ABSTRACT
Background
There have been studies of associations between disease of periodontal tissues and disorders affecting the entire body for instance cardiovascular diseases, stroke, asthma, low preterm birth weight, rheumatoid arthritis, etc. An inflammatory biomarker, plasma Homocysteine, has been identified as a factor that increases the chances for developing cardiovascular diseases. Previous studies have implied that there may be an analogous correlation between the levels of plasma- Homocysteine and chronic generalized periodontitis patients in otherwise systemically stable people. The aim behind this study is to determine the levels of plasma-Homocysteine in patients with chronic generalized periodontitis before and after non-surgical periodontal therapy.
Materials and Methods
36 patients were selected from outpatient department of Periodontology, divided into 2 groups, Group A entailing of 18 healthy control individuals, and Group B comprising of subjects with chronic generalized periodontitis which is further subdivided into Group B1 which includes chronic generalized periodontitis patients at baseline, and Group B2, chronic generalized periodontitis patients 6 months after scaling and root planning. Clinical parameters recorded and blood samples of all the group subjects were collected for analyzing the levels of plasma Homocysteine.
Results
The outcome showed significant differences in the mean plasma Homocysteine levels across the 3 research groups. Levels of plasma Homocysteine were significantly higher in Group BI, which declined after the scaling but not to the levels found among healthy individuals.
Conclusion
The result indicates an early detection of periodontitis by analyzing levels of this biomarker. Also, non-surgical periodontal therapy may supplement traditional plasma Homocysteine lowering therapy.
INTRODUCTION
The annihilation of the periodontal ligament, inflammation of the gingiva, and resorption of the alveolar bone are all symptoms of periodontitis, a chronic inflammatory disease. Studies have examined the relationships between periodontal diseases and systemic disorders. There are common risk factors for periodontitis and cardiovascular disorders, such as diabetes mellitus, hyperlipidemia, obesity, and aging. In periodontitis, periodontal pockets function as a reservoir of gram-negative, anaerobic, lipo-polysaccharide, and inflammatory mediators.1 Inflammation has been demonstrated to play a central role in various diseases and conditions affecting the whole body which is related to persistent infections. As evidence documented periodontitis and CVD are interlinked through common inflammatory cascades.2
A non-proteinogenic sulphur-containing amino acid, namely Homocysteine, was recognized in 1932, and 30 years later, in 1969, its role in pathology was reported for the first time. This enzyme is generated in the human body during the metabolism of methionine and can build up as a result of systemic excess or deficiency in foliate, vitamin B6, or B12.3 Hcy partakes in important processes such as transsulfuration, Cysteine (Cys) formation, transmethylation, etc. Patients with high levels of Hcy also called hyperhomocysteinemia, present with high blood pressure, and develop thromboembolism, premature atherosclerosis, mental retardation, bone fragility, eye disease, and even miscarriage. Clinical studies indicate that every 5 μM increase in Hcy levels is equivalent to a 20 mg/dL increase in blood cholesterol, which doubles the cardiovascular risk.4,5
Many researches, point to reactive oxidative species, as the mediators for the effects of Hcy. It generates reactive species directly or through autoxidation.6,7 These reactive oxygen radicals damage the myocardium of the heart. High levels of homocysteine empirically created (by loading rats with methionine) also cause the production of hydrogen peroxide and reduce the overall antioxidant capacity more in erythrocytes than in plasma.8,9
The premise that Hcy causes vascular injury by encouraging an inflammatory response and then having direct effects on the arterial wall or delayed effects on proteins and DNA components has recently progressed. The tissue destruction in periodontitis is due to the inflammatory response and the release of inflammatory mediators. This may be the possible mechanism that explains the links between the increased levels of Hcy and periodontitis and CVDs.10,11
Regardless of gender or age, the typical range of plasma Hcy is between 5 to 15 micro-mol/litres. Although studies are lacking on age and sex-related issues of Hcy, a study conducted on the Chinese population suggests that the levels of plasma Hcy increase significantly after the age of 50, also the levels of Hcy are higher in males than in females in each age range12 Kilmer McCully analysed data, and they defined the high levels of Hcy as Moderate (15-30 mol/L), Intermediate (30-100 mol/L), and Severe (>100 mol/L).13 The literature has suggested that there may be a connection between patients with plasma-Hcy levels and chronic periodontitis but systemically stable.
Patients suffering from chronic periodontitis have a reduction in inflammatory cytokine level attributable to non-surgical periodontal treatment.14 Therefore, it is conceivable that improving periodontal health might reduce local and systemic inflammation, which would reduce the difficulties associated with these disorders. Given these variables, it was predicted that periodontal intervention treatment may reduce the systemic inflammatory load, which in turn might function as Hcy-lowering therapy and serve a dual purpose in addressing both periodontal disease and other inflammatory disorders.
Therefore, in this study, we aimed to examine the Hcy levels in individuals with chronic periodontitis prior to and after nonsurgical periodontal treatment.
MATERIALS AND METHODS
Cross-sectional research was conducted on patients reporting to the outpatient clinic at Department of Periodontics, Peoples College of Dental Sciences and Research Center in Bhopal. Ethical committee approval obtained with number EC202027. Patients were selected based on the inclusion and exclusion criteria decided. Total number of participants was 36 including 54 sample size of the study, consisted of 3 groups of 18 each. Group A is a healthy or control group consisting of 18 participants and group B is a case group with chronic periodontitis, this group was analyzed pre- and post-treatment and named group B1 and B2.
The subjects included were 18-50 years of age, should be systemically healthy and non-smokers with minimum of 20 teeth should be present, excluding third molars. Clinical parameters such as Gingival Index (GI), Simplified-Oral Hygiene Index (OHI-S), Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL) were recorded. UNC- 15 probe were used to measure at all the six sites of each tooth present in all the subjects.
GI = 0, OHI-S < 1, mean PPD ≤ 3mm, and mean CAL = 0 were included under group A, and subjects with GI >1, OHI-S >3, mean PPD ≥4mm and mean CAL ≥ 3mm were included under group B1. The exclusion criteria of the study includes subjects with aggressive periodontitis, gross oral pathology and who had undergone phase I therapy in the past 3 months, active smokers and tobacco users, pregnant and lactating females, subjects on medication like contraceptives, anti-inflammatory drugs, steroids, immunosuppressants, anti-coagulants, lipid-lowering drugs, vitamin supplementation therapy or any type of antibiotics (within 6 months), subjects suffering from any systemic diseases/ conditions like renal disease, rheumatoid arthritis, cardiovascular diseases, nutritional deficiencies.
Prior to the collection of samples, a written informed consent was taken from the patients. Only those patients were included in the study who willingly volunteers to participate. Patients were advised to come after 12 hr of fasting for sample collection. Blood sample of 5 mL volume of venous blood, were collected by veni-puncture method from median cubital vein located at antecubital fossa. For this purpose, 5mL syringe were used. The collected blood sample then transferred to a vial containing EDTA anticoagulant. The blood sample obtained then centrifuged at 3500 rpm for 10min/1000 rpm for 15 min to isolate the plasma, which were pipetted out in a plastic vial and stored at 2-to-8°C. This extracted plasma then sent for evaluation of plasma homocysteine level with High Performance Liquid Chromatography with Mass Spectrometry (HPLC-MS).
RESULTS
Data analysis
A master chart was created after the data was collated in a Microsoft Excel file. The research groups were used to segment the data. Statistical Package for Social Sciences was used to conduct the statistical analysis (SPSS version 28 IBM). A continuous or ordinal dependent variable is tested using the Kruskal-Wallis H test to see if there are statistically significant variations between any two of an independent variable. A nonparametric measure of the degree and direction of connection between two variables measured on at least an ordinal scale is the Spearman rank-order correlation coefficient. The mean in between groups was compared using a one-way ANOVA. The importance of the distinction between the means of more than 2 groups is tested.
The participants who were in good health had a mean age of 39.3± 5.77 years, whereas those who had chronic periodontitis had a mean age of 34.3 ± 6.56 years. With a test statistic of 10.68 and a p value of 0.005 [Highly Significant], a statistically significant difference was found between the groups when the mean ages of all the groups were compared and their differences were assessed using the Kruskal Wallis test [Table 1].
| AGE | Mean | Standard Deviation | Minimum | Maximum | Kruskal-Wallis statistic | **p value |
|---|---|---|---|---|---|---|
| Group A | 39.33 | 5.77 | 28.00 | 47.00 | 10.68 | p = 0.005 |
| Group B | 34.33 | 6.56 | 27.00 | 49.00 |
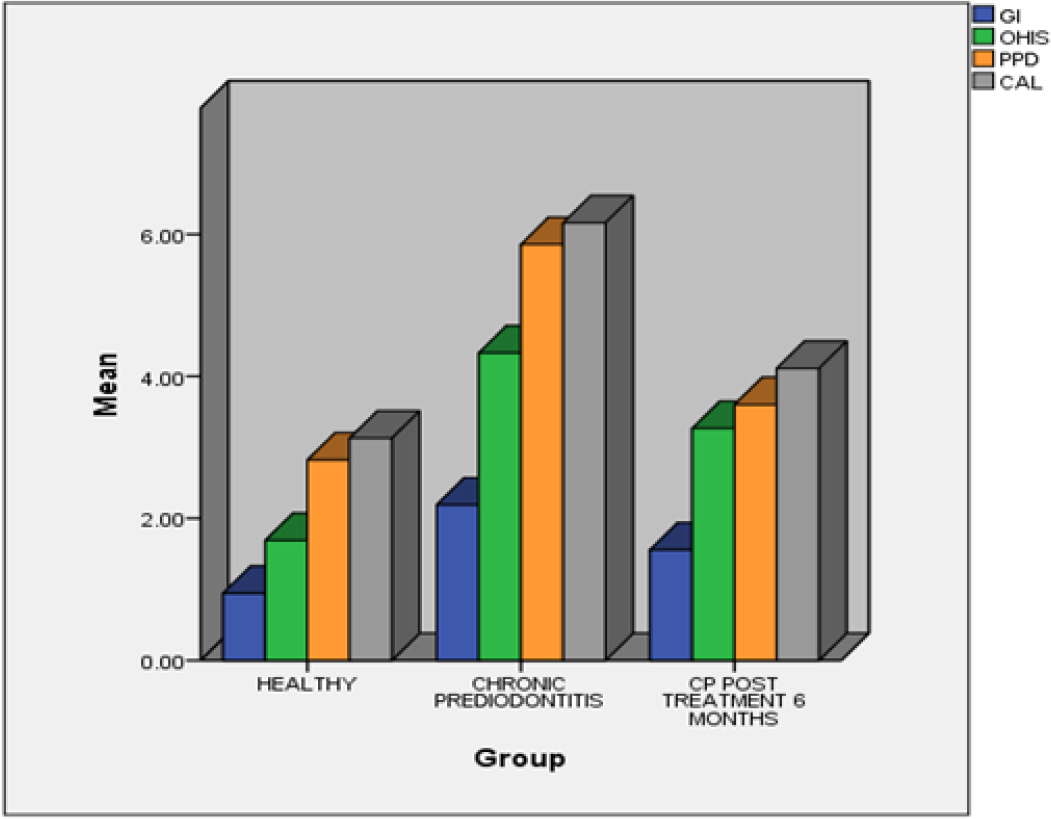
A comparison of mean gingival index score, mean OHI-S score, mean PPD and mean CAL among all the groups (Graph 1) was done and their difference was tested using Kruskal Wallis test. With test values (as given in Table 2) and p value of <0.001 difference between the groups, there was a statistically significant difference.
Graph 1:
Bar graph showing comparison of parameters among groups.
| Groups | Kruskal Wallis statistics | **p value PPP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B1 | Group B2 | |||||||||
| Maximum | Minimum | *SD | Maximum | Minimum | *SD | Maximum | Minimum | *SD | |||
| †GI | 0.30 | 1.90 | ±0.54 | 1.70 | 2.90 | ±0.35 | 1.00 | 2.00 | ±0.34 | 33.26 | <0.001 |
| ‡OHI-S | 0.50 | 2.80 | ±0.70 | 2.90 | 5.80 | ±0.86 | 1.30 | 4.40 | ±0.77 | 37.02 | <0.001 |
| §PPD | 1.90 | 3.50 | ±0.44 | 4.20 | 7.30 | ±1.06 | 2.00 | 5.20 | ±0.73 | 39.23 | <0.001 |
| ¦CAL | 2.30 | 3.70 | ±0.42 | 4.30 | 7.50 | ±1.10 | 3.00 | 6.00 | ±0.90 | 36.6 | <0.001 |
| Hcy | 5.00 | 9.00 | ±1.27 | 14.00 | 19.00 | ±1.41 | 7.00 | 15.00 | ±2.22 | 45.19 | <0.001 |
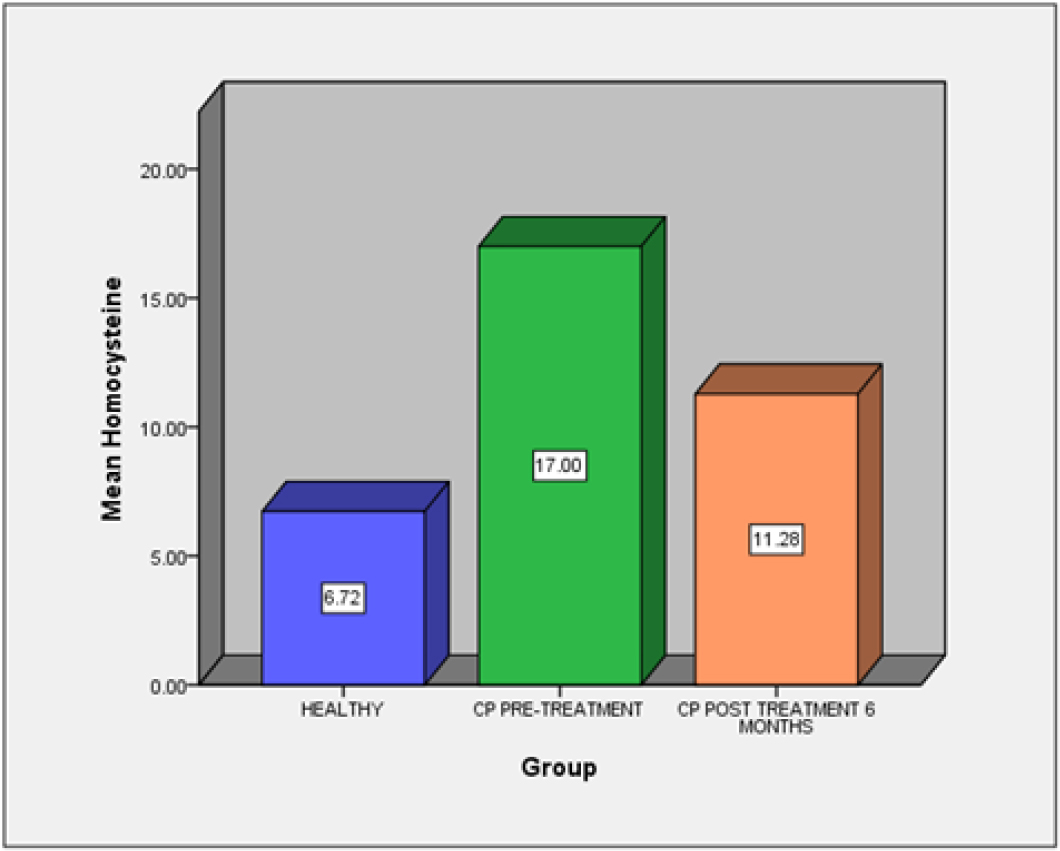
A comparison of mean Hcy levels (Graph 2) among all the groups was done. Between the groups, a statistically significant difference was seen, with a test statistic of 45.19 and a P-value of <0.001 [Highly significant] [Table 2]. Based on data, Group B1 showed higher value, compared to Group A and B2. The correlation between Hcy levels and the clinical parameters was tested. It was found that there was a substantial association between them using the Spearman’s correlation test. With a p value of 0.05, the correlation coefficient was determined to be significant [Table 3].
Graph 2:
Bar graph showing comparison of Plasma homocysteine levels among groups.
| Parameters | Correlation | Plasma Hcy |
|---|---|---|
| AGE | Spearman’s Correlation | -0.315* |
| Sig. (2-tailed) | 0.020 | |
| †GI | Spearman’s Correlation | 0.742 |
| Sig. (2-tailed) | 0.000 | |
| ‡OHI-S | Spearman’s Correlation | 0.769** |
| Sig. (2-tailed) | 0.000 | |
| §PPD | Spearman’s Correlation | 0.770** |
| Sig. (2-tailed) | 0.000 | |
| ¦CAL | Spearman’s Correlation | 0.759** |
| Sig. (2-tailed) | 0.000 |
DISCUSSION
Periodontitis is an inflammatory condition of supporting tissue of the teeth. There are vast literatures that point out the connection of periodontitis with numerous systemic conditions, in particular CVDs. Periodontitis and CVDs are both long-term, non-communicable illnesses. With a global incidence of 45–50% and a severe form affecting 11.2% of the population, periodontitis is the sixth most prevalent illness in humans. 17.9 million fatalities per year are caused by cardiovascular disease globally (one third of all deaths). The systemic inflammatory sequelae of bacteremia, including increases in C-reactive protein and oxidative stress, are proposed as possible explanations for this connection.
At first Hcy was thought to be as one of the factors that link periodontal diseases with cardiovascular conditions.15 Basically, there is inhibition of nitric oxide in the endothelium as a result of increased levels of Hcy which directly alters the vasodilation function resulting in excessive production of reactive oxygen species in the myocardium. As a result, these elevated Hcy levels raise the risk of endothelial cell damage, which is caused by the apoptotic cell death of smooth muscle and endothelial cells16,17 and is generally regarded as an inflammatory response in the vascular system.18
Furthermore, it has been shown in the literature that periodontitis impairs vascular function by damaging blood vessels (through increased, immune cells, cytokines, reactive oxygen species and nitric oxide) as a result of heightened systemic inflammation and bacteremia.19 Similar to Hcy, it has been demonstrated that periodontal bacteria lower nitric oxide bioavailability,19 and this biological route may be an initial step in this connection. Reactive oxygen species are overproduced in both situations, leading to imbalanced conditions that harm tissue.20,21
Although there is a noticeable difference between the groups based on the Gingival Index (GI) score and the Simplified Oral Hygiene Index (OHI-S), Group B1 has a higher value when compared to Group A and B2, which is statistically highly significant with a p value of <0.001. This is a result of the lower levels of plaque in healthy individuals, which subsequently increased as periodontal disease advanced and decreased following non-surgical periodontal treatment. These findings are in line with research by Mallapragada S et al.3 which demonstrates that those with periodontal disease had lower levels of oral hygiene maintenance and greater levels of gingival inflammation. The findings of the current study concur with those of studies by Gautami S. Penmetsa et al.,2 Bhardwaj S et al.,22 and Apurva A et al.23
The Clinical Attachment Level (CAL) and Probing Pocket Depth (PPD) demonstrate a significant difference between the groups, however when the groups are compared, mean Group B1 has a greater value than Group B2 and Group A, that was statistically significant with the value of p <0.001. The mean differences between the groups are consistent with studies by Mallapragada S et al.3 which concluded that the elevated levels of PPD and CAL were found in pretreated periodontitis patients. The current study results are also in agreement with a study conducted by Gautami S. Penmetsa et al.,2 and Bhardwaj S et al.22
When compared to Group B2 and Group A, Group B1 had the highest plasma Hcy levels, which was statistically highly significant (p value <0.001). Numerous other researches in the literature have shown similar outcomes to those of the current study. In a research by Vineet Kotwal et al.,24 it was found that individuals with chronic periodontitis had considerably higher blood Hcy levels. In a research by Rosamma Joseph et al.,25 and several other investigations, it was discovered that patients with periodontitis had the highest levels of serum Hcy.26 A promising biomarker for the early detection and diagnosis of chronic periodontitis is plasma Hcy.
There was a significant positive correlation found between Hcy levels and the OHI-S, PPD and CAL measured. But in Contrast there was no correlation found in between these variables in a study conducted by Gautami S. Penmetsa et al.2 There is a need to conducted further research to conclude the plasma Hcy as a biomarker of the disease.
The current study has certain limitations, including the small sample size used; larger sample sizes from more longitudinal studies are required. The inclusion criteria did not take the aetiology of chronic periodontitis into account, which might have an impact on the plasma Hcy levels. The individuals in the current study varied significantly in age across all groups, which may make comparisons inappropriate.
CONCLUSION
Homocysteine is an inflammatory biomarker, the increased levels of it is regarded as a cardiovascular disease risk factor. Likewise elevated levels of Hcy in chronic generalized periodontitis patients were also found in this study. Periodontitis an inflammatory condition may be linked with cardiovascular diseases and hyperhomocystenemia by a pro-inflammatory state. With the development of this enzyme, periodontitis may now be diagnosed earlier. Furthermore, non-surgical periodontal treatment can be added to traditional homocysteine-reduction medicine. Larger sample numbers should be used in future study on plasma homocysteine to confirm the findings and use the test.
References
- Zope S, Pisal AA. Assessment of nonsurgical periodontal therapy on plasma homocysteine levels in chronic periodontitis. J Citical Rev. 2020;7(7):665-8. [CrossRef] | [Google Scholar]
- Penmetsa GS, Bhaskar RU, Mopidevi A. Analysis of plasma homocysteine levels in patients with chronic periodontitis before and after nonsurgical periodontal therapy using high-performance liquid chromatography. Contemp Clin Dent. 2020;11(3):266-73. [PubMed] | [CrossRef] | [Google Scholar]
- Mallapragada S, Kasana J, Agrawal P. Effect of nonsurgical periodontal therapy on serum highly sensitive capsule reactive protein and homocysteine levels in chronic periodontitis: A pilot study. Contemp Clin Dent. 2017;8(2):279-85. [PubMed] | [CrossRef] | [Google Scholar]
- Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183-98. [PubMed] | [Google Scholar]
- Candido R, Zanetti M, Current p. Current perspective. Diabetic vascular disease: from endothelial dysfunction to atherosclerosis. Ital Heart J. 2005;6(9):703-20. [PubMed] | [Google Scholar]
- Pang X, Liu J, Zhao J, Mao J, Zhang X, Feng L, et al. Homocysteine induces the expression of C–reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis. 2014;236(1):73-81. [PubMed] | [CrossRef] | [Google Scholar]
- Starkebaum G, Harlan JM. Endothelial injury due to cooper-catalyzed hydrogen peroxide generation from homocysteine. J Clin Invest. 1986;77(4):1370-6. [PubMed] | [CrossRef] | [Google Scholar]
- Filip C, Albu E, Nina Zamosteanu M, Irina J, Silion M. Hyperhomocysteinemia’s effect on antioxidant capacity on rats. Cent Eur J Med. 2010;5(5):620-6. [PubMed] | [CrossRef] | [Google Scholar]
- Albu E, Filip C, Zamosteanu N, Jaba IM, Linic IS, Sosa I, et al. Hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2012;78(4):554-5. [PubMed] | [CrossRef] | [Google Scholar]
- Shastry S, James LR. Homocysteine-induced macrophage inflammatory protein-2 production by glomerular mesangial cells is mediated by PI3 kinase and p38 MAPK. J Inflamm (Lond). 2009;6(1):27 [PubMed] | [CrossRef] | [Google Scholar]
- Zhang X, Chen S, Li L, Wang Q, Le W. Folic acid protects motor neurons against the increased homocysteine, inflammation, and apoptosis in SOD1G93A transgenic mice. Neuropharmacology. 2008;54(7):1112-9. [PubMed] | [CrossRef] | [Google Scholar]
- Andersson A, Isaksson A, Hultberg B. Homocysteine export from erythrocytes and its implication for plasma sampling. Clin Chem. 1992;38(7):1311-5. [PubMed] | [CrossRef] | [Google Scholar]
- Xu R, Huang F, Wang Y, Liu Q, Lv Y, Zhang Q, et al. Gender- and age-related differences in homocysteine concentration: a cross-sectional study of the general population of China. Sci Rep. 2020;10(1):17401 [PubMed] | [CrossRef] | [Google Scholar]
- Lahiri KD, Datta H, Das HN. [2013 Feb 3];Reference interval determination of total plasma Hcy in an Indian population. Indian J Clin Biochem. 2014;29(1):74-8. [PubMed] | [CrossRef] | [Google Scholar]
- Kornman KS, Duff GW. Candidate genes as potential links between periodontal and cardiovascular diseases. Ann Periodontol. 2001;6(1):48-57. [PubMed] | [CrossRef] | [Google Scholar]
- Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP, et al. Homocysteine impairs the nitric oxide synthase pathway role of asymmetric dimethylarginine. Circulation. 2001;104(21):2569-75. [PubMed] | [CrossRef] | [Google Scholar]
- Fournier P, Fourcade J, Roncalli J, Salvayre R, Galinier M, Caussé E, et al. Homocysteine in chronic heart failure. Clin Lab.. 2015;61(9):1137-45. [PubMed] | [CrossRef] | [Google Scholar]
- Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14(1):6 [PubMed] | [CrossRef] | [Google Scholar]
- Muñoz Aguilera E, Suvan J, Buti J, Czesnikiewicz-Guzik M, Barbosa Ribeiro A, Orlandi M, et al. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc Res. 2020;116(1):28-39. [PubMed] | [CrossRef] | [Google Scholar]
- Holmlund A, Lampa E, Lind L. Poor response to periodontal treatment may predict future cardiovascular disease. J Dent Res. 2017;96(7):768-73. [PubMed] | [CrossRef] | [Google Scholar]
- Keceli HG, Ercan N, Karsiyaka Hendek M, Kisa U, Mesut B, Olgun E, et al. The effect of the systemic folic acid intake as an adjunct to scaling and root planing on clinical parameters and homocysteine and C-reactive protein levels in gingival crevicular fluid of periodontitis patients: A randomized placebo-controlled clinical trial. J Clin Periodontol. 2020;47(5):602-13. [PubMed] | [CrossRef] | [Google Scholar]
- Bhardwaj S, Prabhuji ML, Karthikeyan BV. Effect of non-surgical periodontal therapy on plasma homocysteine levels in Indian population with chronic periodontitis: a pilot study. J Clin Periodontol. 2015;42(3):221-7. [PubMed] | [CrossRef] | [Google Scholar]
- Pisal AA, Abbayya K, Suragimath G, Varma S, Sameer A, Kale VT, et al. Effect of Non-surgical periodontal therapy on plasma homocysteine levels in patients with chronic periodontitis- A prospective study. IJPHRD. 2020;11(6):84-9. [PubMed] | [CrossRef] | [Google Scholar]
- Kotwal V, Jandial S, Kotwal B, Mengi R, Sharma M, Tomar V, et al. Assessment of impact of nonsurgical periodontal therapy on serum homocysteine levels in patients with chronic periodontitis. Int J Sci Stud. 2020;8(5):114-7. [PubMed] | [CrossRef] | [Google Scholar]
- Joseph R, Nath SG, Joseraj MG. Elevated plasma homocysteine levels in chronic periodontitis: a hospital-based case-control study. J Periodontol. 2011;82(3):439-44. [PubMed] | [CrossRef] | [Google Scholar]
- Gadde S. Elevated plasma homocysteine levels in chronic periodontitis: a pilot study. Int J Sci Res. 2019;8(3):4-5. [PubMed] | [CrossRef] | [Google Scholar]
