ABSTRACT
Background
The experiment was intended to evaluate the effect of Vitex trifolia Linn’s hydroalcoholic extract to treat chronic inflammation.
Materials and Methods
Chronic inflammation was induced by injecting 0.25 mL of Complete Freund’s Adjuvant subcutaneously in the palmar surface of the right-hand paw of Wistar rats. The animals were treated with two different doses of the plant extract and their efficiency and efficacy was compared to standard drugs indomethacin and dexamethasone for 28days. At the end of the study the serum TNF-α and IL-10 using ELISA kits, differential leukocyte count was estimate, vital organs were subjected to histological studies. The anti-tubercular activity of the extracts of V. trifolia was also evaluated by the Microplate Alamar Blue Assay method (MABA) against Mycobacterium tuberculosis.
Results
The extract has also been shown to be effective in controlling levels of chemical cytokines IL-10 and TNF-α and the anti-TB activity was evaluated using Alamar Blue Assay which also exhibited promising results.
Conclusion
The obtained results implicate that hydroalcoholic extracts of V trifolia show promising anti-inflammatory action on chronic stage of inflammation and as well as have proven to be effective as anti-tubercular drug.
INTRODUCTION
Tuberculosis is the 2nd major cause of death in the world due to infectious diseases. Latent infection with MTb (MTb) also is seen commonly in about 2 million people out of which 10% of them develop active TB as a result of weak immune system. The causative micro-organism of the disease is Mycobacterium tuberculosis which is a slow growing bacterium whose cell wall is overly complex composed of peptidoglycans, sulpholipids and cord factor.1
The micro-organism enters the human body on inhalation and targets mainly the dendritic cells and the alveoli, which is followed by phagocytosis by the macrophages in the host cell and in the process generating reactive oxygen and nitrogen species leading to granuloma formation.2–4
The core of any infection is inflammation along with expression and release of mediators of inflammation like Tumor Necrosis Factor alpha (TNF-α), Interleukin (IL)-6, IL-12, IL-1β, IL-10, Interferons (IFN)-γ and Clusters of Differentiation 4 (CD4+) cells. IL-10 is a potent immunomodulator which promotes MTb bacterial growth, along with these cytokines like direct neutrophils, monocytes and lymphocytes are also recruited to the infected site while TNF-α either causes apoptosis of the macrophages or kills the bacilli by generating reactive oxygen species.5,6
When tuberculosis progresses, there is granuloma formation and tissue destruction. The knowledge that controlling or regulating levels of pro-inflammatory mediators’ by using anti-inflammatory drugs or immunomodulatory agents can decrease or control chronic inflammation like Tuberculosis.7
For more than 50 years now a drought is being seen in the discovery of newer anti-TB drugs. The first line of treatment which involves drugs like Rifampin (RIF), Pyrizinamide (PZA) and Ethambutol (ETH) show no activity against dormant bacteria which cause latent tuberculosis. Further drug classes like fluoroquinolones, oxazolidinediones, rifamycin phenothiazine, nitroimidazole and azole derivatives which belong to different classes of chemotherapeutic agents have also emerged recently as probable and effective treatments of tuberculosis.8,9
The plant chosen for study namely Vitex trifolia L. belongs to the genus Vitex which is a shrub about 6m in height and the plant exhibits varied pharmacological activities like thermogenic, astringent, expectorant, carminative, anthelmintic anti-inflammatory, anti-tubercular, anti-pyrogenic and anti-cancer properties.10,11
Various diterepenes vitretifolin D, vitretifolins E have been isolated from the plant and they have shown promising results against several cell lines and promising inhibitory activity on inflammatory mediators, on in vitro cell lines and on IL-6, IL-10 and TNF-α synthesis.12,13
The ethanolic extract of the plant has shown promising activity against acute inflammation induced using carrageenan. Similarly leaves of V. trifolia have depicted time and dose dependent activity of sub-acute inflammation which was introduced by pellets of cotton.14 In vitro studies to evaluate different parameters of inflammation done on cell lines have also shown promising results.15,16 In the present study chronic inflammation was induced using Freund’s adjuvant and levels of cytokines like IL-10 and TNF-α were evaluated after 28 days of treatment and the anti-TB studies were done using the Alamar Blue Assay. The effect the extract has on vital organs like liver, stomach and kidneys was also studied.
MATERIALS AND METHODS
Drugs and Chemicals
Complete Freund’s Adjuvant (CFA) Sigma Aldrich, Bangalore, ELR-TNF-α-1 kit and ELR-IL10 kits from Hysel India Pvt. Ltd., New Delhi, Indomethacin from Micro Labs Ltd., Solan, dexamethasone from MERCK and Co., Inc. Other chemicals used in the study belonged to analytical grade.
Plant material and extract
The Vitex trifolia leaves were obtained from the garden of KLE College of Pharmacy, Vidyanagar, Hubballi. The plant is locally known as Neerlakki which was then authenticated at Department of Botany, P.C. Jabin Science College, Hubballi by Dr. A.B. Sonappanavar. The extract of the leaves was prepared by first shade drying and then grinding the same in a mixer to get the desired powder.
To store the powder, it was transferred in an airtight polyethylene bag. For the preparation of the extract 50g of the drug in powdered form was weighed and then blended with 200 mL of 70% ethanol. This mixture was then refluxed for 1.5 hr at 65-70°C and this cycle was repeated consecutively three times. Extract was obtained in the form of a green semi solid mass and the total percentage yield of the extract was found to be 22.94%.
Experimental Animals
For the study adult male rats belonging to Wistar species and who weighed between 180-200g were obtained from Venkateshwara Enterprises, Bangalore. All experimental procedures were approved by the Institutional Ethics Committee (IAEC). Project Code: KLEU’s 010/IAEC.HBL/31st Aug 2013.
Acute Toxicity Study
For carrying out the acute toxicity studies Organisation for Economic Co-operation and Development (OECD) guidelines 423 were followed and to execute the toxicity studies Swiss albino mice were used which were obtained from the animal house of K.L.E. College of Pharmacy, Vidyanagar Hubballi. The toxicity studies involved using three female mice of weight 20-30g were procured, they were acclimatised after keeping them fasting for 18h being provided only with water. To administer the extract to the animals it was converted into a suspension using Tween 80 and dose of 2000 mg/kg b.w. (body weight) was administered orally to the animals. Further they were observed for 24 hr for behavioural changes, locomotion, muscle spasms, tremors, convulsions, and mortality and further were observed for a period of 14 days for occurrence of any toxic symptoms. At the end of the toxicity studies since there was no mortality at the dose of 2000 mg/kg b.w. for further experimental studies and evaluation of its anti-inflammatory activity 1/10th and 1/20th of the aforesaid dose was used.17
Experimental Design and Drug Treatment
For the evaluation of chronic inflammatory activity of the extract of leaves of Vitex trifolia Linn. CFA was used to induce the inflammation. CFA hyper immunizes the experimental animals leading to generation of high levels of T-lymphocytes along with hyperplasia also causing architectural changes in lymph nodes. The mycobacteria present in the CFA also induces production of cytokines like TNF-α, IL-12, IL-6, IL-10, IFN-γ.
Chronic Inflammation induced in Rats using CFA
Adult Wistar rats weighing around 180-200g were procured from Venkateshwara Enterprises, Bangalore. The rats had free access to water and food, and they were housed in suitable laboratory conditions during the experimental period. After two weeks of acclimatization the rats were divided into five groups of six rats each. To induce chronic inflammation 0.25 mL of CFA was subcutaneously in the palmar surface of the right-hand paw of the rats.
Groups-I and II were given the hydroalcoholic extracts of V. trifolia Linn. at 100 mg/kg b.w.mg/kg and 200 mg/kg dose of the body weight and the treatment was given for 28 days. Group-III and Group-V were treated with indomethacin 10 mg/kg b.w and dexamethasone 0.1 mg/kg b.w. respectively by the p.o route and the treatment were given for a period of 28 days. Group-IV was kept without treatment and served as control group.
At the end of the study blood was collected through the retro-orbital route and tested for the differential leukocyte count. Serum was separated collected and used for the estimation of TNF-α and IL-10 using ELISA kits. Histological studies of the stomach, liver, and kidneys on isolation. For the estimation of TNF-α and IL-10 the procedure/ method outlined in the ELISA kits was followed. During the entire estimation freshly prepared standard and samples were used. The addition was done carefully and slowly to avoid foaming. The anti-mycobacterial assay was conducted at the Dr. Prabhakar Kore Basic Science Research Centre, V.K. Institute of Dental Sciences College Campus KAHER Belagavi. The anti-mycobacterial activity was assessed using Microplate Alamar Blue Assay method (MABA) against Mycobacterium tuberculosis-MTCC300 (Microbial Type Culture Collection and Gene Bank). The method uses an oxidation reduction dye Alamar Blue which is an indicator of cellular growth and viability. For this experiment about 200 μL of deionized water which was sterile was added to all outer perimeter wells which are 96 in a plate, further to minimize evaporation of the medium in the test wells during incubation the 96 wells plate were added with 100mg/ kg b.w. μL of Middlebrook 7H9 broth and this was followed by serial dilution of compounds which were made directly on plate. The dilution series was made between concentrations (100 mg/kg b.w. to 0.19μ), further they were sealed and incubated for 24 hr. The change in colour is visually noticeable wherein the blue non-fluorescent colour on reduction changes to pink on reduction. MIC is defined as the lowest drug concentration at which the colour changes from blue to pink. Blue colour is indicative of no bacterial growth while pink colour is scored as growth.18
Statistical Analysis
The results and outcomes of the experimental values was expressed as mean ± standard deviation and evaluated using statistical one-way ANOVA followed by Post-Hoc Dunnet’s ‘t’ test or Bonferroni’s test using Graph pad Prism 5 software. A p value of < 0.05 was measured and considered as statistically significant.
RESULTS
Acute Toxicity Study
On treating animals with the hydroalcoholic extract of leaves of V. trifolia L. they demonstrated no behavioural changes and on administering the maximum dose of 2000mg.kg body weight there was no mortality reported even after 14days hence conclusion was drawn that 2000mg/kg is a safe dose and 1/10th and 1/20th of this dose i.e., 100mg/kg b.w. and 200mg/kg b.w. can be administered further to carry out the pharmacological investigations.
Effect of CFA induced Chronic inflammation
The current study was planned to assess how the effect of hydroalcoholic extracts of V. trifolia Linn. on chronic inflammation induced by CFA. The paraffin oil on being injected produces tumour necrosis and stimulates chemokines production. The treatment with the plant extract on two doses was given for a period of 28days after inducing chronic inflammation.
On the 28th day blood was collected through the retro-orbital route tested for differential leucocyte count, serum was separated to estimate the levels of TNF-α and IL-10 using ELISA kits and histological studies were carried out on stomach, liver, and kidneys on isolation.
Evaluation of Leucocyte count
On completion of the study period samples were collected from those animals of control group and treatment groups and were studied for DLC count. Elevated levels of Leucocytes as well as Polymorphonucleocytes were seen and the same has been depicted in Table 1 and Figures 1 and 2 respectively. Increase in the leucocyte and PMN count demonstrates aggravated inflammation in samples of animals belonging to the control group. Similarly, the lymphocyte values are seen to be below normal levels in the control group.
| Group | Differential Leucocyte Count (cells/cmm) | % of Polymorpholeucocytes | % of Lymphocytes |
|---|---|---|---|
| Normal | 6300±13 | 21±0.68 | 80±20 |
| Control | 15550±88 | 48±10 | 48±20 |
| V trifolia (100mg/kg b.w.mg/kg) b.w. | 9583±39* | 38±20* | 69±10* |
| V trifolia (200mg/kg)b.w. | 8967±33* | 33±10* | 77±10* |
| Indomethacin | 11717±43* | 31±03* | 75±02* |
| Dexamethasone | 11100mg/kg b.w.±57* | 31±03* | 74±02* |
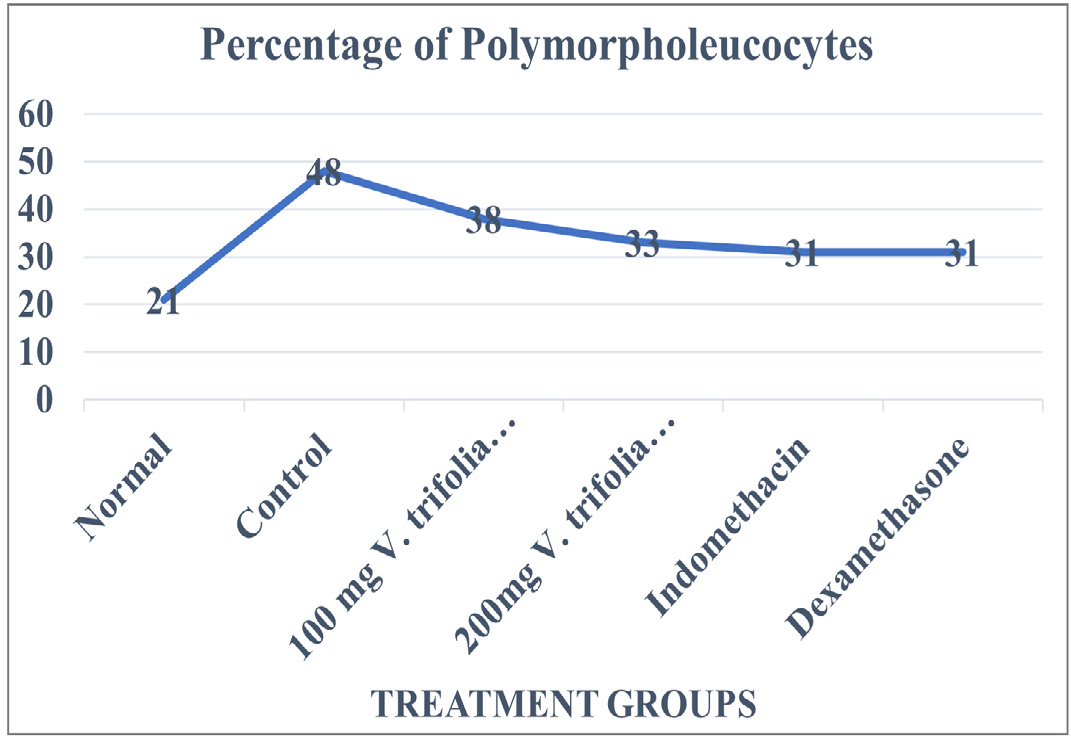
Figure 1:
Percentage of Polymorpholeucocytes in normal animals, animals induced with inflammation, animals receiving treatment with hydroalcoholic extracts of V. trifolia.
Linn at 100mg/kg b.w.mg/kg b.w*., 200mg/kg b.w*., Std. Drug Indomethacin* and Dexamethasone*.
Values are expressed as mean±SE (n=6). *Significantly different from control p<0.05.
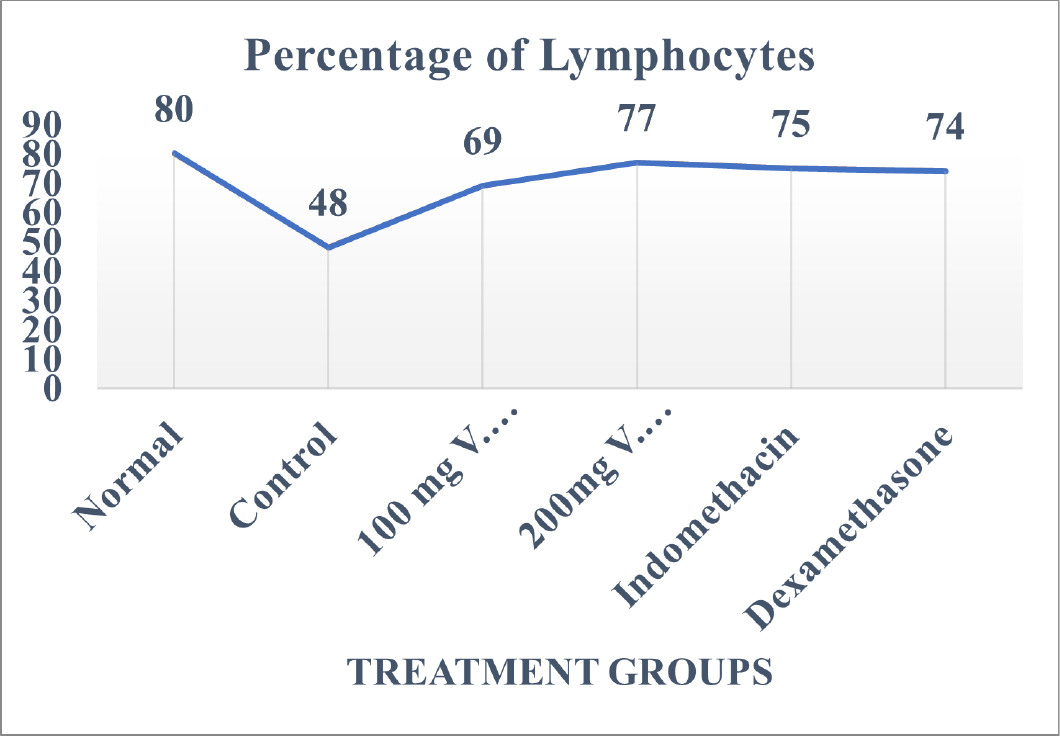
Figure 2:
Percentage of Lymphocytes in normal animals, animals induced with inflammation, animals receiving treatment with hydroalcoholic extracts of V. trifolia.
Linn at 100mg/kg b.w.mg/kg b.w*., 200mg/kg b.w*., Std. Drug Indomethacin* and Dexamethasone*.
Values are expressed as mean±SE (n=6). *Significantly different from control p<0.05.
Leucocytes count in control group was found to be 15550±88 cells/cmm while in group of animals who were treated with hydroalcoholic extract of V. trifolia Linn. at 100mg/kg b.w. and 200mg/kg b.w. the leucocyte count in them was found to be 8967±33 cells/cmm and 9583±39 cells/cmm respectively.
Leucocyte value in group of animals treated with Indomethacin and Dexamethasone was 10417±78 cells/cmm and 11100mg/ kg b.w.±57 cells/cmm, respectively. The percentage of PMN and lymphocytes was found to be 48±1% and 48±2% respectively for animals in control group while the percentage of PMN in the group of animals treated with Indomethacin and Dexamethasone was 31% for both. The Lymphocyte count for these groups was 75% and 74% respectively. The PMN percentage for group of animals treated with hydroalcoholic extract of V. trifolia Linn treated at 100 mg/kg b.w.mg/kg b.w. and 200 mg/kg b.w. was found to be 38±2% and 33±1% percent while that of Lymphocytes was 69±1% and 77±1% percent, respectively.
The group of animals who received treatment with hydroalcoholic extract of V. trifolia Linn. at 100 mg/kg b.w. and 200 mg/kg b.w. showed a decrease in the percentage levels of lymphocyte count as well as PMN count at every dose depicting that this action of the extracts is not dose dependable.
Assessment of levels of TNF-α and IL-10
The effect of the extract on the levels of TNF-α and IL-10 is graphically represented in Figures 3 and 4 respectively.

Figure 3:
Value of TNF-α in normal animals, animals induced with inflammation, animals receiving treatment with hydroalcoholic extracts of V. trifolia.
Linn at 100mg/kg b.w.mg/kg b.w***., 200mg/kg b.w***., Std. Drug Indomethacin*** and Dexamethasone***.
Values are expressed as mean±SE (n=6). ***Significantly different from control p<0.01.
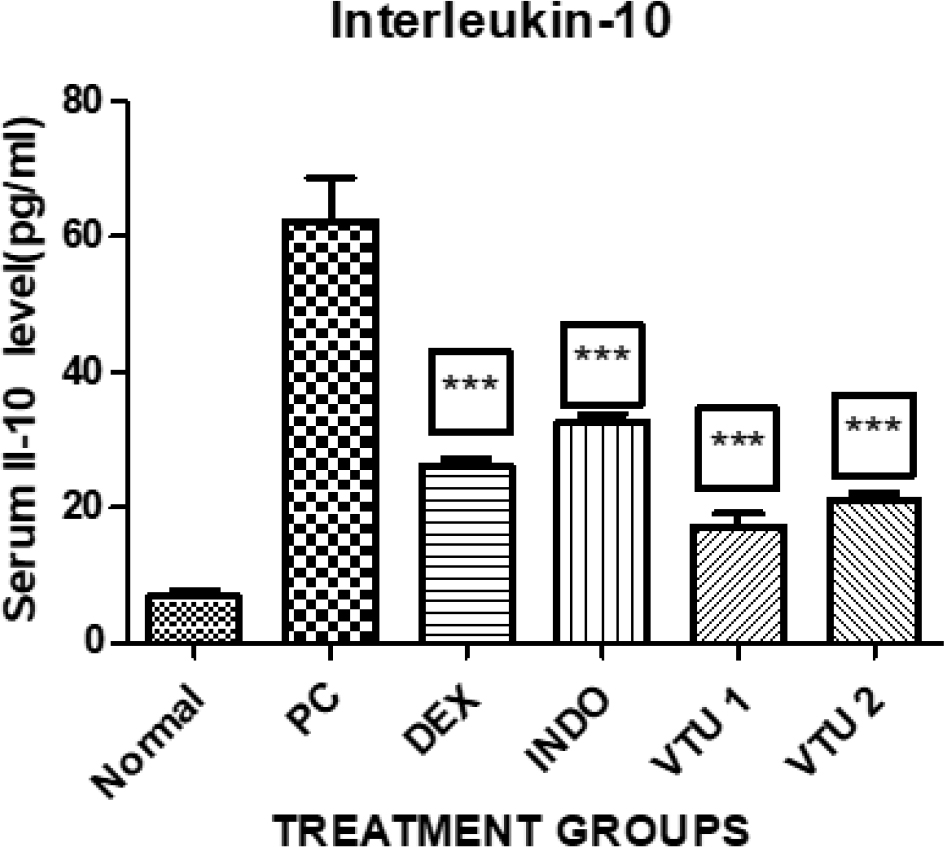
Figure 4:
Value of IL-10 in normal animals, animals induced with inflammation, animals receiving treatment with hydroalcoholic extracts of V. trifolia.
Linn at 100mg/kg b.w.mg/kg b.w***., 200mg/kg b.w***., Std. Drug Indomethacin*** and Dexamethasone***.
Values are expressed as mean±SE (n=6). ***Significantly different from control p<0.01.
Group of animals who received treatment with the hydroalcoholic extracts of V. trifolia Linn at the dose of 100mg/kg b.w.mg and 200 mg/kg b.w have shown significant inhibition (p< 0.01) in controlling the levels of TNF-α in these animals as compared to the animals belonging to the control group. In group of animals which received 100 mg/kg b.w.mg/kg body weight of hydroalcoholic extract of V. trifolia Linn the IL-10 values were seen to be of 36±6 pg/dL and the animals which received 200 mg/kg body weight of V. trifolia Linn. extract showed IL-10 mean value of 55±5 pg/dL. The Dexamethasone treated animals have shown a mean value of 106±15 pg/dL and those animals treated with Indomethacin have shown 100 mg/kg b.w.±12 pg/ dL respectively.
The group of animals treated with 100 mg/kg b.w.mg/kg body weight of hydroalcoholic extract of V. trifolia Linn. has shown mean value of 17±1 pg/dL while the group treated with 200mg/ kg body weight of V. trifolia Linn extract has shown mean value of 21±1 pg/dL. The group of animals treated with Dexmethasone and Indomethacin have shown mean value of 26±1pg/dL and 30±1pg/dL respectively.
The use of Freud’s adjuvant for inducing inflammation has caused a steep surge in the value of IL-10 which is visible in control group animals. However, in animals which received the treatment of hydroalcoholic extract of V. trifolia Linn. the effect is dose dependent low values seen in animals treated with 200mg/ kg of extract when compared to the animals which were treated with 100mg/kg b.w.mg/kg of extract (p<0.01). Groups which received Dexamethasone and Indomethacin for treatment also shown significant decrease in IL-10 values on comparing them to control group (p< 0.01).
Histological Studies
Vital organs like Liver, Stomach and kidneys were isolated and studied for their histological parameters. The study was done at 100mg/kg b.w.x. For the same a 5μ section of the tissue to be studied was isolated using a standard microtome and fixed using 10% formalin, immersed in paraffin. For staining purpose of the tissue haematoxylin and eosin was used and then histological studies were performed.
DISCUSSION
This experimental study aimed to analyse and authenticate the use of hydroalcoholic extract of leaves V. trifolia Linn. on chronic stage of inflammation and it’s in vitro anti-tubercular activity. Amongst the various methods available to induce chronic inflammation use of CFA has the advantage of inducing prolonged inflammation along with altering leukocyte proliferation and differentiation leading to exudation of cytokines like IL-1β, IL-6, 8, 10 and 12 along with TNF-α, and phagocytosis.
After the completion of treatment for 28 days for chronic inflammation the animals who received treatment with the extract and the group of animals belonging to control group were evaluated for their haematological parameters. The animals belonging to the control group exhibited aggravated inflammation which was correlated to the elevated levels of WBC and polymorphonucleocytes. The hydroalcoholic extracts of V. trifolia Linn leaves have exhibited considerable dose related activity on the levels of leucocytes and polymorphonucleocytes decreasing the count of both. Also, the animals who received the treatment with indomethacin and dexamethasone have shown a fall in the levels of WBCs along with a significant surge in the levels of lymphocytes.
In histological studies, on completing the 28 days study period of chronic inflammation the animals were sacrificed and the stomach, kidneys and liver were isolated further subjecting the same to histological studies.19–22
On isolating the liver tissue and studying under light microscope the group of animals who did not receive any treatment for inflammation exhibited acute congestion and deposition of RBCs. The liver sections of animals belonging to the group which received treatment with std. drugs like indomethacin and dexamethasone exhibited structural damage to the hepatic cells, damaged hepatic arteries and hepatic sinusoids along with acute congestion. The sections of liver obtained from animals which received treatment with 100mg/kg b.w.mg/kg b.w. of hydroalcoholic extracts of leaves of V. trifolia Linn show regular and standard appearing of the lobules of the hepatic cells along with normal structure of the hepatic cells and clear sinusoids while those which received and 200 mg/kg b.w. of the extract showed mild hepatic sinusoid damage Figure 5. The isolated kidney sections under light microscopy of sections obtained from animals in various group, the group of animals who were treated with V. trifolia extract of 100 mg/kg b.w.mg/kg b.w. of extract depicted an unremarkable cut section along with mild congestion, while group of animals who were treated with 200 mg/kg b.w. of extract depicted an unremarkable cut section but with congestion and distortion of renal tubules and glomeruli. However, the animals from the groups that belonged to positive control group and those which received treatment with indomethacin and dexamethasone showed distortion of cytoarchitecture, congestion, glomerular contraction and vacuolation of the cell lining Figure 6. When the transverse section of animals who received treatment with Indomethacin and Dexamethasone were studied the group of animals who received Indomethacin showed damaged epithelial cells and necrosis, while comparatively group of animals who received Dexamethasone unremarkable structural but with mild necrosis. The control group of animals who received no treatment depicted congestion, acute mucosal necrosis along with infiltration of neutrophils. The group of animals which received hydroalcoholic extract of V. trifolia Linn leaves at the dose of 100mg/kg b.w.mg/kg and 200mg/kg b.w. as treatment showed normal mucin producing glands and normal muscular layer Figure 7.
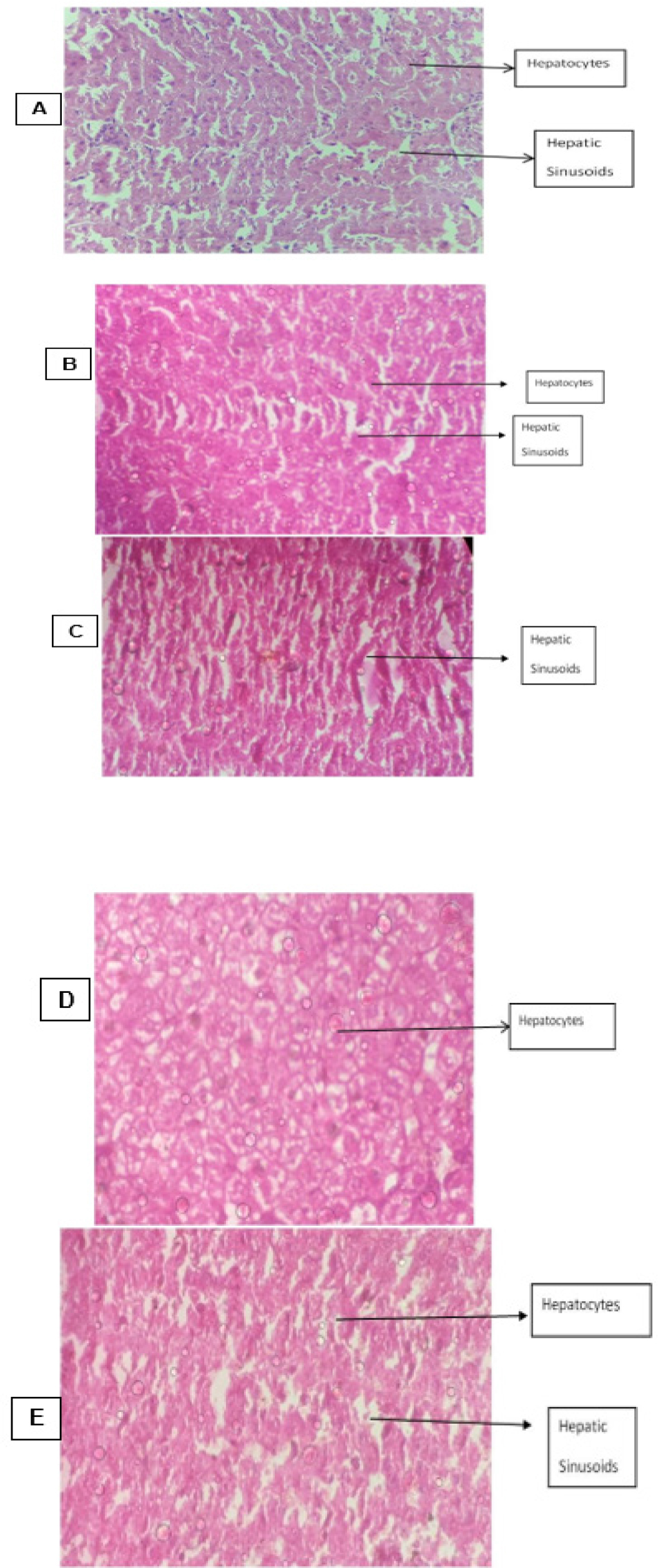
Figure 5:
T.S. of Liver Tissue from animals belonging to different groups.
T.S. of Liver: A: Disease control group B: Group treated with Indomethacin C: Group treated with Dexamethasone D: Group treated with 100mg/kg b.w.mg/ kg b.w. of Vitex trifolia E: Group treated with 200mg/kg b.w. of Vitex trifolia.
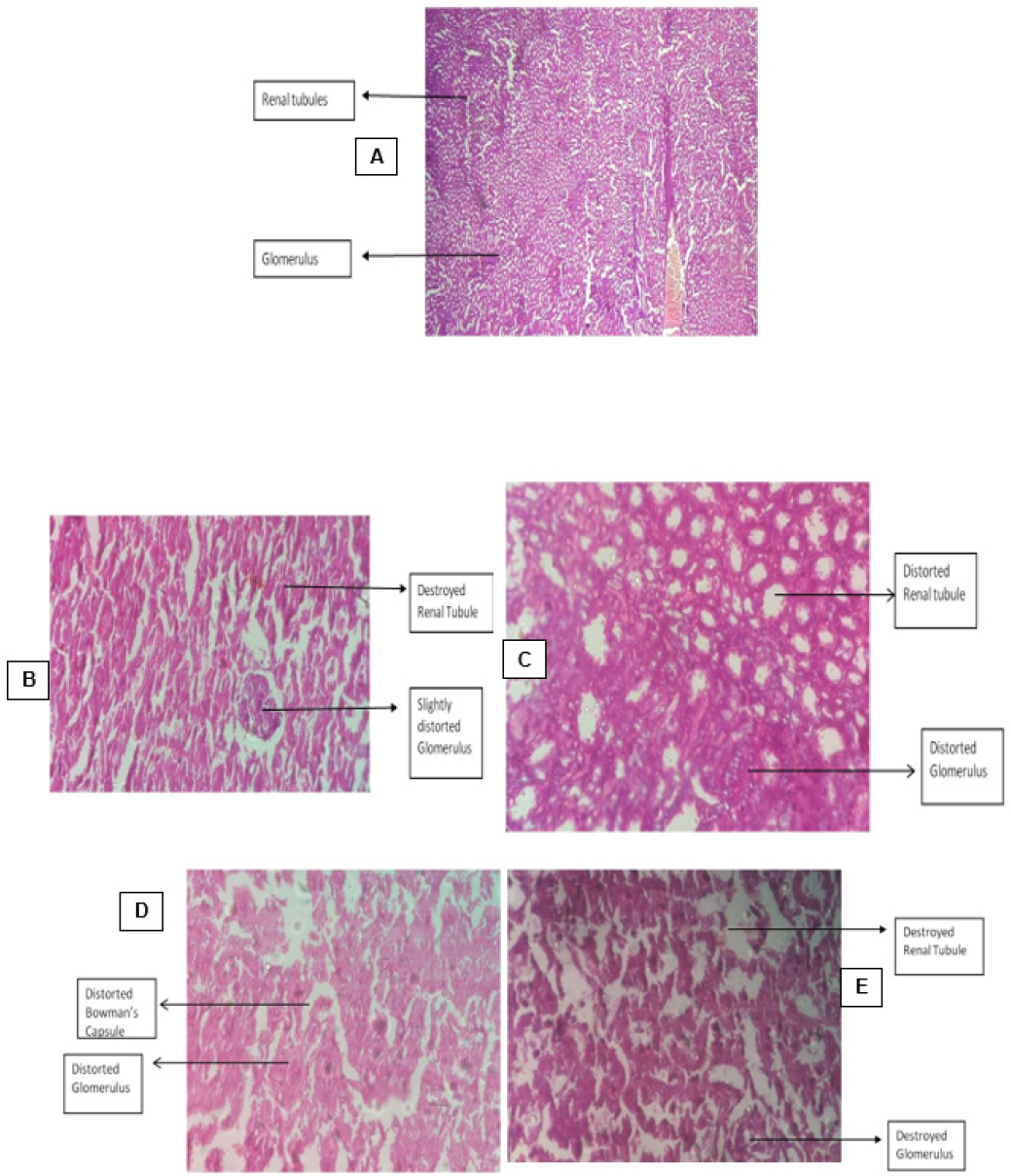
Figure 6:
T.S. of Kidney Tissue from animals belonging to different groups.
T.S. of Liver: A: Disease control group B: Group treated with Indomethacin C: Group treated with Dexamethasone D: Group treated with 100mg/kg b.w.mg/ kg b.w. of Vitex trifolia E: Group treated with 200mg/kg b.w. of Vitex trifolia.
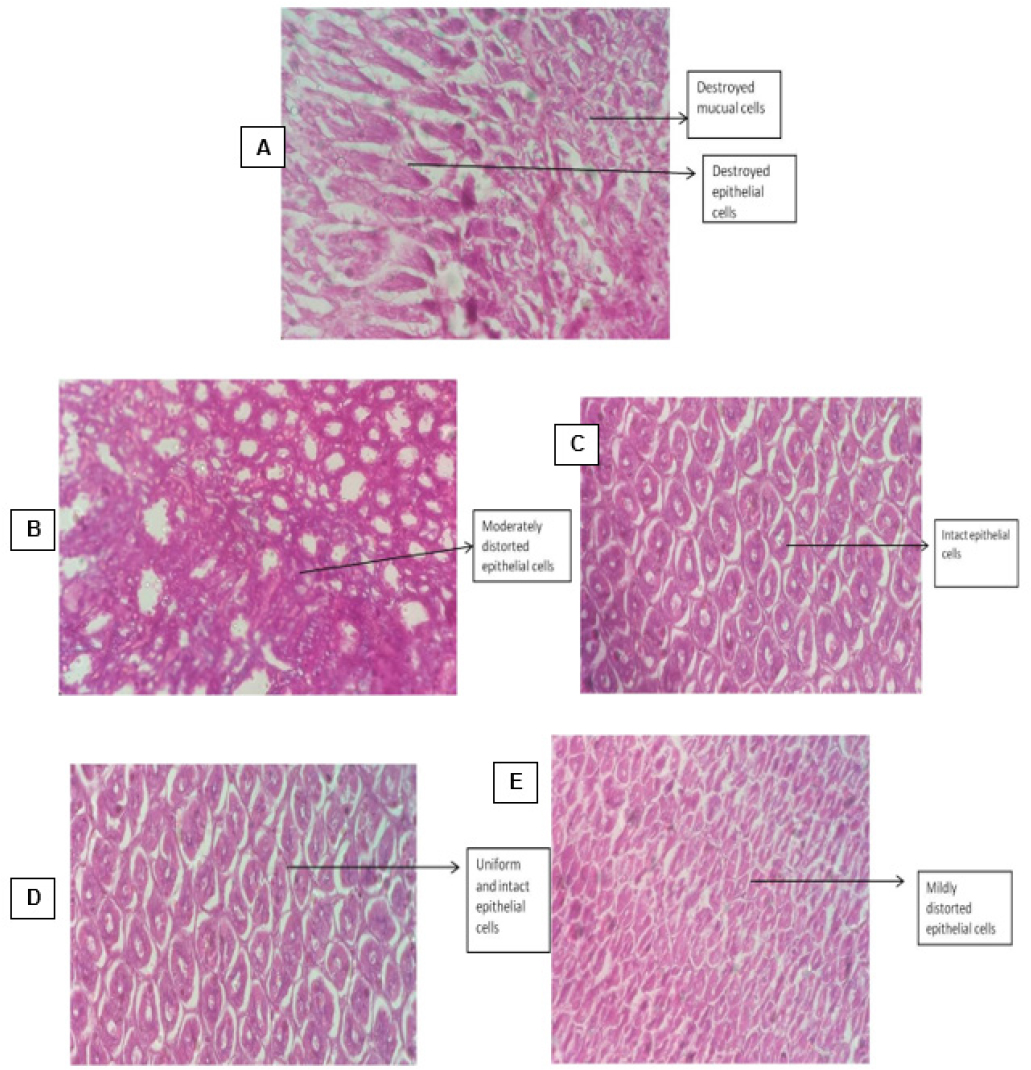
Figure 7:
T.S. of Stomach Tissue from animals belonging to different groups.
T.S. of Stomach: A: Disease control group; B: Group treated with indomethacin; C: Group treated with dexamethasone; D: Group treated with 100mg/kg b.w.mg/kg b.w. of Vitex trifolia; E: Group treated with 200mg/kg b.w. of Vitex trifolia.
To analyse and study the anti-tubercular activity of hydroalcoholic extract of leaves of V. trifolia Linn the use of Microplate Alamar Blue Assay was done. The procedure is a non-toxic and sensitive compared to the other MTT assays. The assay is based on the principle of visual colour change in the well shown by a reduction-oxidation indicator which fluoresces, and a cellular metabolic reduction is recorded colorimetrically. The fluorescence irradiated is direct in proportion to the number of viable cells which is measured on completion of the incubation period. This study showed that the hydroalcoholic extract of V. trifolia Linn possesses inhibitory anti-TB activity at 125 μg/ mL concentration inhibiting 50% growth of the M. tuberculosis. These results are at par with studies which reported isolation of diterpenoids and their effective anti-tubercular activity against the M. tuberculosis H37Rv strain in BACTEC-460 assay at MIC value of 100mg/kg b.w. and 25 μg/mL.23–25
CONCLUSION
The present experimental study the extract has shown that at the end of 28 days of treatment the hydroalcoholic extract of leaves of Vitex trifolia has lowered the levels leucocytes, polymorphonucleocytes and has maintained the level of lymphocytes in normal range when CFA was used to induce chronic inflammation. The extract has also shown to be effective in controlling levels of chemical cytokines IL-10 and TNF-α which are predominantly seen in the case of chronic inflammatory stage and tuberculosis.
Cite this article
Ankalikar AA, Viswanathswamy AHM, Ronad PM. Effect of Hydroalcoholic Extracts of Leaves of Vitex trifolia Linn on Chronic Inflammation and Tuberculosis. J Young Pharm. 2023;15(4):671-8.
ACKNOWLEDGEMENT
The authors are grateful to The Principal, KLE College of Pharmacy, Vidyanagar Hubli for facilitating research amenities.
ABBREVIATIONS
| IL-1 | Interleukin |
|---|---|
| TNF | Tumor Necrosis Factor |
| TB | Tuberculosis |
| MTb | Mycobacterium tuberculosis |
| IFN-γ | Interferon-gamma |
| IL-10 | Interleukin 10 |
| MDR-TB | Multi drug resistant tuberculosis |
| XDR-TB | Extensively drug resistant |
| NF-κB | Nuclear factor kappa B |
| CPCSEA | Committee for the Purpose of Control and Supervision of Experiments on Animals |
| LD50 | Lethal Dose |
| MIC | Minimum Inhibitory Concentration |
| MHB | Muller Hinton Broth |
| mg/kg | Milligram per kilogram |
| p.o | per oral |
| °C | Degree centigrade mm millimeter |
| CFA | Complete Freund’s Adjuvant |
| ELISA | Enzyme-linked immunosorbent assay |
| IC50 | Maximal inhibitory concentration |
| μg/mL | Microgram per milliliter |
| S.E | Standard error |
| WBC | White Blood Cells |
| DLC | Differential Leucocyte Count |
| PMN | Polymorphonucleocytes |
| pg/dl | Picogram per deciliter |
| T.S. | Transverse section |
| INH | Isoniazid |
| NSAIDS | Non-steroidal anti-inflammatory drugs |
| RBCs | Red Blood Cells |
| VTU1 | 100mg/kg b.w.mg/kg b.w. dose of hydroalcoholic extract of V. trifolia Linn |
| VTU | 200mg/kg b.w. dose of hydroalcoholic extract of V. trifolia Linn |
| PC | Positive Control group |
| DEX | Dexamethasone INDO Indomethacin |
References
- . J Med Microbiol. Array;Array(6):Array-75. [CrossRef]
- Rothschild BM, Martin LD, Lev G, Bercovier H, Bar-Gal GK, Greenblatt C, et al. Mycobacterium tuberculosis complex DNA from an extinct bison dated 17,000 years before the present. Clin Infect Dis. 2001;33(3):305-11. [PubMed] | [CrossRef] | [Google Scholar]
- Sasindran SJ, Torrelles JB. Mycobacterium tuberculosis infection and inflammation: what is beneficial for the host and for the Bacterium?. Front Microbiol. 2011;2(2):2 [PubMed] | [CrossRef] | [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19(1):683-765. [PubMed] | [CrossRef] | [Google Scholar]
- Olobo JO, Geletu M, Demissie A, Eguale T, Hiwot K, Aderaye G, et al. Circulating TNF-alpha, TGF-beta, and IL-10 in tuberculosis patients and healthy contacts. Scand J Immunol. 2001;53(1):85-91. [PubMed] | [CrossRef] | [Google Scholar]
- Goren MB, D’Arcy Hart P, Young MR, Armstrong JA. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976;73(7):2510-4. [PubMed] | [CrossRef] | [Google Scholar]
- Pahwa R, Goyal A, Jialal I. Chronic inflammation. 2022 [PubMed] | [CrossRef] | [Google Scholar]
- Moreira AL, Tsenova L, Aman MH, Bekker LG, Freeman S, Mangaliso B, et al. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect Immun. 2002;70(4) 2100mg/kg b.w.-7:2100-7 [PubMed] | [CrossRef] | [Google Scholar]
- Heifets L, Lindholm-Levy P. Heifets LB L-LPJ. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis populations. Am Rev Respir Dis. 1992;145(5):1223-5. [CrossRef] | [Google Scholar]
- Warrier PK. Indian medicinal plants: V. 3: A compendium of 500 species. 1995 [CrossRef] | [Google Scholar]
- Agarwal R. Anticholinesterase, antioxidant and nitric oxide scavenging activity of the aqueous extract of some medicinal plants. Br J Pharm Res. 2013;3(4):807-16. [CrossRef] | [Google Scholar]
- Nadkarni AK. Indian materia medica. 1976;1:1281-2. [CrossRef] | [Google Scholar]
- Tandon VR, Gupta RK. An experimental evaluation of anticonvulsant activity of Vitex negundo. Ind J Physiol Pharmacol. 2005;49(2):199-205. [PubMed] | [Google Scholar]
- Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, et al. Injury primes the innate immune system for enhanced toll-like receptor reactivity. J Immunol. 2003;171(3):1473-83. [PubMed] | [CrossRef] | [Google Scholar]
- Saklatvala J, Dean J, Clark A. Control of the expression of inflammatory response genes. Biochem Soc Symp. 2003;70(70):95-106. [PubMed] | [CrossRef] | [Google Scholar]
- de Fries R, Mitsuhashi M. Quantification of mitogen induced human lymphocyte proliferation: comparison of alamar Blue assay to 3H-thymidine incorporation assay. J Clin Lab Anal. 1995;9(2):89-95. [PubMed] | [CrossRef] | [Google Scholar]
- Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2(2):74-9. [PubMed] | [CrossRef] | [Google Scholar]
- Michels da Silva D, Langer H, Graf T. Inflammatory and molecular pathways in heart failure-ischemia, HFpEF and transthyretin cardiac amyloidosis. Int J Mol Sci. 2019;20(9):2322 [PubMed] | [CrossRef] | [Google Scholar]
- Zhang X, Wu X, Hu Q, Wu J, Wang G, Hong Z, et al. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019;236:116464 [PubMed] | [CrossRef] | [Google Scholar]
- Cutolo M, Soldano S, Smith V. Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev Clin Immunol. 2019;15(7):753-64. [PubMed] | [CrossRef] | [Google Scholar]
- Needham EJ, Helmy A, Zanier ER, Jones JL, Coles AJ, Menon DK, et al. The immunological response to traumatic brain injury. J Neuroimmunol. 2019;332:112-25. [PubMed] | [CrossRef] | [Google Scholar]
- Okamoto T, Gohil K, Finkelstein EI, Bove P, Akaike T, van der Vliet A, et al. Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286(1):L198-209. [PubMed] | [CrossRef] | [Google Scholar]
- Ducati RG, Ruffino-Netto A, Basso LA, Santos DS. The resumption of consumption — a review on tuberculosis. Mem Inst Oswaldo Cruz. 2006;101(7):697-714. [PubMed] | [CrossRef] | [Google Scholar]
- Barry PJ, O’Connor TM. Novel agents in the management of Mycobacterium tuberculosis disease. Curr Med Chem. 2007;14(18):2000-8. [PubMed] | [CrossRef] | [Google Scholar]
- Janin YL. Antituberculosis drugs: ten years of research. Bioorg Med Chem. 2007;15(7):2479-513. [PubMed] | [CrossRef] | [Google Scholar]
