ABSTRACT
Background
The current study compared the anti-arthritic efficacy of formulated Solid Lipid Nanoparticles (SLNP) of α-mangostin to its pure ethanolic extract of α-mangostin. Because of its anti-inflammatory property, Alpha-mangostin (α-mangostin) from Garcinia mangostana should be beneficial in the treatment of Rheumatoid arthritis (RA’s). Formulating a solid lipid nanoparticle with improved bioavailability will be beneficial for the efficacy of the formulation.
Materials and Methods
α-mangostin and α-mangostin Solid Lipid Nanoparticle (MSLNP) were tested in a rat model at doses of 75 mg/kg and 75 mg/kg, respectively, for their effectiveness against Freund’s Complete Adjuvant (FCA)-induced rheumatoid arthritis compared with the standard group (Prednisolone). On the 0th, 8th, 15th, 22nd, and 28th days, paw volumes, paw thicknesses, body weight, stair climbing test, mobility test, and arthritis score were measured. Histopathological examination of ankle joints was done after the completion of the 28th day of study.
Results
MSLNP showed a significant anti-arthritic effect when compared to pure α-mangostin, possibly by lowering paw edema, paw thickness, and the arthritis score index. It also reduced hyperalgesia by improving mobility and stair climbing abilities in experimental rats. The results of histopathology confirmed the significance of MSLNP compared with α-mangostin for anti-arthritic effect. In experimental animals, the MSNLP had a better anti-rheumatoid effect compared with the pure extract.
Conclusion
The observations of the present study contribute to the understanding that MSLNP has shown superior anti-arthritic activity in comparison to the pure ethanolic extract of α-mangostin.
INTRODUCTION
Rheumatoid Arthritis (RA’s) is an auto-immune disease that mostly affects the tiny synovial joints of the hands and foot.1 Genetics and ecological conditions are both essential variables in the progress of RA’s. Tobacco use and smoking are still key risk factors in the disease development.2 Tobacco use, combined with other factors such as pollution, toxins, irradiation, and chemotherapy drugs, results in the production of reactive oxygen species, which promote ligament and cartilage deterioration by increasing the levels of synovial fluid lipid peroxides. It can also result in the production of increased glycation and oxidative protein products, both of which may contribute to the inflammatory responses seen in RA’s. The nuclear factor-B pathway is also activated by reactive oxygen species, which adds to the auto immune responses in RA’s. Thus, administering anti-oxidants to counteract the effects of reactive oxygen species might be one of the treatment methods for RA’s.3 The integrity of the gut, along with its homeostasis, is maintained by the gut bacteria. Irritation of gut mucosa and changes in gut flora as a result of infection or tobacco use are additional well known causes of autoimmunity, and they play an important for the development of RA’s.4 One of the genetic variables thought to trigger autoimmunity, enhance inflammation, and so contribute to RA’s risk is “Shared Epitope (SE) alleles and MHC region expressing HLA’s protein”. Personalized therapy must be designed for each patient due to a variety of causal variables. Because precise drug medication is not yet widely employed in clinical practice,5 the ultimate aims in RA’s care are still to relieve inflammation and associated pain, prevent and manage joint deterioration, and maintain the joint’s functionality. Analgesics, NSAIDs, glucocorticoids, and synthetic and/or biological disease RA’s is now treated with medications that modify anti-rheumatic drugs.6
These medications, on the other hand, have a limited impact on preventing joint deterioration. NSAID’s affect the gut lining by producing ulceration. Adrenal insufficiency (acute) and withdrawal syndrome are caused by glucocorticoids. Anti-rheumatic drugs can upset the gut, affecting immune responses and increasing the risk of infection. As a result, each has its own set of limitations, and RA’s treatment must be found to be both safe and efficacious. The new compound should also be toxic free and have excellent PK data.
The pericarp of the Garcinia mangostana fruit contains the therapeutic xanthones Alpha-mangostin (α-mangostin). It is highly safe and successful in treating a range of pathological diseases.7 The anti-inflammatory potential of α-mangostin has been highlighted in a number of preclinical investigations, which is interesting.8 α-mangostin may be a useful therapeutic drug for the treatment of RA, according to scientific evidence. The presence of α-mangostin (anti-inflammatory and antioxidant moiety) in Garcinia mangosteena, suggests that it might be used to treat RA’s. Furthermore, the traditional system prescribes it for the treatment of auto immune disorders such as RA’s. Poor bioavailability of α-mangostin extract limits its usage as an effective treatment option for anti-arthritis. Increasing bioavailability by formulating a solid lipid nanoparticle of α-mangostin overcomes this limitation and maximizes the drug’s availability at the site of action. The formulated solid lipid nanoparticle is then used to evaluate its anti-arthritis effect in comparison with a pure extract of α-mangostin.
In this study, we compared the anti-arthritic effect of α-mangostin Solid Lipid Nanoparticles (MSLNP) with α-mangostin pure extract. The study included five groups: the normal control group, the FCA induction control group, the α-mangostin treatment group, the MSLNP treatment group, and the Prednisolone standard group. The study was carried out for 28 days (excluding the acclimatization and grouping periods). Induction of FCA was started after the seventh day of dosing with treatment groups (vehicle for the control group). The anti-arthritic potential was evaluated for parameters such as paw volume, body weight, paw thickness, stair climbing ability, mobility test, and arthritis score. The evaluated parameters were compared for their level of significance using a two-way ANOVA and Dunnett’s test multiple correlations (for comparing significance with every group). On the last day of the study, animals were killed, their ankle joints were separated, and the histopathological changes after treatment were examined.
MATERIALS AND METHODS
Materials
Saline solution, Prednisolone, Freund’s complete adjuvant (from sigma Aldrich, USA), Garcinia mangosteena ethanolic extract (Laila Nutracueticals, Vijayawada, India).
Animals
Healthy male Sprague Dawley rats (150–200 g) aged between 6 to 8 weeks were purchased from Mahaveere Enterprises (Hyderabad, India). The IAEC (Institutional Animal Ethics Committee Registration Number- LN/IAEC/Efficacy/LN220305) gave its approval to all of the research and guidelines provided by the CPCSEA (committee for the purpose of control and supervision of experimental animals). The animals were kept at 22 ± 2°C, with a humidity of 45-55% and a 12 hr dark-light cycle period. Prior to the studies, the rats were given unrestricted access to water and feed. A total of 30 rats were used for the study.
Acclimatization and Grouping of rats
Before being enrolled in the trial, the animals will undergo a three-day acclimatization period. Rats will be monitored daily for any clinical symptoms during this time, and body weight was measured on the first days of the acclimatization phase.
Randomized rats into five groups (6 animals/group). The groups were labeled as follows:
Group 1 – Normal Control group,
Group 2 – Induction control group (FCA control group),
Group 3 – 75mg/Kg α-mangostin ethonolic extract treated group,
Group 4 – 75 mg/Kg Solid lipid nanoparticle of α-Mangostin (MSLNP) treated group,
Group 5 – 10mg/Kg Prednisolone treated group.
On day 7 (seven days after the administration of the test, reference, or vehicle), arthritis is induced in all groups (except Group 1) by administering a single subcutaneous injection of FCA (Sigma-Aldrich, USA) diluted to a concentration of 50 μL under a light isoflurane anesthetic into the sub plantar region of the hind limb. 50 μL of normal saline was used as the vehicle for the normal control group (Group 1). The vehicle, test items, and Prednisolone were given to the respective study group animals for a period of 28 days. All experimental rats were euthanized by an overdose of CO2 (gas asphyxiation) inhalation at the end of a 28-day study. After complete removal of blood, the animals’ ankle joints were separated for histopathology evaluation.
α-Mangostin Solid Lipid Nanoparticle (MSLNP) preparation
To formulate α-mangostin-SLNPs (MSLNP), hot melt homogenization followed by ultrasonication was utilized. Briefly, solid lipid (2:3 ratio of stearic acid to Precirol ATO5) was heated to a melting temperature of 60-65°C (approximately 5°C above the solid lipid melting point) in order to produce SLNPs. The liquefied lipid was mixed for 5 min at high rpm using a high-speed stirrer (15,000 rpm). The required amount of α-mangostin (1 part) was added to the molten substance while being constantly stirred at the same temperature, and the mixture was thoroughly mixed for an additional 5 to 10 min. Poloxamer 407-Sodium Taurocholate (1:1) surfactant and co-surfactant were mixed in distilled water, heated to the lipid phase temperature, added to the molten lipid, and homogenised at 15,000 rpm for 30 min to create a coarse emulsion. The pre-emulsion was then sonicated at 50% amplitude for 3 min, six times for 30 sec each, with 1 min breaks in between at 4-8°C. The emulsion was then lyophilized using mannitol as a cryoprotectant for 24 hr in a freeze dryer.
Preparation of Suspension
The required quantities of ingredient (α-mangostin extract/ MSLNP/Prednisolone) were weighed and transferred to a mortar and pestle. To obtain the final concentration of (mg/mL) suspension, the necessary volume of vehicle containing 0.5% w/v Sodium Carboxymethylcellulose (SCMC) in water was added and triturated. To create a homogeneous suspension, the suspension was moved to a suitable container and spun in a vortex.
Measurement of Paw Volume by Digital Plethysmometer
A plethysmometer (Pan Lab, LE7500) was used to assess paw volume on day 0 (one day prior to treatment), 08, 15, 22, and 28 after the administration of vehicle, test items, and reference compound. At each time point, the volume of the paws was measured twice, and an average was computed. The difference between the final and initial paw volumes was used to define the edema volume. To assess the severity of secondary lesions, the right hind paw’s volume was measured. The percentage inhibition of edema volume was calculated using the following formula:
Where,
V0 – Edema volume of normal control group; Vc- Edema volume of FCA control group; Vt – Edema volume of treatment group.
Evaluation of Body Weight
The animal’s body weight was calculated the day before treatment began, once a week for the duration of the trial, and before it ended.
Stair Climbing Test
Rats were taught to climb wooden stairs in the observational cage for 7 days, with three steps that were 5 cm, 10 cm, and 15 cm high, with water on the step-2 and feed on the step-3.9 Stair climbing score details are given in Table 1. After the FCA injection, this test was done on days 0, 08, 15, 22, and 28.
| Sl. No. | Score | Climbing ability |
|---|---|---|
| 1 | 0 | Unable to climb. |
| 2 | 1 | Climbs onto step-1. |
| 3 | 2 | Climbs onto step1 and step 2. |
| 4 | 3 | Climbs onto step-1, 2, and 3. |
Evaluation of Mobility Test
For the mobility test, each rat was trained for 7 days in a wooden box. Each rat’s mobility was tested on days 0, 08, 15, 22, and 28 following the FCA injection. In a 5 min observation cage, the mobility of animals was studied.10 The mobility testing was scored in accordance with the description in Table 2.
| Sl. No | Score | Mobility |
|---|---|---|
| 1 | 0 | If rat is sitting. |
| 2 | 1 | If rat is crawling. |
| 3 | 2 | If rat is walking. |
| 4 | 3 | If rat races and climb with difficulty. |
| 5 | 4 | If rat races and climb well. |
Evaluation of Paw thickness
Using digital Vernier callipers (IITC Life Science Inc., CA), the paw thickness of the hind limbs will be assessed on days 0, 08, 15, 22, and 28 after administration of the test item. At each time point, one measurement of paw thickness was taken.
Evaluation of arthritis score
The visual arthritis grading methods were utilized to determine the severity of the arthritis. The arthritis score varied from 0 to 4, with the following grades given in Table 3.11
| Sl. No | Score | Swelling | Erythema |
|---|---|---|---|
| 1 | 0 | No swelling | No erythema |
| 2 | 1 | Slight swelling | Slight erythema |
| 3 | 2 | Moderate swelling | Moderate erythema |
| 4 | 3 | Significant swelling | Significant erythema |
| 5 | 4 | Major deformity | Major incapability |
Histopathological evaluation of ankle joints
Rats were treated with isoflurane anaesthesia on the 28th day (final day of the study), then killed, and their left hind leg joints were removed and preserved in 10%-formalin solution. Tissue treated with formalin was dehydrated. The tissues were decalcified further, trimmed, embedded in paraffin wax, and sectioned at 3-5 μM intervals. Hematoxylin and eosin staining was used to evaluate these sections for inflammation in synovial junctions, deterioration of the bone and cartilage, and proteoglycan loss.
Statistical Analysis
The experimental results were expressed as mean ± standard error. A two-way ANOVA followed by Dunnett’s-tests were used for statistical analysis in GraphPad Prism to determine the level of significance for one variable from each group. p values of 0.05 or lower were required for significance.
RESULTS
Effect on Paw Volume
Treatment with FCA, generated arthritis (primary) in the rats, as evidenced by considerable swelling in the paw (p<0.0001), which lasted for 28-days when compare to the normal control group. Although the paw volume of FCA-treated rats increased, it was not large enough to be classified as secondary arthritis. When compared to FCA-induced control animals, the MSLNP and Prednisolone treatment groups reduced paw edema from the 8th to the 28th day (p<0.0001), whereas the α-mangostin treated group did not show significance (i.e., a p value of 0.525). However, the decrease in paw edema is lower compared in MSLNP treatment group compared with the standard (Prednisolone) treatment groups. Effect of MSLNP on Paw volume results were displayed in Figure 1.
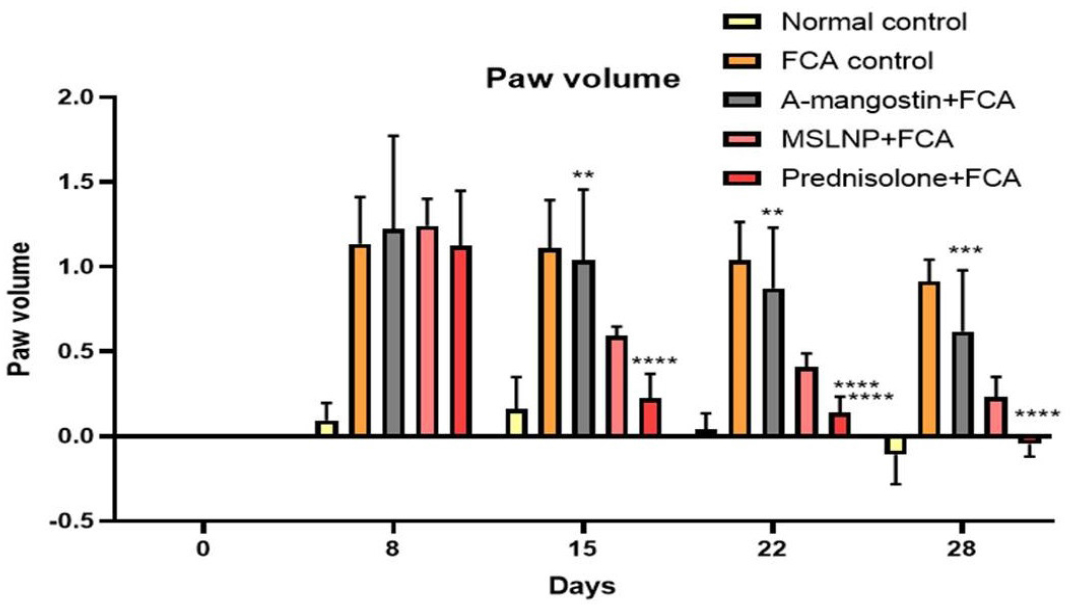
Figure 1:
Effect of MSLNP on Paw volume of FCA induced arthritis in rat model. Data represented as Mean ± SE; ****p<0.0001, ***p<0.0005, **p<0.005 compared to FCA control group (two-way ANOVA followed by Dunnett’s test).
Effect on Body Weight
Treatment with FCA lowered body weight relative to normal controls, although the difference was not significant. However, as demonstrated in Figure 2, treatment with α-mangostin, MSLNP, and Prednisolone resulted in a considerable weight gain from the 15th to the 28th day when compared to FCA control rats. The Dunnett’s correlation confirms the significance with treatment groups with a p-value of 0.0017 for α-mangostin, 0.0007 for MSNLP and 0.0005 for Prednisolone treatment groups.
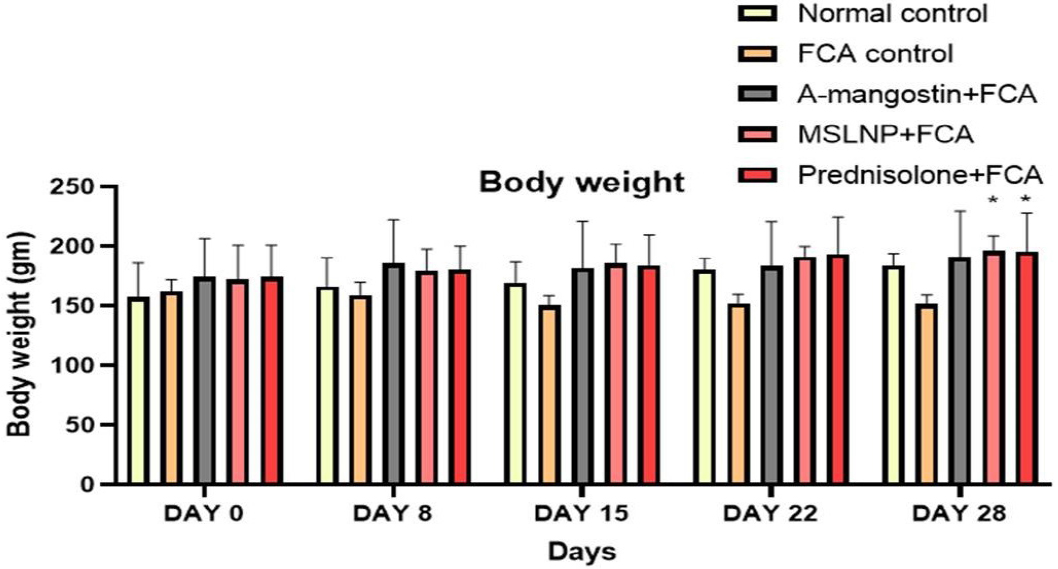
Figure 2:
Effect of MSLNP on Body weight of FCA induced arthritis in rat model. Data represented as Mean ± SE; *p<0.05 compared to FCA control group (two-way ANOVA followed by Dunnett’s test).
Effect on Stair Climbing and Motility Test
When compared to normal control groups (Group 1), FCA administration dramatically reduced stair climbing behavior from day 8 to day 28, demonstrating the formation of hyperalgesia, as given in the data from Figure 3. Treatment with α-mangostin, MSLNP, and prednisolone showed a significant decline in scores compared with the FCA induction group. Dunnett’s multiple correlation confirms the clear significance (p<0.0001) of every treatment group and normal control group when the results are compared with the FCA induction control group.
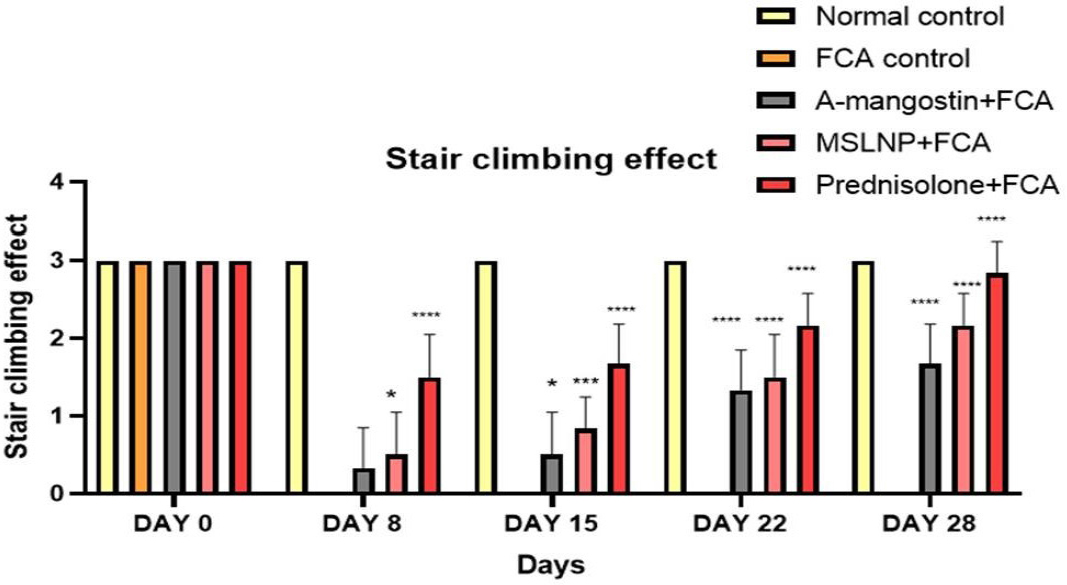
Figure 3:
Effect of MSLNP on Stair climbing effect of FCA induced arthritis in rat model. Data represented as Mean ± SE; ****p<0.0001, ***p <0.0005, *p<0.05 compared to FCA control group (two-way ANOVA followed by Dunnett’s test).
When compared to normal control group, FCA-administered groups showed a substantial decrease in mobility from day 8 to day 28 as given in Figure 4. The mobility score of all groups except the control group decreased after induction of FCA (i.e., on the 8th day). MSLNP treatment groups showed a significant increase in mobility score when the results were compared with α-mangostin treatment groups. Dunnett’s multiple correlation confirms the clear significance (p<0.0001) of every group when the mobility test results are compared with the FCA induction control group.
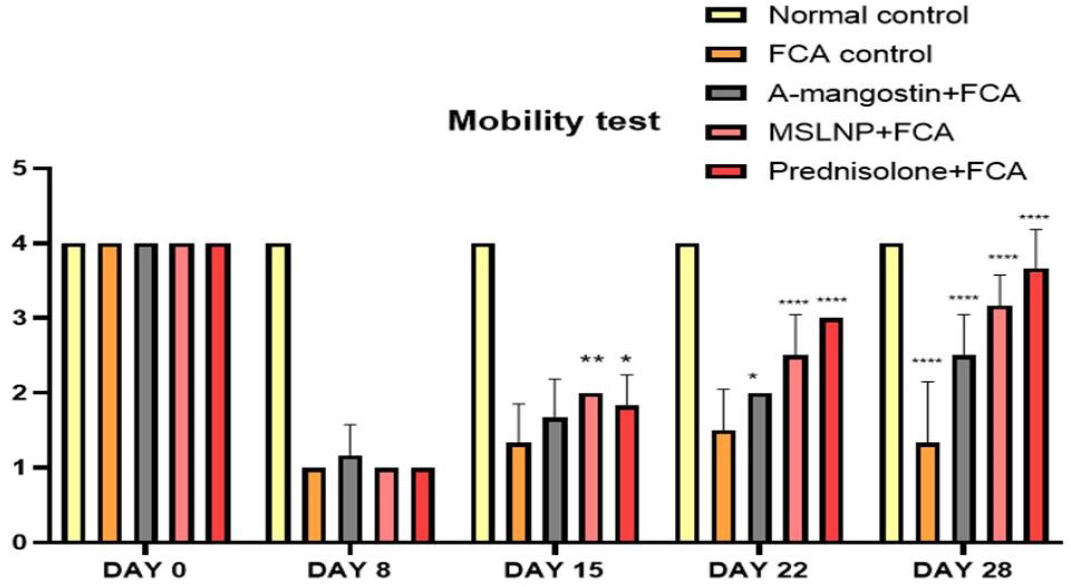
Figure 4:
Effect of MSLNP on Mobility test of FCA induced arthritis in rat model. Data represented as Mean ± SE; ****p<0.0001, **p<0.005, *p <0.05 compared to FCA control group (two-way ANOVA followed by Dunnett’s test).
Effect on Paw thickness
The results of paw thickness are represented as % inhibition of paw edema compared with the FCA-induced group as given in Figure 5. Dunnett’s multi correlation confirms that with treatment with the α-mangostin group, there is no significance in the p-value (0.1134) against the FCA-induced control group. The MSLNP (p < 0.05) and Prednisolone (p < 0.0001) groups were significantly different from the FCA induction group, indicating that these treatments have an anti-arthritic effect on paw thickness.
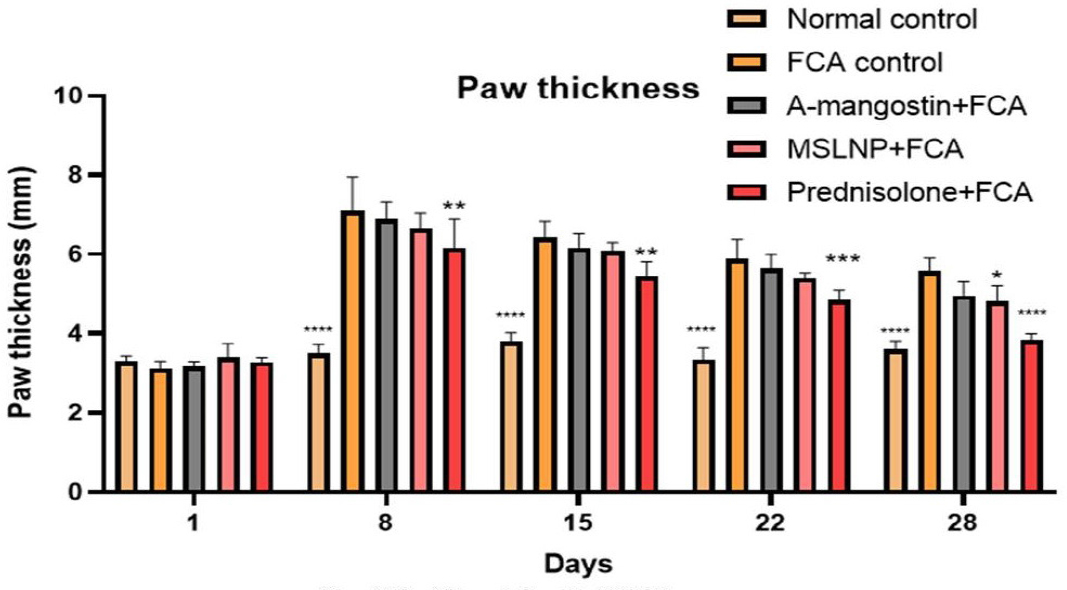
Figure 5:
Effect of Paw thickness on Paw thickness of FCA induced arthritis in rat model. Data represented as Mean ± SE; ****p<0.0001, ***p<0.0005, **p<0.005, *p <0.05 compared to FCA control group (two-way ANOVA followed by Dunnett’s test).
Effect on Arthritis score
The results of the arthritis score were given in Figure 6. There are significant differences in the mobility of the induction group and that of the treatment groups. There is significant swelling and erythema in the induction group compared to all other groups. Among the treatment groups, prednisolone-treated groups showed better grades compared to the other two treatment groups. The results of MSLNP- treated groups are better compared with those of α-mangostin treated groups. Dunnett’s multiple correlation confirms clear significance of all groups with FCA-induction control group (p < 0.0001)
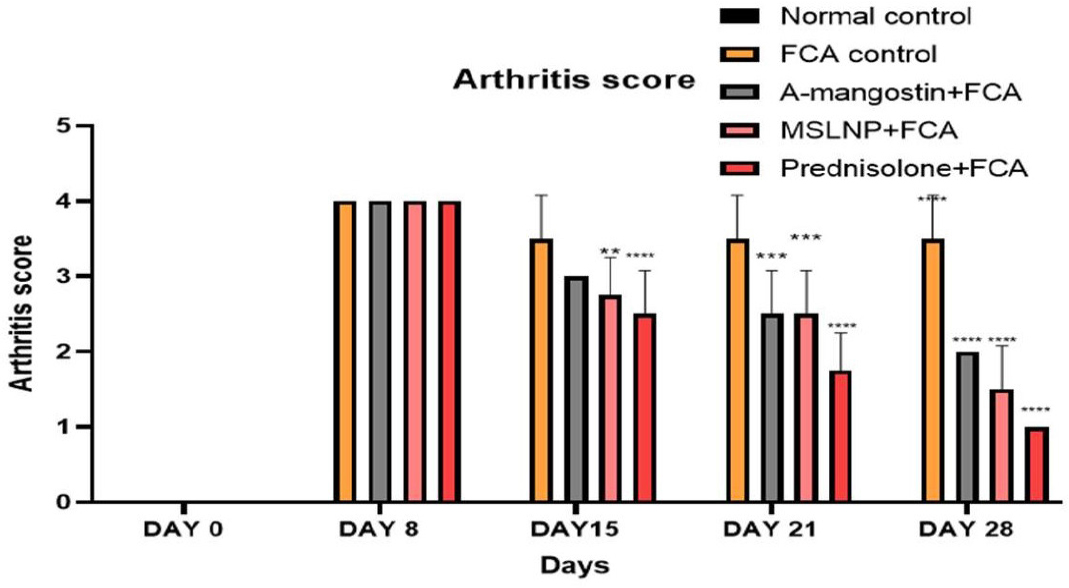
Figure 6:
Effect of Paw thickness on Arthritis score of FCA induced arthritis in rat model. Data represented as Mean ± SE; ****p<0.0001, ***p<0.0005, **p<0.005 compared to FCA control group (two-way ANOVA followed by Dunnett’s test).
Histopathological evaluation
The histopathological abnormalities of the ankle joint in rats from various treatment groups are depicted in Figure 7. In control rats, there was no evidence of synovial inflammation or bone or cartilage erosion, and the synovial lining displayed a normal synovial space. The synovial membrane cells in FCA-induced arthritic rats proliferated, forming a thick, multilayered synovial cell lining with reduced synovial space. Additionally, inflamed synovial tissue was seen.
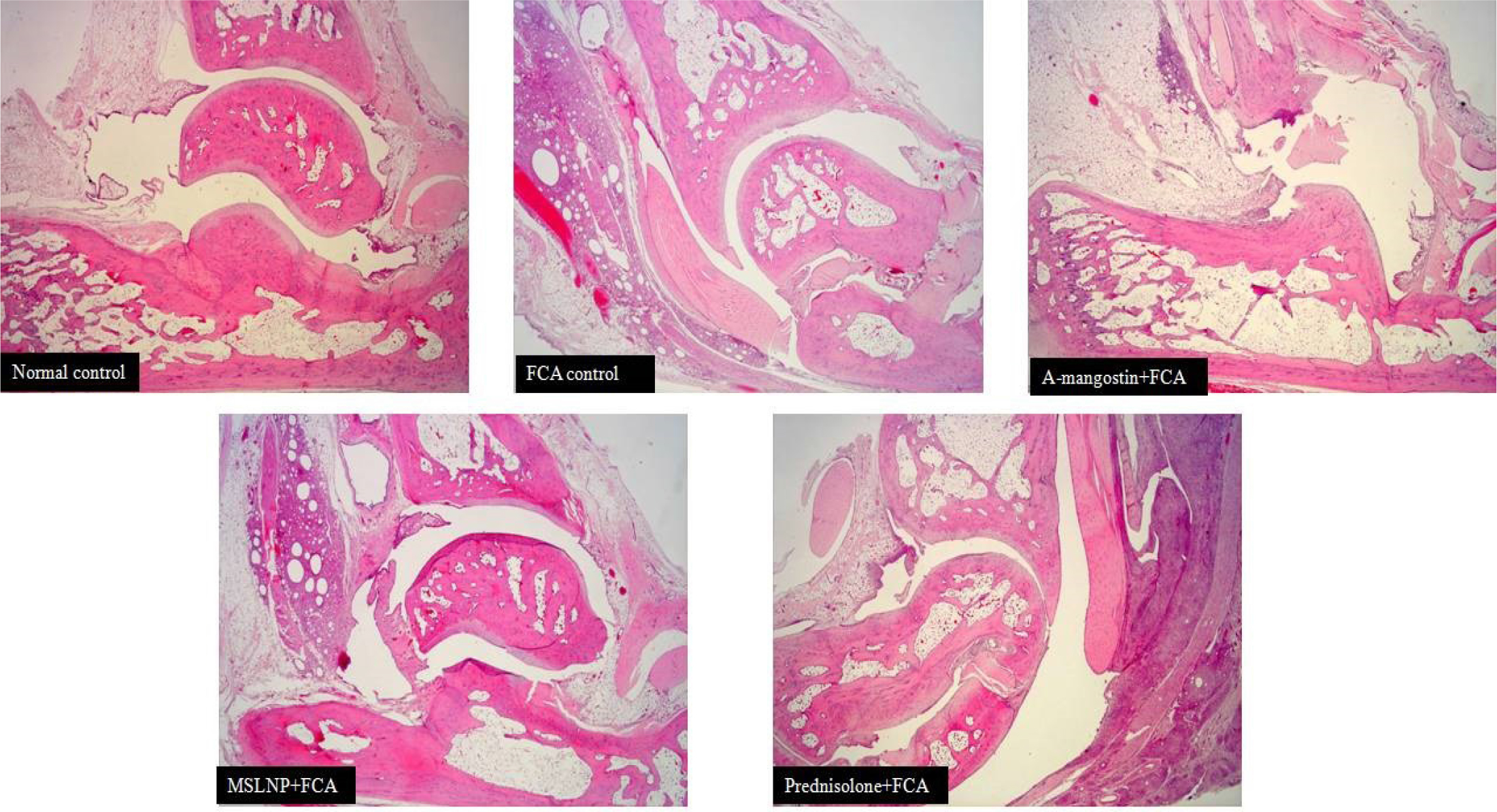
Figure 7:
Histopathology section of tarsotibial joint of left hind paw from different animal groups. Syonovial inflammation (s), Caritlage erosion (c), Bone erosion (b), and Proteoglycan loss (p).
Compared to the FCA-induced arthritic group, the prednisolone, MSLNP, and α-mangostin treated groups showed less synovial hypergenesis, inflammation, accumulation of infection in synovial fluid, bone and cartilage erosion, and narrowing of the joint space.
DISCUSSION
Between 1980 and 2019, the RA’s prevalence was 460 per 1 lakh people worldwide, with variations due to study design and geographic region.12 It has a close relationship with immunological and other organs in addition to having an impact on joints. Drug development for RA requires a therapeutic strategy that takes into account the pathological and metabolic aspects of the disease.13 FCA-induced arthritis has been shown to be helpful in assessing the uniqueness of a drug used to treat RA. The effectiveness of anti-inflammatory and anti-arthritic medications used to treat RA is frequently evaluated using the FCA-induced RA model.14 Rats with FCA-induced arthritis share several characteristics with people with RA, making it a more suitable method for research.15 When FCA is applied subcutaneously under the subplantar region of the hind limb (left) to the paw of the animal, it causes a localized inflammatory response as well as a chronic systemic response. It also exhibits a high reaction to heat response proteins and cartilage’s proteoglycan. The local reactions that follow FCA induction subside in 3– 4 days, while the chronic inflammation might last for 2 weeks to a few months.16
NSAID’s that suppress cyclooxygenase enzymes (COX 1 and 2) can effectively treat acute and chronic pain, and are thus frequently used.17 Opioid analgesics continue to be the most effective therapeutic option for the treatment of moderate to severe pain. On the other hand, undesirable side effects continue to be an issue. Acute and inflammatory responses have been treated with a variety of innovative medicines in recent decades. Nonetheless, the number of such medications is limited, and their usage is restricted due to the fact that they all have negative effects. As a result, the development of new pain management procedures may provide doctors with more pain management options. α-mangostin has anti-arthritic efficacy in FCA driven arthritis in rats (chronic inflammation). Formulating a solid lipid nanoparticle of α-mangostin with decreased particle size and in more bioavailable form makes the formulation to be better efficacious towards its anti-arthritic effect. Hence, we for the first time formulated MSLNP and compared the efficacy of the formulation (MSLNP) against pure ethanolic extract of α-mangostin for RA treatment.
From the day of acclimatization, the animals received oral treatment for 28 days at the doses listed in Table 2.
The assessment of various parameters, such as paw volume, body weight, stair climbing test, mobility test, paw thickness, and arthritis score, can be used to determine the anti-arthritis activity of treatment groups against the FCA-induced groups. In the acute stage after FCA induction, swelling of the paw (the 1° RA’s lesion) was used to assess the inflammatory response, and the increased volume of the paw (the 2° RA’s lesion) was measured to determine the immune response resulting in polyarthritis in the chronic phase. The paw edema values of FCA-induced groups are compared with all other groups. Except for the α-mangostin group, where the observed p-value is > 0.0001 using Dunnett’s test, the FCA-induced group showed a significant difference (p < 0.0001) in paw edema compared with all other groups (including the normal control group).
The day after the FCA induction there is a considerable loss of weight in rats, but after that, a normal weight increase is seen.18 The outcome of the current study also suggests a strong correlation between the degree of inflammation and weight loss. Weight loss implies an active sickness while weight gain shows healing, so changes in the rats’ body weight were tracked as one of the parameters used to evaluate the disease’s course. The fact that all induction groups experienced less weight gain than the control group was consistent with the notion that rheumatoid cachexia, a significant comorbidity of RA, was linked to the reduction of lean mass. This latter condition is assumed to be the result of cytokine-driven hypermetabolism, which in turn raises the activity of the Ubiquitin proteasome proteolytic cascade in response to muscle wasting and consequent weight loss.19 During the final phase of the experiment (4th week), the MSLNP-treated groups lost the least amount of body weight compared to the α-mangostin-treated groups. The MSLNP-treated group’s results are more similar to the prednisolone-treated group’s trend. These findings suggest that MSLNP was more effective than α-mangostin in the body weight parameter in the treatment of RAs.
The arthritis score, stair climbing ability, and mobility tests were evaluated to quantify the physical impairment of walking ability, which is linked with arthritis. FCA-induced RA in rats resulted in an elevated arthritic score and a lower stair climbing and mobility score. MSLNP-treated groups improved more in terms of walking ability and arthritis score than α-mangostin-treated groups. Even better motor ability, climbing ability, and arthritis score were observed with the prednisolone-treated groups compared to all other groups, which was persistent up to the last day of the study (28th day). Dunnett’s test confirms there is a significant difference (p value <0.0001) in the arthritis score, stair climbing ability, and mobility test when the results of the FCA-induced group are compared with the remaining four groups.
Histopathological investigation was utilized to diagnose joint deformities, erosions, swelling, and tissue edema in arthritis patients. This provides information on the lesion in the soft tissue that is seen as an early indication of RA. Additionally, typical pathologic alterations in human arthritis are bone degeneration and erosions.20 Swelling in tissues (soft tissue) and joint space constriction were observed in arthritic patients produced by FCA, which suggests that bone degradation occurs in arthritic disease.
The histology of Group 1 (Normal Control Group) revealed normal, healthy synovial membranes without any inflammatory cell infiltrations. The undamaged bone surfaces and totally stained Saf 0 in the intact cartilage of the Group I rat demonstrated no signs of bone degradation.
Group 2 (FCA induction control arthritic group) rat joints displayed severe synovial membrane hyperplasia as well as a significant buildup of inflammatory cells throughout the joints. Moderate red staining loss, affecting about two thirds of the top layer of uncalcified cartilage, and increased localised subchondral bone erosion Cortical bone penetration ranging from partial to complete, with a slight break through to the bone marrow.
Group 3 (FCA + α-mangostin ethanolic extract group) demonstrated that there is increased synovial membrane thickness, enhanced infiltration of inflammatory cells, and a mild to moderate loss of the non-calcified cartilage layer that affects up to 2/3 of the superficial cartilage area. At the cortical bone’s outer surface, there is minor superficial bone degradation.
Only 3-5 cell layers of synovial membrane were visible in Group 4 (FCA + MSLNP), as were mild inflammatory cell infiltrations into the sub-lining and individual articular tissues, as well as a mild loss (Proteoglycan loss) of staining, which affected approximately 1/3 of the superficial cartilage zone. There is a slight roughening of the superficial, non-calcified cartilage layer on the cortical bone’s outer surface, as well as small suprafacial bone erosion.
Group 5 (FCA + Prednisolone) had 3-5 synovial membrane cell layers, mild inflammatory cell infiltrations into the sub-lining and per articular tissue, and mild protoglycan staining loss, which contributed to about 1/3 of the superficial cartilage zone. Healthy, intact cartilage and bone surfaces with fully stained Hematoxylin and eosin
The histopathology confirms there is significant improvement in efficacy of the formulation (MSNLP) when compared with its pure ethanolic extract α-mangostin for its anti-arthritic effect.
CONCLUSION
The observations of the present study contribute to the understanding that MSLNP has shown superior anti-arthritic activity in comparison to the pure ethanolic extract of α-mangostin. According to the results, MSNLP had greater bioavailability and tissue distribution (ankle joint) than pure extract, which accounts for its anti-arthritic potential. The histopathological analysis supports the hypothesis since there is excellent protection against synovial inflammation, erosion of cartilage and bone. It also demonstrates its beneficial effects during recovery from RA’s by including paw volume, body weight, stair climbing ability, mobility test, paw thickness, and an arthritic score. Even though MSLNP outcomes are not better than those of prednisolone-treated groups, significant results are achieved with MSLNP when compared with α-mangostin pure extract.
References
- O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med.. 2004;350(25):2591-602. [PubMed] | [CrossRef] | [Google Scholar]
- Ruiz-Esquide V., Sanmartí R.. Tabaco y otros factores ambientales en la artritis reumatoide. Reumatol Clin.. 2012;8(6):342-50. [CrossRef] | [Google Scholar]
- Phull AR, Nasir B, Haq IU, Kim SJ. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact. 2018;281:121-36. [PubMed] | [CrossRef] | [Google Scholar]
- Taneja V. Arthritis susceptibility and the gut microbiome. FEBS Lett. 2014;588(22):4244-9. [PubMed] | [CrossRef] | [Google Scholar]
- Goulielmos GN, Zervou MI, Myrthianou E, Burska A, Niewold TB, Ponchel F, et al. Genetic data: the new challenge of personalized medicine, insights for rheumatoid arthritis patients. Gene. 2016;583(2):90-101. [PubMed] | [CrossRef] | [Google Scholar]
- Bansback NJ, Regier DA, Ara R, Brennan A, Shojania K, Esdaile JM, et al. An overview of economic evaluations for drugs used in rheumatoid arthritis: focus on tumour necrosis factor-α Antagonists. Drugs. 2005;65(4):473-96. [PubMed] | [CrossRef] | [Google Scholar]
- Hafeez BB, Mustafa A, Fischer JW, Singh A, Zhong W, Shekhani MO, et al. α-mangostin: A Dietary Antioxidant Derived from the Pericarp of L. inhibits Pancreatic Tumor Growth in xenograft Mouse Model. Antioxid Redox Signal. 2014;21(5):682-99. [PubMed] | [CrossRef] | [Google Scholar]
- John OD, Mushunje AT, Surugau N, Guad RM. The metabolic and molecular mechanisms of α-mangostin in cardiometabolic disorders [review]. Int J Mol Med. 2022;50(3):120 [PubMed] | [CrossRef] | [Google Scholar]
- Warriar P, Barve K, Prabhakar B. Anti-arthritic effect of garcinol enriched fraction against adjuvant induced arthritis. Recent Pat Inflamm Allergy Drug Discov. 2019;13(1):49-56. [PubMed] | [CrossRef] | [Google Scholar]
- De Castro Costa M, De Sutter P, Gybels J, Van Hees J. Adjuvant-induced arthritis in rats: A possible animal model of chronic pain. Pain.. 1981;10(2):173-85. [PubMed] | [CrossRef] | [Google Scholar]
- Singh VS, Dhawale SC, Shakeel F, Faiyazuddin Md, Alshehri S. Antiarthritic potential of Leaf fractions in FCA-induced arthritic rats: involvement of cellular inflammatory mediators and other biomarkers. Agriculture. 2021;11(1):68 [CrossRef] | [Google Scholar]
- Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41(5):863-77. [PubMed] | [CrossRef] | [Google Scholar]
- Bax M, Van Heemst J, Huizinga TWJ, Toes REM. Genetics of rheumatoid arthritis: what have we learned?. Immunogenetics. 2011;63(8):459-66. [PubMed] | [CrossRef] | [Google Scholar]
- Lin B, Zhao Y, Han P, Yue W, Ma XQ, Rahman K, et al. Anti-arthritic activity of L. extract on complete Freund’s adjuvant induced arthritis in rats. J Ethnopharmacol. 2014;155(1):248-55. [PubMed] | [CrossRef] | [Google Scholar]
- Tag HM, Kelany OE, Tantawy HM, Fahmy AA. Potential anti-inflammatory effect of lemon and hot pepper extracts on adjuvant-induced arthritis in mice. J Basic Appl Zool. 2014;67(5):149-57. [CrossRef] | [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783-801. [PubMed] | [CrossRef] | [Google Scholar]
- Hoogstraate J, Andersson LI, Berge OG, Jonzon B, Öjteg G.. COX-Inhibiting Nitric Oxide Donators (CINODs)-a new paradigm in the treatment of pain and inflammation. Inflammopharmacology.. 2003;11(4):423-8. [PubMed] | [CrossRef] | [Google Scholar]
- Yoshikawa T, Tanaka H, Kondo M. The increase of lipid peroxidation in rat adjuvant arthritis and its inhibition by superoxide dismutase. Biochem Med. 1985;33(3):320-6. [PubMed] | [CrossRef] | [Google Scholar]
- Granado M, Priego T, Martín AI, Villanúa MA, López-Calderón A. Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes and gene expression in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;289(6):E1007-14. [PubMed] | [CrossRef] | [Google Scholar]
- Almarestani L, Fitzcharles MA, Bennett GJ, Ribeiro-da-Silva A. Imaging studies in Freund’s complete adjuvant model of regional polyarthritis, a model suitable for the study of pain mechanisms, in the rat. Arthritis Rheum.. 2011;63(6):1573-81. [PubMed] | [CrossRef] | [Google Scholar]
