ABSTRACT
Background
Itraconazole, a broad-spectrum antifungal agent, exhibits poor aqueous solubility and limited skin permeability, restricting its effectiveness in conventional topical formulations. Film-Forming Gels (FFGs) represent a promising strategy for enhancing cutaneous drug delivery by forming a sustained-release reservoir on the skin.
Objectives
The study aimed to develop and optimize an itraconazole-based Film-Forming Gel (FFG) for enhanced transdermal drug delivery using a Quality by Design (QbD) approach, targeting improved diffusion, antifungal efficacy, and formulation stability.
Materials and Methods
A 3² full factorial design was utilized to evaluate the effects of two independent variables-Eudragit RS PO and Carbopol 940 concentrations-on drug diffusion and viscosity. The formulations were evaluated for pH, viscosity, spreadability, drying time, drug content, in vitro drug diffusion, and antifungal activity using standard protocols. Statistical analysis and formulation optimization were performed using Design Expert® 7.0 software.
Results
The optimized formulation, comprising 15% Eudragit RS PO and 4.5% Carbopol 940, achieved 99.03% cumulative drug diffusion with a viscosity of 6368 cp. Regression models confirmed the significance of model terms (p<0.05) for both responses, with high model fit (R²=0.9931 for diffusion and 0.9404 for viscosity). The optimized FFG showed a significantly larger inhibition zone (26.87±0.93 mm) against C. albicans compared to the control (14.59±1.25 mm), with statistical confirmation (p<0.001). Stability studies indicated minimal variation in key parameters over three months, maintaining formulation integrity.
Conclusion
The QbD-based development successfully optimized a film-forming gel of itraconazole, demonstrating improved drug diffusion, mechanical properties, and antifungal activity. This formulation offers a promising alternative to conventional topical antifungal therapies and supports further clinical investigation.
INTRODUCTION
The skin is a widely utilized route for both systemic and local drug delivery due to its ease of access, avoidance of gastrointestinal disturbances, and circumvention of first-pass metabolism (Alkilaniet al., 2015). However, its barrier function poses significant challenges for the effective transdermal transport of many therapeutic agents (De Oliveiraet al., 2020). Current topical antifungal dosage forms, including creams, ointments, and patches, often suffer from limitations such as poor retention, frequent reapplication, variable drug release, and patient non-compliance (Benson, 2005; Choudhary and Singh, 2021; Shahet al., 2023). These issues are further exacerbated when dealing with hydrophobic drugs like itraconazole, a broad-spectrum triazole antifungal agent used for treating both superficial and systemic fungal infections (Madhaviet al., 2023).
Itraconazole’s poor aqueous solubility and limited skin permeability hinder its therapeutic effectiveness in conventional dermal formulations (Nguyenet al., 2021). There remains a critical need for a topical delivery system that not only enhances local drug retention and penetration but also improves patient adherence and minimizes systemic side effects (Punnel and Lunter, 2021).
Film-Forming Gels (FFGs) have emerged as a promising alternative, capable of forming a transparent, adherent film upon solvent evaporation, offering sustained drug release and enhanced therapeutic outcomes (Sharma and Rana, 2021). They combine the advantages of gels (ease of application and comfort) with the benefits of films (prolonged residence time and occlusion), making them particularly suitable for treating chronic dermatological conditions (Rehmanet al., 2024; Kathe and Kathpalia, 2017).
In this context, the present study aims to develop and optimize a QbD-based film-forming gel formulation of itraconazole using Carbopol 940 as a viscosity enhancer and Eudragit RS PO as the film-forming polymer. A 3² full factorial design was employed in conjunction with response surface methodology to systematically evaluate the influence of formulation variables on critical quality attributes (Montgomery, 2013). The objective is to achieve an itraconazole-loaded FFG with optimal spreadability, film integrity, drying time, and drug content, thereby enhancing antifungal efficacy and patient acceptability.
MATERIALS AND METHODS
Materials
Itraconazole was received as a gift sample by Glenmark Pharmaceuticals Limited, Nashik. Eudragit RS PO was procured from Amishi Drugs and Chemical Pvt. Ltd., Ahmedabad whereas Carbopol 940, HPMC K4M, Propylene glycol, and Triethyl citrate, Triethanolamine were obtained from S. D. Fine Chem. Ltd., Mumbai. All other chemicals were of analytical grade and were obtained commercially.
Methods
Quantification of Itraconazole by UV-Spectroscopy
The UV analysis and calibration curve for itraconazole was created by measuring absorbance of resulting solution prepared in methanol by employing a UV-Spectrophotometer (Shimadzu A11454500) at 262 nm. A standard stock solution was prepared by dissolving 10 mg of Itraconazole 10 mL of Methanol to yield 1000 µg/mL. Aliquot of 1 mL of this solution was diluted to 10 mL using the methanol to obtain a working standard solution of 100 µg/mL. Lastly, 5 dilutions in the concentration range of 5-25 µg/mL were prepared from the working stock solution and then the absorbance of these dilutions was determined with a UV-Spectrophotometer at 262 nm to plot the calibration curve.
Solubility studies and partition coefficient
The solubility of itraconazole in several solvents was evaluated, like methanol, ethanol, Dimethyl Sulfoxide (DMSO), and water. To conduct the solubility studies, 20 mL of selected solvent was placed in a flask. 10 mg of itraconazole was added, and the mixture was stirred at 37ºC using a hot plate and magnetic stirrer until dissolved. Additional drug was added until a saturated solution was achieved, which was stirred for 2-3 hr to obtain clarity. Another 10 mg of itraconazole was then added, and stirring continued for 24 hr. Absorbance was measured at 262 nm to quantify drug.
For the partition coefficient, a small amount of itraconazole was dissolved in 5 mL of water and added to a separating funnel along with 5 mL of octanol, then shaken vigorously for about 15 min. The funnel was left to stand for 24 hr, after which the separate layers were collected into two test tubes and their absorbance was determined at 262 nm. This process was conducted three times (Shahet al., 2023).
Preparation of itraconazole-loaded film-forming gel
The film-forming gel formulation was prepared using a dispersion method with a pestle and mortar for each batch. A constant 1.42 µM itraconazole (mol. wt., 705.64 g/mol) was used across all formulations, along with specified amounts of Eudragit RS PO and Carbopol 940 as outlined according to the experimental design in Table 1. HPMC K4M (1% w/w) was added to a solvent mixture of ethanol and water (70:30 w/w) and stirred at 1200 RPM for 30 min. Triethyl citrate was dissolved in propylene glycol, and Eudragit RS PO was mixed into this solution until homogenized. The required amount of Carbopol 940 was dispersed in the ethanol-water solvent with continuous stirring to avoid lumps and allowed to swell for 2-3 hr. The gel was neutralized by adding triethanolamine dropwise while stirring until the pH reached approximately 6.5 to 7. Itraconazole was dissolved in the same ethanol-water mixture at 37ºC for 1 min. Combine the prepared Carbopol gel, Eudragit RS PO solution, and HPMC K4M gel in a beaker. The polymeric solutions were stirred continuously with a magnetic stirrer at room temperature. Itraconazole solution was gradually added to the polymer mixture while stirring to ensure uniform distribution, followed by homogenization at 1200-1500 RPM for 15 min, resulting in an opaque gel (Parhi and Goli, 2020).
| Independent Variables | |||||
|---|---|---|---|---|---|
| Label | Factors | Unit | Level | ||
| Low level -1 | High level +1 | ||||
| A | Eudragit RS PO | g | 1 | 2 | |
| B | Carbopol 940 | g | 0.3 | 0.6 | |
| Dependent Variables | |||||
| Responses | Goal | ||||
| Y1 | Diffusion % | Maximize | |||
| Y2 | Viscosity cP | Maximize | |||
Experimental Design
The impact of two independent variables including concentrations of variables such as Eudragit RS PO (X1) and Carbopol 940 (X2) on responses such as diffusion (Y1) and viscosity (Y2) were optimized by response surface modelling. In this study, the experiments were performed using the Central Composite Design (CCD). The levels to the experimental units employed in this investigation and their coded values (Srivastavaet al., 2021) are given in Table 1.
Experimental data were analyzed by multiple regression equation to fit a second-order polynomial model. The model equation used is given below (Formula 1):
Where, Y is the predicted response, β0 is the intercept, n is the number of factors analyzed, βi, βii and βij are the linear (main effect), quadratic and interactive model coefficients, respectively.
Characterization of FFG
The FFG was then evaluated for various quality parameters such as pH, viscosity, drug content, diffusion test, spreading and drying properties to ensure its safety, stability, and therapeutic effectiveness. pH ensures skin compatibility, while viscosity affects spreadability and retention (Taksandeet al., 2016). Drug content confirms uniform dosing (Sarohaet al., 2013), and the diffusion test predicts drug release and permeation (Tran and Tran, 2019). Spreading and drying properties determine ease of application and film formation time, which influence patient comfort and compliance (Babyet al., 2022). Together, these tests help in developing a stable and effective topical formulation.
Antifungal efficacy studies
Antifungal efficacy of the optimized FFG and marketed conventional formulation was determined by the agar well diffusion method. A sterile 10 mm cork borer was used to make wells on the Sabouraud Dextrose Agar plate. The standard C. albicans strain was inoculated on the plate by streaking method. Marketed conventional formulation and the optimized FFG were dissolved in methanol to obtain 10 µg/mL concentration of each formulation. These samples were poured into the previously bored plates and were incubated at 30ºC for 48 hr in aseptic conditions. The zone of inhibition was measured and compared to the control group. The control sample was prepared with ITZ in oil blend (Oleic acid and isopropyl myristate in the ratio of 1:1) (Jayet al., 2017; Moriet al., 2017).
Stability study
The optimized formulation of FFG were subjected to accelerated stability testing as per ICH Q1A(R2) guidelines (Tatode et al., 2021). The samples were stored in a stability chamber maintained at 40±2ºC and 75±5% Relative Humidity (RH) for a period of three months. All measurements were performed in triplicate. Data were statistically analyzed using one-way ANOVA to assess the significance of any changes over time (p<0.05 considered statistically significant). The results were compared to the initial values to evaluate the physical and chemical stability of the formulation under accelerated conditions.
Statistical analysis
Design Expert 7.0 software was used for the experimental design and statistical analysis. The coefficients of determination (R2) and Analysis of Variance (ANOVA) were used to evaluate the goodness of fit of the regression model. The Center Combination Design (CCD) of the Response Surface Methodology (RMS) was used to estimate the optimal formulation conditions of the two independent variables and each dependent variable (Tatode et al., 2021).
RESULTS
UV-spectroscopic method of analysis of Itraconazole
The method was validated according to the ICH guidelines, Q2 (R1). The linearity (R²=0.9982) was observed in the concentration range of 5-25 µg/mL (Figure 1). Precision and repeatability studies indicated a percent relative standard deviation of<2%, thereby complying with requirements of the validated method.
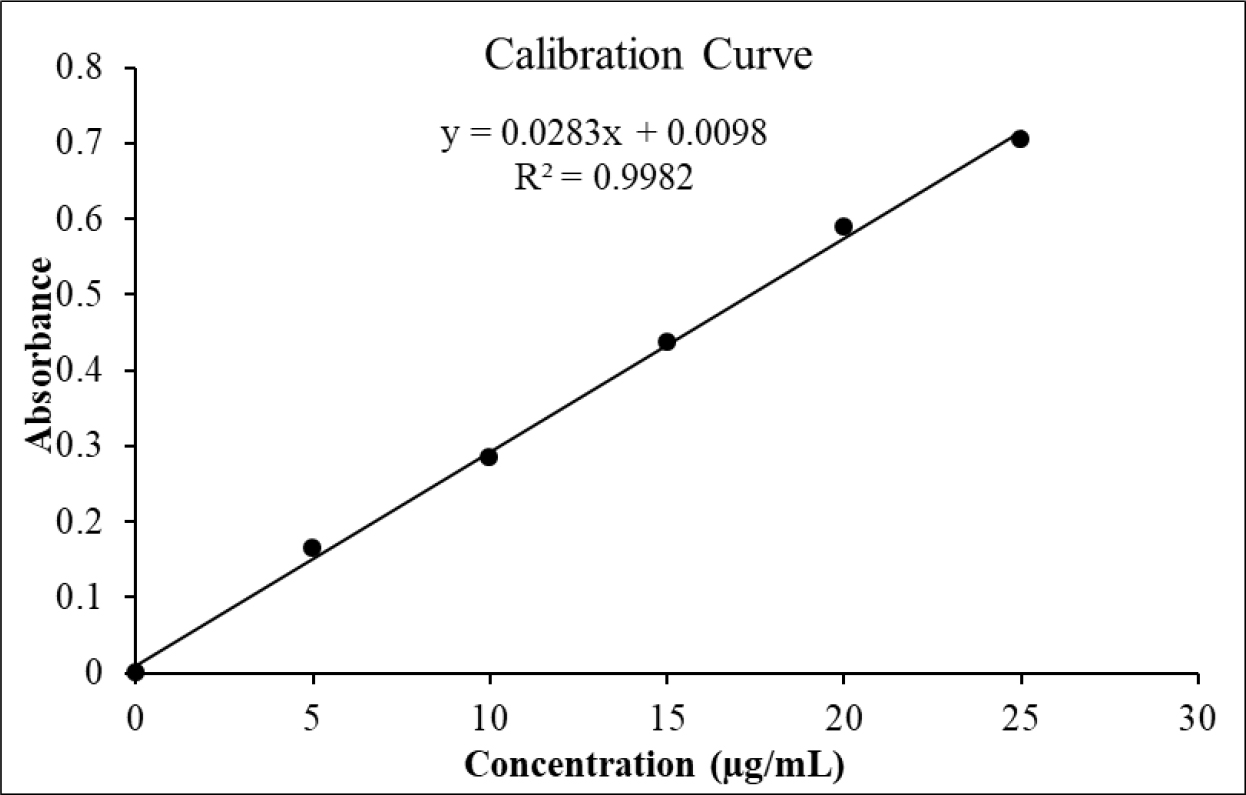
Figure 1:
Linear response standard calibration curve of different concentration of itraconazole.
Solubility studies and partition coefficient
The extent of solubilisation of itraconazole was measured, revealing values of 6.73 × 10-3 mg/mL in water, 0.386 mg/mL in Dimethyl Sulfoxide (DMSO), 0.515 mg/mL in methanol, and 0.533 mg/mL in ethanol. The sequence of non-polar nature of studied solvents was in order of water<dimethyl sulphoxide<methanol<ethanol. In Film-Forming Gels, the partition coefficient of itraconazole influences its solubility, release from the gel matrix, and skin or other tissue penetration. Thus, balancing lipophilicity and other formulation parameters is crucial for optimizing itraconazole’s pharmacokinetics and therapeutic efficacy. The partition coefficient of itraconazole was found to be 5.19±0.628, which is close to a previously reported value of 5.66, indicating optimal penetration and therapeutic effectiveness (Chivateet al., 2021).
Modelling and Analysis of Variance (ANOVA)
Enormous datasets can be processed by RSM software within a very short time, to obtain optimal conditions (Vyavahareet al., 2018). In the current study, design expert 7.0 software was used to analyze the impact of independent variables on responses. Nine experimental runs (Formulation F1-F9) (Table 2) were designed to study the influence of Eudragit RS PO (A) and Carbopol 940 (B) on diffusion (%) and viscosity (cP).
| F. Code | Factor 1 | Factor 2 | Response 1 | Response 2 |
|---|---|---|---|---|
| A: Eudragit RS PO (g) | B: Carbopol 940 (g) | Diffusion % | Viscosity cP | |
| F1 | 1 | 0.3 | 95.21±0.17 | 5232.0±11.0 |
| F2 | 1.5 | 0.6 | 96.62±0.06 | 6909.0±5.3 |
| F3 | 2 | 0.6 | 82.12±0.15 | 7451.0±9.5 |
| F4 | 1 | 0.45 | 94.16±0.15 | 5741.0±3.6 |
| F5 | 1 | 0.6 | 93.75±0.06 | 6483.0±2.0 |
| F6 | 1.5 | 0.3 | 98.71±0.10 | 5598.0±3.0 |
| F7 | 2 | 0.3 | 85.45±0.06 | 5656.0±4.6 |
| F8 | 2 | 0.45 | 83.24±0.06 | 6522.0±3.0 |
| F9 | 1.5 | 0.45 | 99.03±0.12 | 6368.0±3.6 |
Impact of formulation variables on diffusion
The experimental data were analyzed by multiple regression analysis and the coefficients of model were used for the significance levels (Table 3). The drug diffusion (Y1) across the 9 runs ranged from 82.12±0.15 to 99.03±0.12%. Quadratic model fitting indicated a significant effect on diffusion (F=168.64, p=0.0008). The ANOVA (Table 4) confirmed significance for factors A, B, and A². The F-value for the model obtained through regression analysis was 240.75, which implies the model was significant.
| Response Variable | Model | F Value | Sequential p-value | Adjusted R² | Predicted R² |
|---|---|---|---|---|---|
| Diffusion | Quadratic | 168.6419 | 0.0008 | 0.9890 | 0.9775 |
| Viscosity | Linear | 47.32373 | 0.0002 | 0.9205 | 0.8729 |
| Source | Sum of Squares | df | Mean Square | F Value | p-value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Diffusion | ||||||
| Model | 348.6531 | 3 | 116.2177 | 240.7493 | <0.0001 | significant |
| A-Eudragit RS PO | 173.9894 | 1 | 173.9894 | 360.4254 | <0.0001 | |
| B-Carbopol 940 | 7.889067 | 1 | 7.889067 | 16.34249 | 0.0099 | |
| A2 | 166.7747 | 1 | 166.7747 | 345.4799 | <0.0001 | |
| Residual | 2.413667 | 5 | 0.482733 | |||
| Corrected total | 351.0668 | 8 | ||||
| Viscosity | ||||||
| Model | 4102430 | 2 | 2051215 | 47.32373 | 0.0002 | significant |
| A-Eudragit RSPO | 938521.5 | 1 | 938521.5 | 21.6527 | 0.0035 | |
| B-Carbopol 940 | 3163908 | 1 | 3163908 | 72.99477 | 0.0001 | |
| Residual | 260065.9 | 6 | 43344.31 | |||
| Corrected total | 4362496 | 8 | ||||
The final predictive equation obtained using significant terms for diffusion was given as:
This equation provides a comprehensive view of how the factors A and B, along with A2, influence the diffusion process in study. The negative coefficients suggest that increases in these factors lead to a decrease in diffusion, which can be critical for understanding the dynamics of the system being analyzed in the context of DOE.
High R² (0.9931), Pred R² (0.9775), and adequate precision (36.29) confirmed robustness. Thus, this model can be used to navigate the design space.
Impact of formulation variables on viscosity
Viscosity (Y2) values were observed in the range of 5232.0±11.0 to 7451±9.5 cP, indicating appropriate consistency for dermal application. The Linear model was significant (F = 47.32, p=0.0002) as shown in Table 3. ANOVA revealed that both A and B significantly affected viscosity, with Carbopol 940 having a more pronounced effect (Table 4).
The final predictive equation obtained using significant terms for viscosity was given as:
This equation provides a clear relationship between the viscosity of the fluid and the coded factors A and B. The positive coefficients suggest that increases in either factor A or B lead to an increase in viscosity.
The R² value of 0.9404 signifies the model’s robustness. The predicted R² of 0.8729 aligns well with the adjusted R² of 0.9205. Adequate precision value of 18.663 confirms a strong signal. Thus, this model can effectively guide navigation through the design space.
The relationship between the dependent and independent variables was further elucidated using contour and response surface plots (Figure 2).
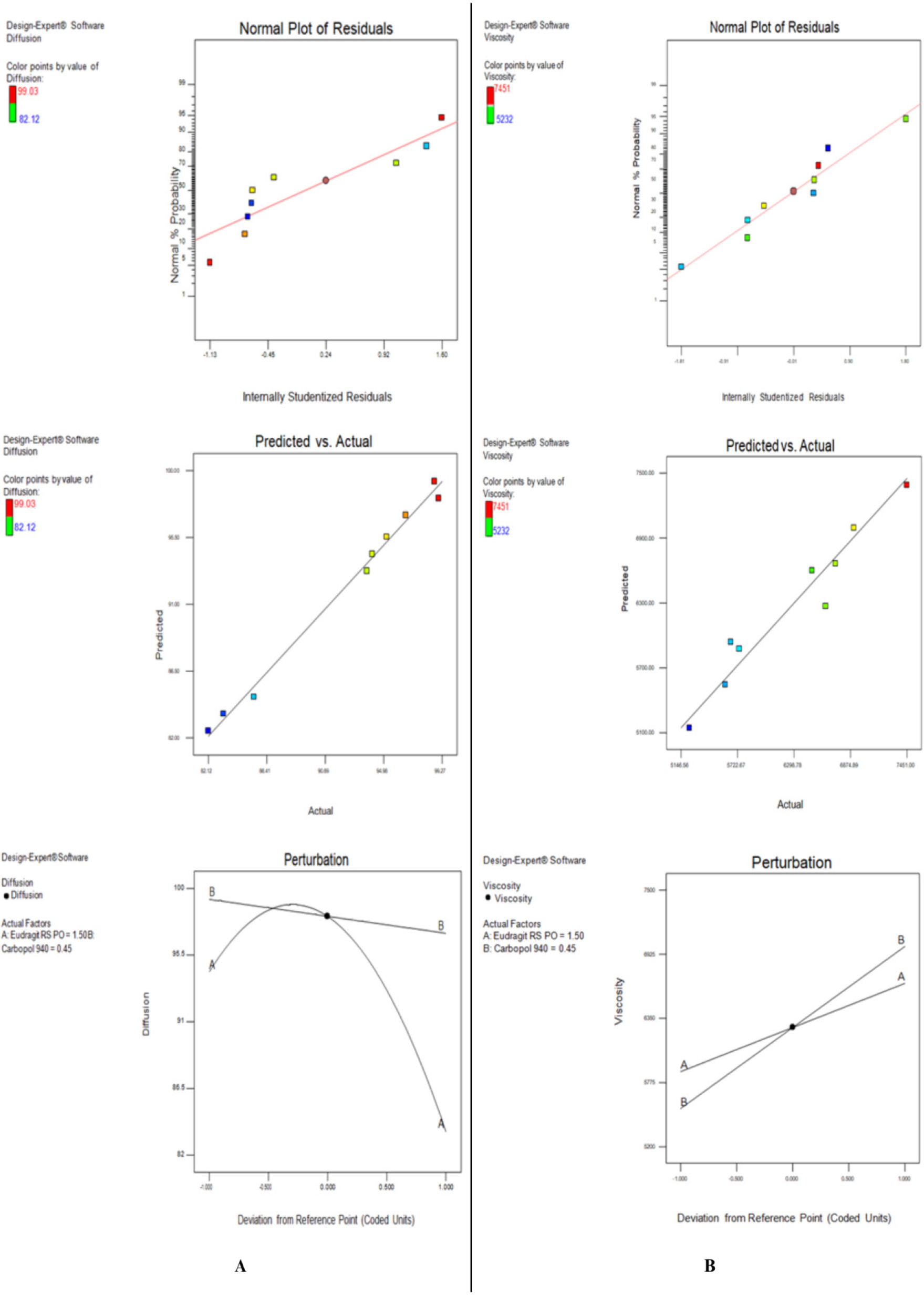
Figure 2:
Contour plots illustrating the effects of formulation variables on critical quality attributes of itraconazole-loaded FFG: (A) Effect of diffusion and (B) Effect of viscosity.
Statistical optimization of itraconazole-loaded FFG formulation
The validated optimally formulated itraconazole-loaded FFG relied on experimental comparison with predicted critical responses results. When using Eudragit RS PO (1.5 g) and Carbopol 940 (0.45 g) researchers obtained high diffusion with 99.03% along with desirable viscosity of 6368 cp. Laboratory measurements of the optimized formulation (F9) revealed values that were near predicted values of diffusion and viscosity (98.12%, 6268.22 cp) within 3% relative error range proving the optimization model was accurate and reliable. The narrow deviation values between predicted and experimental data verified the acceptance of these models.
Characterization of itraconazole-loaded FFG formulation
The physicochemical evaluation of itraconazole-loaded FFGs confirmed their suitability for topical application, as evidenced by the data presented in Table 5. The pH of all formulations ranged between 5.8±0.1 and 6.2±0.1. Spreadability values varied from 7.4±0.2 to 9.8±0.1 g·cm/sEC, with an inverse correlation to viscosity, as higher polymer content resulted in reduced ease of spreading. The drying time, a critical parameter for film formation and patient compliance, was found to be between 1.45 min±5 sec and 3.05 min±10 sec across all batches, reflecting efficient film formation upon application. Drug content was consistently within the pharmacopoeial limits of 95-105%, with all formulations showing values ranging from 97.77% to 101.22%, thereby confirming content uniformity and accurate dosing. In vitro drug diffusion of itraconazole was determined from all prepared batches of film-forming gels (F1-F9) as shown in Figure 3.
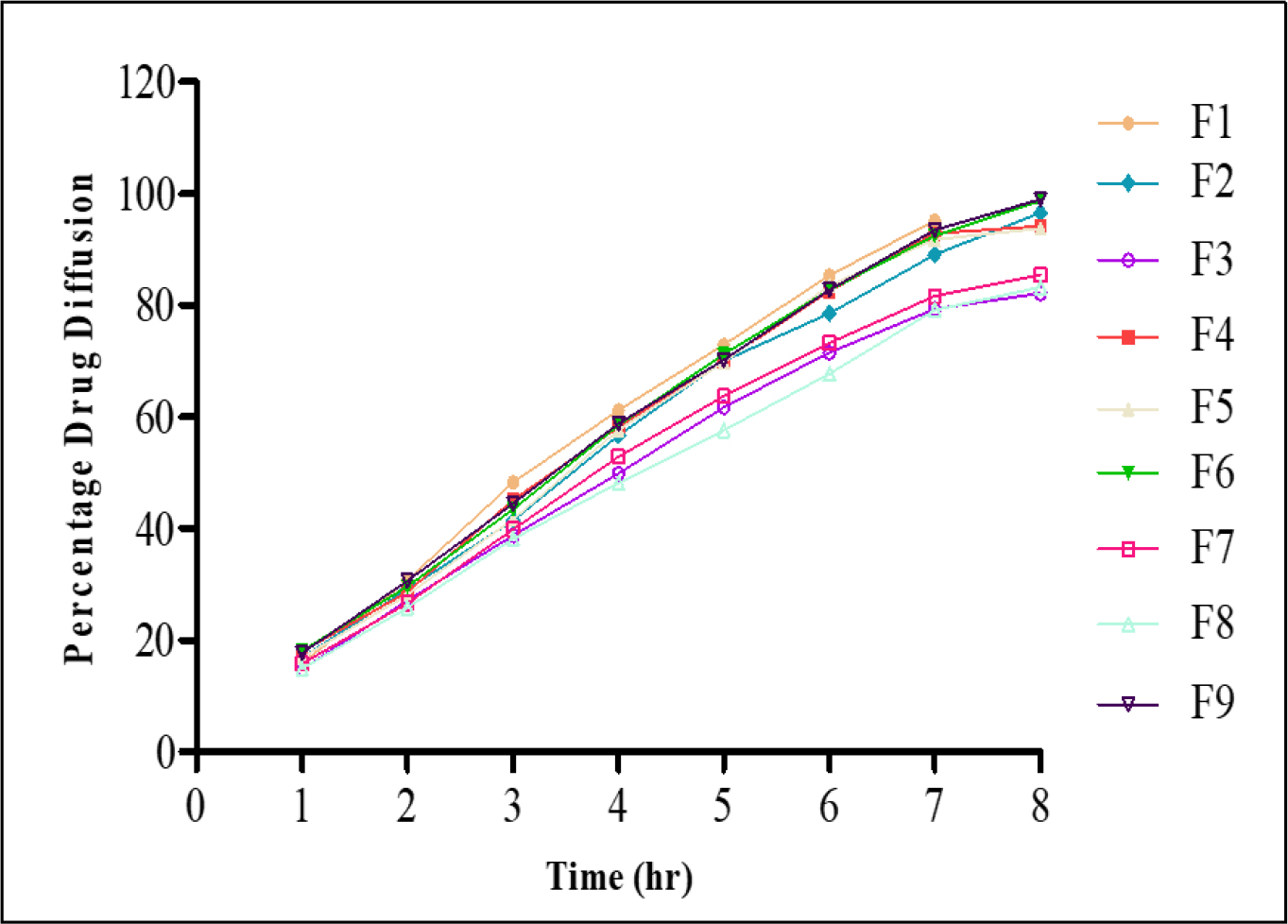
Figure 3:
Percentage drug diffusion of itraconazole from prepared batches of FFGs.
| Batches | pH | Spreadability (g.cm/s) | Drying time (min) | Drug content (%) |
|---|---|---|---|---|
| F1 | 5.8±0.1 | 9.2±0.2 | 1.45 m±5 sec | 96.45 |
| F2 | 6.2±0.1 | 9.8±0.1 | 2.15 min±5 sec | 98.15 |
| F3 | 6.0±0.1 | 7.9±0.1 | 3.05 min±10 sec | 97.78 |
| F4 | 5.9±0.1 | 8.0±0.1 | 1.50 m±5 sec | 97.77 |
| F5 | 6.1±0.1 | 9.5±0.2 | 1.52 m±3 sec | 99.84 |
| F6 | 6.0±0.1 | 7.4±0.2 | 2.05 min±5 sec | 101.22 |
| F7 | 6.1±0.1 | 7.6±0.2 | 2.45 min±5 sec | 100.33 |
| F8 | 6.2±0.1 | 8.3±0.1 | 2.55 min±10 sec | 99.25 |
| F9 | 6.0±0.1 | 8.5±0.2 | 2.10 min±10 sec | 99.21 |
Evaluation of antifungal efficacy
The optimized FFG exhibited significantly enhanced antifungal activity against Candida albicans, with a mean inhibition zone of 26.87±0.93 mm compared to 14.59±1.25 mm for the control (Figure 4). Statistical analysis using Welch’s t-test (t=21.05, p=0.00007) confirmed the difference as highly significant (p<0.001), indicating superior efficacy likely due to improved drug delivery and retention at the application site.
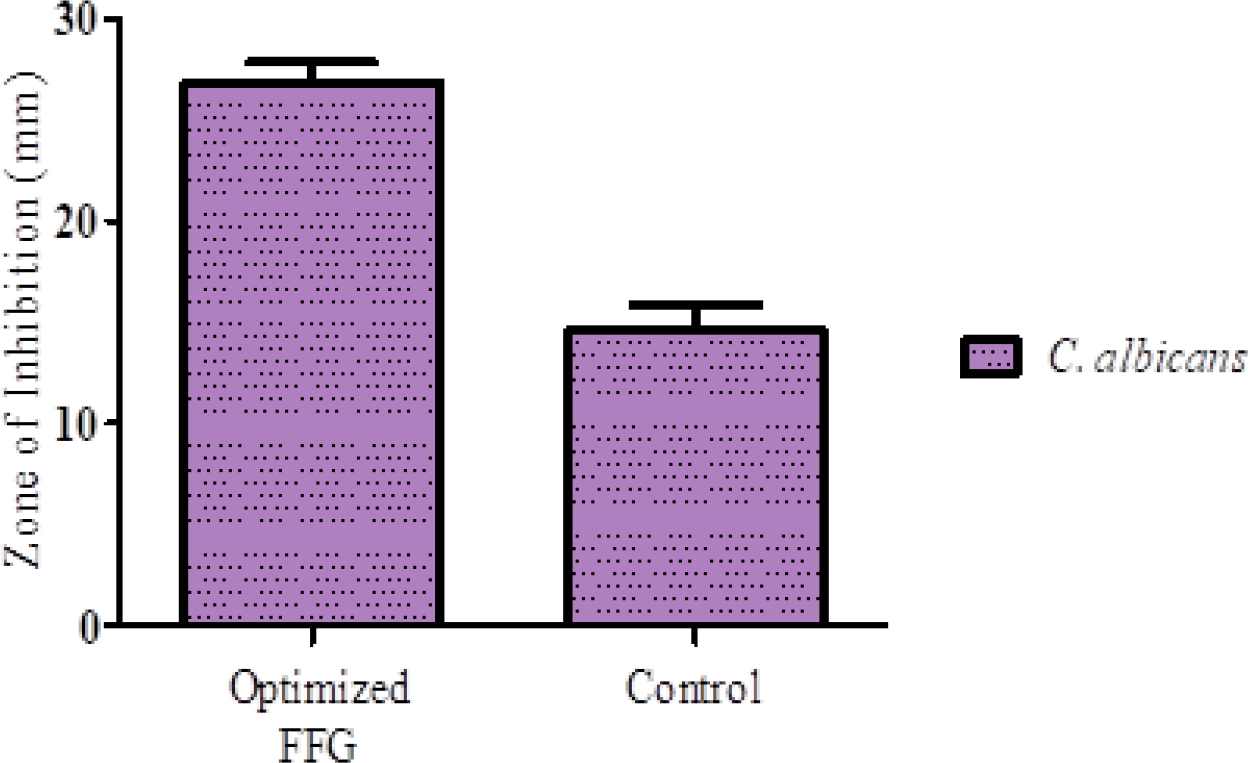
Figure 4:
Antifungal activity of optimized FFG against C. albicans.
Stability study
The optimized FFG was subjected to accelerated stability testing (40±2ºC / 75±5% RH) over 3 months. Minor reductions were observed in pH (6.09 to 6.02), viscosity (6559 to 6487 cP), drug content (99.96% to 99.62%), and diffusion (99.17% to 98.71%), all remaining within acceptable limits (Figure 5). One-way ANOVA revealed significant changes in pH (p=0.0003), drug content (p=0.0366), and diffusion (p=0.0025), while viscosity change was non-significant (p=0.0527). These findings confirm the formulation’s stability under stress conditions.
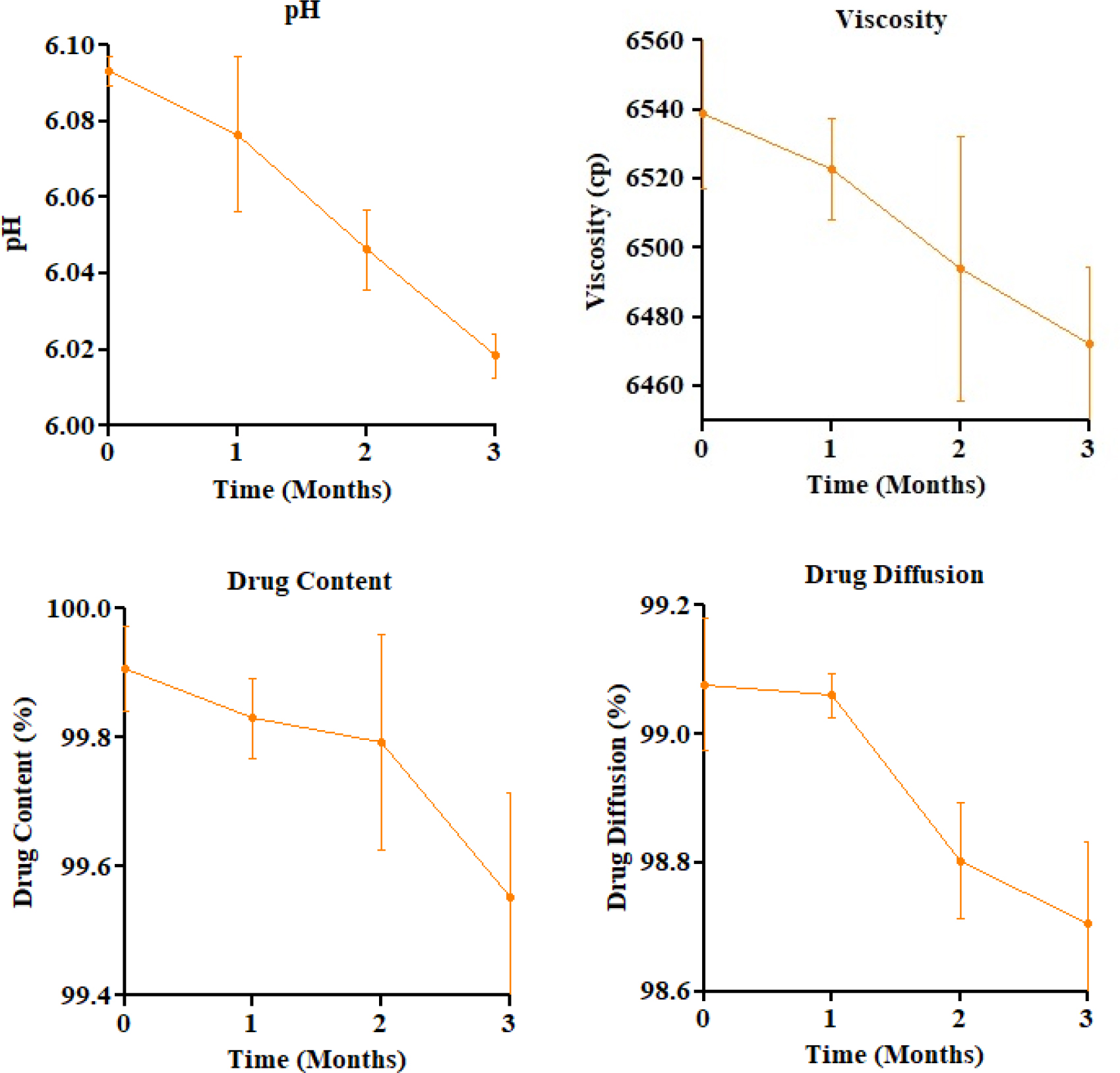
Figure 5:
Stability study-parameter trends over 3 months (Mean±SD).
DISCUSSION
The development and optimization of an itraconazole-loaded FFG were successfully achieved using a QbD approach. The validated UV-spectroscopy method ensured reliable drug estimation. Physicochemical characterization (pH, viscosity, spreadability, drying time, and drug content) were all within acceptable limits, supporting the development of a stable and user-friendly topical formulation. The pH is satisfactory, aligning well with the physiological pH of human skin (~5.0), thereby minimizing the risk of barrier disruption, irritation, or inflammation. Itraconazole-containing FFG formulations dried in 1-3 min, which aligns with the literature suggesting an optimal skin drying time within 5 min.
Drug diffusion studies indicated that higher concentrations of Eudragit RS PO and Carbopol 940 increased formulation viscosity, thereby reducing drug diffusion. Among all, batch F9 demonstrated the most favorable balance, achieving 99.03% cumulative release in 8 hr, representing an optimal balance between polymer content and release characteristics. This behavior supports the hypothesis that polymer concentration must be finely tuned to achieve controlled drug delivery in film-forming systems.
To further optimize the formulation and understand the role of key variables, a statistical modeling approach was employed. The application of DoE through central composite design enabled a systematic and quantitative understanding of the influence of formulation variables-Eudragit RS PO (X₁) and Carbopol 940 (X₂)-on the key quality attributes of the Film-Forming Gel (FFG), namely drug diffusion (Y₁) and viscosity (Y₂). DOE results supported the use of a quadratic model for predicting drug diffusion and a linear model for viscosity. Significant factors affecting diffusion were Eudragit RS PO, Carbopol 940, and their quadratic term. For viscosity, both polymers had a direct positive influence.
As percentage of Eudragit RS PO and Carbopol 940 in formulation increases, diffusion gets decreases. It is due to the fact that Eudragit RS PO contributes to the film’s mechanical strength and stability, which can hinder the release of the drug. Higher concentrations lead to a more compact structure, reducing the diffusion rate (Saudagar and Gangurde, 2017). Studies also indicate that formulations with higher Carbopol concentrations exhibited lower drug release rates, following a diffusion-controlled mechanism (Shaikhet al., 2020). Eudragit RS PO is having high impact on diffusion as compared to Carbopol 940 as its P value is very low as compare to Carbopol 940. It may due to the formation of Interpolyelectrolyte Complexes (IPEC) between Eudragit and Carbopol can alter the diffusion rates, with Eudragit RS PO providing a more stable matrix for drug encapsulation (Mustafinet al., 2010).
The percentage of Eudragit RS PO and Carbopol 940 in the formulation affects the drug’s viscosity. Increasing the amounts of Eudragit RS PO and Carbopol 940 in the formulation raises the viscosity due to stronger IPEC network formation, which stabilizes the system through ionic bonds, leading to increased viscosity (Mustafinet al., 2010). Carbopol 940 significantly influences viscosity more than Eudragit RS PO, as evidenced by its much lower P value. The lower P-value associated with Carbopol 940 indicates a more statistically significant effect on viscosity compared to Eudragit RS PO, suggesting that Carbopol 940 is more effective in achieving desired viscosity profiles in formulations (A-sasutjaritet al., 2005; Royet al., 2022).
Overall, the response surface methodology enabled identification of critical factors influencing both drug diffusion and viscosity. The developed polynomial equations provided insight into the nature and magnitude of interactions between formulation variables. These findings demonstrate that Eudragit RS PO predominantly governs drug release behavior due to its film-forming capacity, while Carbopol 940 primarily dictates the viscosity and textural attributes of the gel. The validated models provide a rational basis for formulation optimization, aligning with QbD principles and ensuring robustness and reproducibility in future scale-up or clinical development.
The antifungal activity of the optimized formulation was significantly superior to that of the control (26.87±0.93 mm vs. 14.59±1.25 mm, p<0.001). The enhanced zone of inhibition is attributed to the improved drug permeation and retention provided by the film-forming matrix, which maintains prolonged contact with the skin and facilitates sustained drug release. These findings are consistent with previous reports that indicate film-forming gels can enhance topical bioavailability and therapeutic efficacy (Maliet al., 2017).
The stability study conducted under ICH-recommended accelerated conditions (40±2ºC / 75±5% RH) over three months demonstrated that the optimized formulation remained physically and chemically stable.
This study lays the groundwork for advancing itraconazole-based topical therapy. Future research should focus on in vivo and clinical evaluations to confirm therapeutic efficacy and safety. The film-forming gel platform can be adapted for other drugs or combined therapies. Further studies on polymer-drug interactions, scale-up, and regulatory validation are essential for commercialization. The QbD approach used here also provides a valuable model for optimizing similar semisolid drug delivery systems.
CONCLUSION
The present study successfully developed and optimized a QbD based FFG containing itraconazole for enhanced topical antifungal delivery. The use of a central composite design and response surface methodology enabled systematic evaluation of the effects of Eudragit RS PO and Carbopol 940 on critical quality attributes such as drug diffusion and viscosity. The optimized formulation demonstrated superior physicochemical properties, including acceptable pH, appropriate viscosity, rapid drying time, and uniform drug content. In vitro diffusion studies confirmed sustained drug release, while antifungal efficacy assays revealed a significantly enhanced inhibitory effect against Candida albicans compared to the control group (p<0.001). Stability studies showed no statistically significant deterioration in formulation performance, supporting its shelf stability. Collectively, the results indicate that the QbD-driven itraconazole FFG is a robust and efficacious formulation with the potential to overcome the limitations of conventional topical antifungal preparations and warrants further clinical evaluation.
Cite this article:
Somwanshi SB, Donde HK. Quality by Design-Driven Formulation and Evaluation of an Itraconazole Film-Forming Gel for Enhanced Antifungal Activity. J Young Pharm. 2025;17(3):636-45.
ACKNOWLEDGEMENT
We are thankful to the institute for providing all facilities required during conducting this experiment.
ABBREVIATIONS
| FFG | Film-Forming Gel |
|---|---|
| QbD | Quality by design |
| ANOVA | Analysis of variance |
| CCD | Center Combination Design |
| RMS | Response Surface Methodology |
| RH | Relative Humidity |
| ICH | International Conference on Harmonization. |
References
- Alkilani A. Z., McCrudden M. T. C., Donnelly R. F.. (2015) Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 7: 438-470 https://doi.org/10.3390/pharmaceutics7040438 | Google Scholar
- A-sasutjarit R., Sirivat A., Vayumhasuwan P.. (2005) Viscoelastic properties of Carbopol 940 gels and their relationships to piroxicam diffusion coefficients in gel bases. Pharmaceutical Research 22: 2134-2140 https://doi.org/10.1007/s11095-005-8244-2 | Google Scholar
- Baby A., Shivakumar H. N., Alayadan P.. (2022) Formulation and evaluation of film forming solution of diphenhydramine hydrochloride for transdermal delivery. Indian Journal of Pharmaceutical Education and Research 56: 43-49 https://doi.org/10.5530/ijper.56.1.6 | Google Scholar
- Benson H. A. E.. (2005) Transdermal drug delivery: Penetration enhancement techniques. Current Drug Delivery 2: 23-33 https://doi.org/10.2174/1567201052772915 | Google Scholar
- Chivate A., Garkal A., Hariharan K., Mehta T.. (2021) Exploring novel carrier for improving bioavailability of itraconazole: Solid dispersion through hot-melt extrusion. Journal of Drug Delivery Science and Technology 63: Article 102541 https://doi.org/10.1016/j.jddst.2021.102541 | Google Scholar
- Choudhary N., Singh A. P.. (2021) Transdermal drug delivery system: A review. Indian Journal of Pharmacy and Pharmacology 8: 5-9 https://doi.org/10.18231/j.ijpp.2021.002 | Google Scholar
- De Oliveira F. F. D., De Menezes L. R., Tavares M. I. B.. (2020) Film-forming systems in topically administered pharmaceutical formulations. Materials Sciences and Applications 11: 576-590 https://doi.org/10.4236/msa.2020.118038 | Google Scholar
- Jay B., Patel A., Sinha P., Suthar B., Narkhede S.. (2017) Formulation and evaluation of film forming gel of bifonazole for local drug delivery. Pharmaceutical Sciences. Monitor 8: 173-189 https://doi.org/10.4236/msa.2020.118038 | Google Scholar
- Kathe K., Kathpalia H.. (2017) Film forming systems for topical and transdermal drug delivery. Asian Journal of Pharmaceutical Sciences 12: 487-497 https://doi.org/10.1016/j.ajps.2017.07.004 | Google Scholar
- Madhavi N., Sudhakar B., Sravani U.. (2023) Development and evaluation of itraconazole solid dispersion gel cutaneous delivery. International Journal of Applied Pharmaceutics 15: 334-341 https://doi.org/10.22159/ijap.2023v15i6.48978 | Google Scholar
- Mali K. K., Dhawale S. C., Dias R. J.. (2017) Microemulsion based bioadhesive gel of itraconazole using tamarind gum: and evaluation. Marmara Pharmaceutical Journal 21: 688-688 https://doi.org/10.12991/marupj.323593 | Google Scholar
- Montgomery D. C.. (2013) Design and analysis of experiments https://doi.org/10.12991/marupj.323593 | Google Scholar
- Mori N. M., Patel P., Sheth N. R., Rathod L. V., Ashara K. C.. (2017) Fabrication and characterization of film-forming voriconazole transdermal spray for the treatment of fungal infection. Bulletin of Faculty of Pharmacy, Cairo University 55: 41-51 https://doi.org/10.1016/j.bfopcu.2017.01.001 | Google Scholar
- Mustafin R., Kabanova T. V., Zhdanova E. R., Bukhovets A. V., Garipova V. R., Nasibullin S. F., Kemenova V. A., et al. (2010) Diffusion-transport properties of a polycomplex matrix system based on Eudragit® EPO and carbomer 940. Pharmaceutical Chemistry Journal 44: 147-150 https://doi.org/10.1007/s11094-010-0419-4 | Google Scholar
- Nguyen K. T., Tran P. H. L., Ngo H. V., Tran T. T. D.. (2021) Hydrophobic and hydrophilic film-forming gels for the controlled delivery of drugs with different levels of hydrophobicity. Anti-Cancer Agents in Medicinal Chemistry 21: 2082-2088 https://doi.org/10.2174/1871520621666201231141842 | Google Scholar
- Parhi R., Goli V. V. N.. (2020) Design and optimization of film-forming gel of etoricoxib using response surface methodology. Drug Delivery and Translational Research 10: 498-514 https://doi.org/10.1007/s13346-019-00695-2 | Google Scholar
- Pünnel L. C., Lunter D. J.. (2021) Film-forming systems for dermal drug delivery. Pharmaceutics 13: 932 https://doi.org/10.3390/pharmaceutics13070932 | Google Scholar
- Rehman M., Tahir N., Sohail M. F., Qadri M. U., Duarte S. O. D., Brandão P., Esteves T., Javed I., Fonte P., et al. (2024) Lipid-based nanoformulations for drug delivery: An ongoing perspective. Pharmaceutics 16: 1376 https://doi.org/10.3390/pharmaceutics16111376 | Google Scholar
- Roy D., Das S., Panda M., Sultana S., Mandal M., Sil I., Majumder B., et al. (2022) Formulation, evaluation and release kinetics of low viscosity metronidazole gel with varying amount of Carbopol. Indian Journal of Science and Technology 15: 2690-2698 https://doi.org/10.17485/IJST/v15i48.2145 | Google Scholar
- Saroha K., Singh S., Aggarwal A., Nanda S.. (2013) Transdermal gels-An alternative vehicle for drug delivery. International Journal of Pharmaceutical Chemical and Biological Sciences 3: 495-503 https://doi.org/10.17485/IJST/v15i48.2145 | Google Scholar
- Saudagar R. B., Gangurde P. A.. (2017) Formulation, development and evaluation of film-forming gel for prolonged dermal delivery of miconazole nitrate. Research Journal of Topical and Cosmetic Sciences 8: 19-29 https://doi.org/10.5958/2321-5844.2017.00003.6 | Google Scholar
- Shah N., Ahmad Z., Syed A., Aslam A., Zafar N., Arif A., et al. (2023) Development of film forming gel for the delivery of 5-fluorouracil: / evaluation. Polymer Bulletin 81: 1-17 https://doi.org/10.1007/s00289-023-05004-z | Google Scholar
- Shaikh S., Mohd A., Khan G. J., Patel S., Ahmad S., Jadhav L., et al. (2020) Formulation and characterization of piroxicam emulgel for topical drug delivery. International Journal of Pharma and Bio Sciences 10: 46-54 https://doi.org/10.22376/ijpbs/lpr.2020.10.2.P46-54 | Google Scholar
- Sharma R., Rana V.. (2021) QbD steered fabrication of pullulan-Terminalia catappa-Carbopol® 971P film forming gel for improved rheological, textural and biopharmaceutical aspects. International Journal of Biological Macromolecules 1: 1301-1312 https://doi.org/10.1016/j.ijbiomac.2021.10.179 | Google Scholar
- Srivastava S., Verma U., Kumar R., Bhatt N.. (2021) Preparation and evaluation of econazole nitrate containing film-forming gel. European Journal of Molecular and Clinical Medicine 8: 2881-2895 https://doi.org/10.1016/j.ijbiomac.2021.10.179 | Google Scholar
- Taksande J. B., Gupta M., Trivedi R. V., Wadher K. J., Umekar M. J.. (2016) Formulation and characterization of organic–inorganic hybrid film for transdermal drug delivery. Journal of Applied Pharmaceutical Research 4: 8-15 https://doi.org/10.1016/j.ijbiomac.2021.10.179 | Google Scholar
- Tatode A. A., Patil A. T., Umekar M. J.. (2018) Application of response surface methodology in optimization of paclitaxel liposomes prepared by thin film hydration technique. International Journal of Applied Pharmaceutics 10: 62-69 https://doi.org/10.22159/ijap.2018v10i2.24238 | Google Scholar
- Tran T. T. D., Tran P. H. L.. (2019) Controlled release film forming systems in drug delivery: The potential for efficient drug delivery. T. and T. Pharmaceutics 11: 290 https://doi.org/10.3390/pharmaceutics11060290 | Google Scholar
- Vyavahare G. D., Gurav R. G., Jadhav P. P., Patil R. R., Aware C. B., Jadhav J. P., et al. (2018) Response surface methodology optimization for sorption of malachite green dye on sugarcane bagasse biochar and evaluating the residual dye for phyto and cytogenotoxicity. Chemosphere 194: 306-315 https://doi.org/10.1016/j.chemosphere.2017.11.180 | Google Scholar
