only 3 FDCs of paracetamol were listed in CDSCO approved FDC list. The FDCs should be registered based on the definitive criteria. In developed countries, there is specific criteria for registration of FDCs and a strict control on manufacturing, but in India, there is an urgent need to develop the afore mentioned guidelines, as evident from this study and similar studies.
CONCLUSION
Though FDCs have many advantages, irrational combinations may result in reduced efficacy and a high risk of adverse drug reactions. Though the government of India has taken the right step forward by banning 328 FDCs in 2018, still there are many irrational FDCs present in India.16
In this study we have observed about 40% of irrational combinations. However, only FDCs listed in the drug databases were considered for the study. A study which includes all the marketed FDCs should be conducted in the future. Pharmacists have an important role to play in spreading awareness among practitioners and the general public regarding the judicious use of FDCs available in the market.
Cite this article
Praveena KS, Pradhan S, Samson PL, Ofuoma O, Shwetha S, Dongre SK. Study of Fixed Dose Combinations of Paracetamol Available in India. J Young Pharm. 2022;14(4):444-6.
ACKNOWLEDGEMENT
We are very thankful to the management, College of Pharmaceutical Sciences, Dayananda Sagar University, for providing all the facilities to conduct this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- Gupta YK, Ramachandran SS. Fixed dose drug combinations: issues and challenges in India. Indian J Pharmacol. 2016;48(4):347-9. [PubMed] | [CrossRef] | [Google Scholar]
- Gautam CS, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol. 2008;65(5):795-6. [PubMed] | [CrossRef] | [Google Scholar]
- Balat JD, Gandhi AM, Patel PP, Dikshit RK. A study of use of fixed dose combinations in Ahmedabad, India. Indian J Pharmacol. 2014;46(5):503-9. [PubMed] | [CrossRef] | [Google Scholar]
- Dalal K, Ganguly B, Gor A. Assessment of rationality of fixed dose combinations approved in CDSCO list. J Clin Diagn Res. 2016((4):FC05-8) [PubMed] | [CrossRef] | [Google Scholar]
- Prajapati K, Shah S, Desai M. Critical analysis of cardiovascular and central nervous system fixed dose combinations available in Indian market. J Clin Diagn Res. 2016;10(12) FC36-FC39 [PubMed] | [CrossRef] | [Google Scholar]
- Shah DD, Sorathia ZH. Tramadol/diclofenac fixed-dose combination: a review of its use in severe acute pain. Pain Ther. 2020;9(1):113-28. [PubMed] | [CrossRef] | [Google Scholar]
- Debbarma R, Gari M, Sinha P, Mandal S. Fixed dose combination of ibuprofen and paracetamol induced toxic epidermal necrolysis. Int J Basic Clin Pharmacol. 2016;5:2697-9. [CrossRef] | [Google Scholar]
- CDSCO. [[cited [Mar 11 2021]].];New Drugs Approved by CDSCO. Available from: https://cdscoonline.gov.in/CDSCO/Drugs [CrossRef] | [Google Scholar]
- CDSCO. [[cited [May 15 2021]]];Fixed dose combinations approved by DCG (I). Since 1961 to 28th June 2019 [internet]. Available from: https://cdsco.gov.in/opencms/opencms/en/Approval_new/Approved-New-Drugs/ [CrossRef] | [Google Scholar]
- World Health Organization. [[cited [Apr 17 2021]]];WHO model lists of essential medicines [internet]. Available from: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists [CrossRef] | [Google Scholar]
- Auwal F, Dahiru MN, Abdu-Aguye SN. Availability and rationality of fixed dose combinations available in Kaduna, Nigeria. Pharm Pract (Granada). 2019;17(2):1470 [PubMed] | [CrossRef] | [Google Scholar]
- Bhuyar P, Rahim MH, Sundararaju S, Ramaraj R, Maniam GP, Govindan N, et al. Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni Suef Univ J Basic Appl Sci. 2020;9(1):1-5. [PubMed] | [CrossRef] | [Google Scholar]
- Poudel A, Mohamed Ibrahim MI, Mishra P, Palaian S. Assessment of the availability and rationality of unregistered fixed dose drug combinations in Nepal: a multicenter cross-sectional study. Glob Health Res Policy. 2017;2:14 [PubMed] | [CrossRef] | [Google Scholar]
