ABSTRACT
Background: The core structure of benzo[7] annulene contains a number of natural products, and this moiety is helpful in the treatment of many pharmacological activities. The thiazolidine ring has strong antibacterial activity, multi-drug resistance in several bacterial strains, and it shows effective in pharmacological treatment. Materials and Methods: A novel series of compound were synthesized 2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo[7]annulen-8- yl)-3-thiazolidin-4one (9a-i) (10a-i) and its scaffold. All the synthesized compound are purified by using ethanol for recrystallization. The protein is download from PDB 5C5S (human myosin 9b RhoGAP) and it predicted in silico studies using Autodock Vina PyRx (0.8). Results: All the derivatives are characterized by TLC and spectral analysis 1H NMR, 13C NMR, IR, MASS. All the synthesized compounds were screened as in vitro anti-cancer, and anti-bacterial activity. Among the series of compound 9h, 9i, 10h, 10i exhibit more potent against two cancer cell lines MCF7, A549. IC50μg/mL 9h (21.16 and 19.26μg/mL), 9i (24.21 and 20.44μg/mL), 10h (18.32 and 16.32μg/ mL), 10i (17.46 and18.28μg/mL). with the reference drugs as doxorubicin (15.29 and 12.26μg/ mL) and also screened antibacterial activity most of the compound shows promising active with reference ciprofloxacin and the molecular docking analysis results shows a good binding affinity with 5c5s protein. Conclusion: The synthesized compounds were screened in vitro evaluation of anticancer activity by using MTT assay and antibacterial evaluation of different bacterial strain by using agar diffusion method. The synthesized compound were show more active when carbon chain increases activity also increases.
INTRODUCTION
One of the most occurring cancers is breast and lung cancer. Most of the research is challenging on breast and lung cancer. Medicinal chemist shows very interested to discover a new drug in anti-proliferative or anti-cancer activity. According to World Health Organization (WHO) report in the past two decade, the overall number of people diagnosed with cancer nearly doubled, from an estimated 10 million in 2000 to 19.3 million in 2020. Today one in 5 people worldwide will develop cancer during their life-times.1
Benzo[7]annulene2 and their utility in heterocyclic chemistry and its scaffold shows as a diverse pharmacological activity such as anti-cancer,3 anti-microbial,4 anti-inflammatory,5 anti-convulsant,6 anti-rheumatoid,6 anti-hypertensive agents.6 Benzo[7]annulene derivatives are also known as anti-cancer agents or anti-proliferative agents. Benzo[7]annulene nucleus contains multiple natural products as core structure and its obtained from natural alkaloids it shows medicinally and pharmacologically active such as theaflavin, brussonol, colchicine, TAK779 Figure 1. This are the compound are proven as clinically anti-proliferative activity.7 Benzo[7]annulene nucleus inhibit apoptotic inducers, tubulin polymerization inhibitor and anti-mitotic effect.8 In our research Benzo[7]annulene contain 9 position chlorine atom8 Because of its electron with-drawing group it has ability to bind the receptors and shows as agonist effect to their receptors.
Fused heterocyclic moieties like thiazolidine ring is important in drug discovery process. Thiazolidine has five membered ring structure and it contain Sulphur, amine and ketone group.9 It has a diverse pharmacological activity. The nucleus contain a therapeutic importance in Figure 2.10 It is very potent in anti-bacterial,11 anti-cancer activity.12 Thiazolidine ring is attached with β-lactam is a penicillin antibiotics.13 It directly inhibit bacterial cell wall synthesis as bactericidal effect.14 Sulphur, amino group act as electron-withdrawing group it shows more potent on antiproliferative activity.15 Thiazolidine nucleus has a potential to inhibit tubulin polymerization, apoptosis inducers activity.16
In our research a great privileged Benzo[7]annulene ring fused with thiazolidine ringand its scaffolds shows a very significant and potential biol ogically active. Extremely efforts to develop synthesis via knoevanagel condensation mechanism and its scaffold were promising towards breast cancer cell line (MCF-7) and lung cancer cell line (A-549) by using MTT assay and in vitro anti-bacterial activity were evaluated most of the scaffolds are potent against different bacterial strain by using agar diffusion method and build up on the in silico molecular docking analysis were predicted and it show a good binding affinity.
MATERIALS AND METHODS
All the chemical are purchased from SD Fine and AURA Laboratories. The synthesis compounds are characterized by TLC methods (silica gel plates). Then further characterization different analytical technique were performed by Spectral analysis like IR spectroscopy (Shimadzu), 1H-NMR spectroscopy (300 MHZ), 13C spectroscopy (300 MHZ), Mass spectrometry (Shimadzu).
Synthesis of 9-chloro-Benzo[7]annulene containing thiazolidine derivatives
The procedure consists of five steps;
Step1: Preparation of 5-(3,4-dimethylphenyl)-5-oxopentanoic acid
o-xylene (1) 10g (0.094 moles) was added slowly to an ice-cooled suspension of AlCl3 (27g, 0.268 moles) in DCM (100 mL) and then glutaric anhydride (2) (10.76g, 0.094 moles) was added to the reaction mixture The mixture was poured into ice-water and was extracted with DCM. Separated organic layer was dried (Na2SO4). The compound was dissolved in DCM and filtered over silica gel to remove the dicarboxylic acid formed during work up then giving a yellow colour solid.
Step2: Preparation of 5-(3,4-dimethylphenyl) pentanoic acid
Mossy Zinc 18.71g (0.283 moles) by shaking it with a mixture of HgCl2 (1.76g, 0.008 moles), Conc. HCl (1 mL) and water (2.35 mL) for 20min., aqueous layer was throughout, and the residue washed with water. And then add distilled water (3 mL), Toluene (15 mL), Acetic acid (4.5 mL), conc. HCl (10 mL) and compound -(Benzoyl)-Butyric Acid (3) (10g, 0.045 moles) was reflux 4hr with adding conc. HCl (10 mL). The reaction mixture was cooled, the organic layer was separated and the aqueous layer was extracted with Benzene (2 x 40 mL). The combined organic layers were washed with water to neutralize and dried over (Na2SO4). Obtained product (5-(3, 4-dimethyl phenyl)-pentanoic acid.
Step3: Preparation of 2, 3-dimethyl- 6,7, 8, 9-tetrahydro-5H-benzo[7]annulen-5-one
Poly phosphoric acid was added and heated on a steam bath at the temperature of 96oC. To the molten reaction mass, compound 5-(3,4-dimethylphenyl) pentanoic acid (4) was added with continuous stirring. The reaction was heating on steam bath for 4 hr at 96°C. After complete the reaction the contents were poured into crushed ice and neutralized with 5% NaOH. The aqueous layer was extracted with ethyl acetate. The resultant compound was purified using TLC chromatography. Solvent system: (1: 9) Ethylacetate: Hexane.
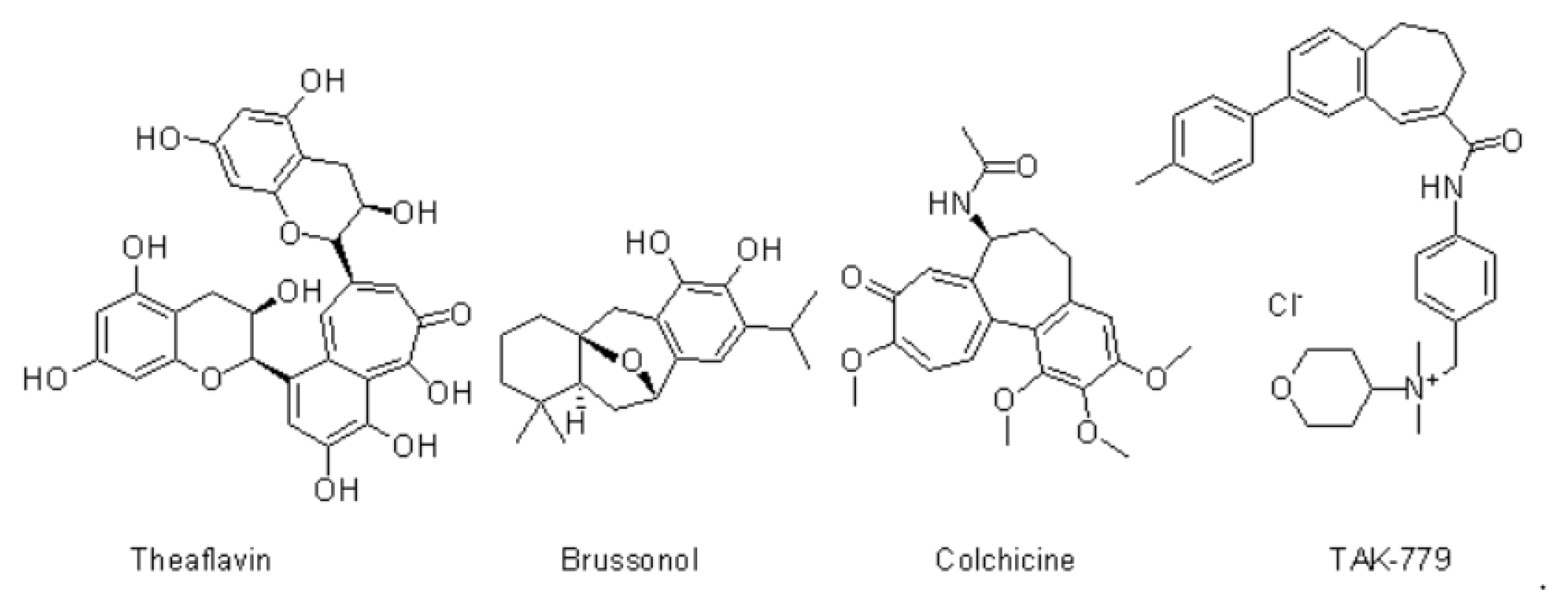
Figure 1.
Benzo[7]annulene contain natural products as core structure.
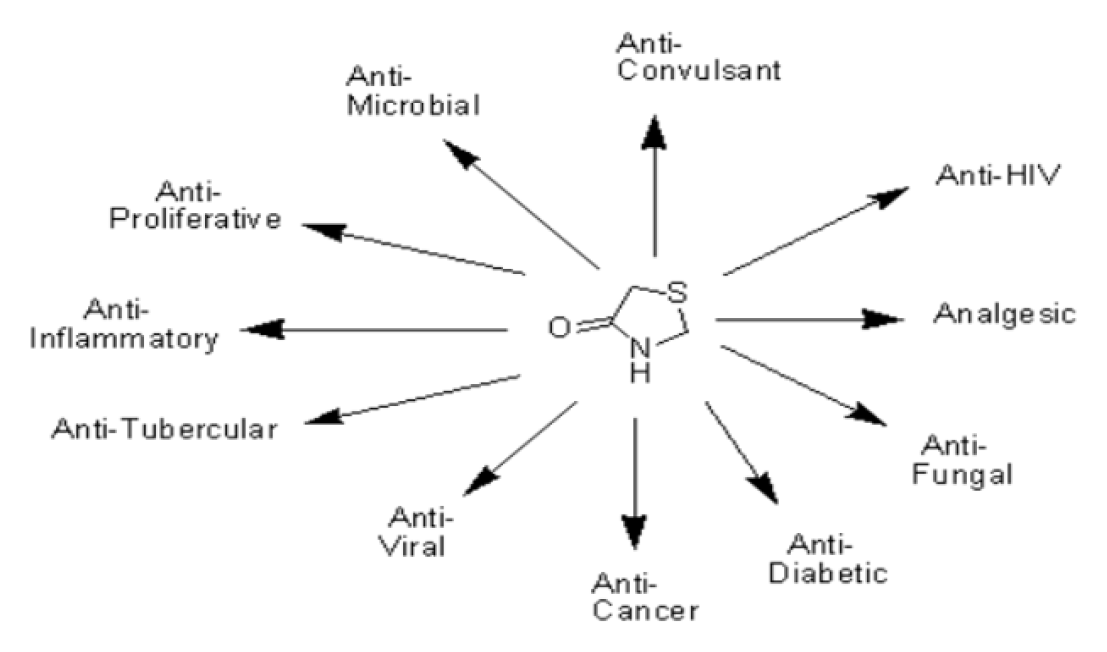
Figure 2.
Therapeutic importance of Thiazolidine.
Step4: Preparation of 9-chloro-2, 3-dimethyl-6, 7-dihydro-5H-benzo [7] annulene-8 carbaldehyde
DMF (20 mL) and POCl3 (2.97mL, 0.031 moles) was added at 0°C in ice bath and is stirred for 15 minutes. under inert conditions. Then disubstituted-6, 7, 8, 9-Tetrahydro Benzocyclo hepten-5- one (5) (6g, 0.031moles) was added drop wise and is refluxed at 80°C for 5hr. After complete reaction, 20% NaOH solution was 2-(9-chloro-2, 3-dimethyl-6,7-dihydro-5H-benzo[7]annulen-8- yl)-3-phenylthiazolidin-4-oneadded for neutralization. The compound was extracted with ethyl acetate and the organic layer was dried over Na2SO4.
Step5: Preparation of 2-(9-chloro-2, 3-dimethyl- 6,7-dihydro-5H-benzo[7]annulen-8-yl)-3-thiazolidin-4one
9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo[7]annulene-8- carbaldehyde (100 mg, 1eq), Ammonia (0.038 ml, 0.5eq) and thioglycolic acid (0.091 mL, 1.5eq) were added and reacted for 1 hr in the presence of toluene (3 mL) to give the compound and were extracted with ethyl acetate and the organic layer was dried over Na2SO4. The scheme represent in Figure 3.
Biological activity evaluation
Anticancer activity
Anticancer activity of 2-(9-chloro-2,3-dimethyl- 6,7-dihydro-5H-benzo[7]annulen-8-yl)-3-thiazolidin- 4one(9a-i) (10a-i) was evaluated as in vitro against two cell lines breast cancer (MCF7) and lung cancer (A-549) by using MTT assay method, Cells were trypsinized and perform the trypan blue assay to know viable cells in cell suspension and cells were counted by haemocytometer and seeded at density of 5.0 X 10 3 cells / well in 100 μl media in 96 well plate culture medium and incubated overnight at 37°C. Discard the drug solution and add the fresh media with MTT solution (0.5 mg/ mL-1) was Added to each well and plates were incubated at 37°C for 3hr. At the end of incubation time, precipitates are formed as a result of the reduction of the MTT salt to chromophore Formosan crystals by the cells with metabolically active mitochondria. The optical density of solubilised crystals in DMSO was measured at 570 nm on a microplate reader. The percentage growth inhibition was calculated using by standard formula and concentration of test drug needed to inhibit cell growth by 50% values (IC50) Interestingly, (9a-i) (10a-i) the compounds 9h, 9i and 10h, 10i were found more potent active against both the cell lines. The results show IC50 μg/mL 9h (21.16 and 19.26μg/mL), 9i (24.21 and 20.44μg/mL), 10h (18.32 and 16.32 μg/mL), 10i (17.46 and 18.28μg/mL). The compared with reference drugs as doxorubicin (15.29 and 12.26μg/mL).
Antibacterial activity
Antibacterial activity 2-(9-chloro-2, 3-dimethyl- 6,7-dihydro-5H-benzo[7]annulen-8-yl)-3-thiazolidin- 4one(9a-i) (10a-i) was evaluated against two gram-positive S. aureus, B. subtilis and two gram-negative bacteria E. coli, P. aeruginosa by using agar plate method. Interestingly, (9a-i) (10a-i) The compounds 9d, 9g, 9h, 9i and 10d, 10g, 10h, 10i were promising active against gram positive and gram negatives strains reference with ciprofloxacin. Furthermore, it was observed that the displayed a dose dependent inhibition of bacterial growth in the investigation. The results shows that the substituted of 9d, 10d Ethyl benzene 9g, 10g Methoxyethane 9h, 10h Methyl propionate and 9i, 10i propionic acid substituted to Thiazolidine scaffold.
Molecular docking analysis
The docking analysis was performed by using Autodock vina PyRx 0.8 software (Academic free version).17 The software was calculated and analyze how the molecule binds to receptor through its 3D and 2D structure. The protein was selected from WWW.RCSBPDB.COM PDBID:5C5S (human myosin 9b RhoGAP)20 and docking visualization result analysis was performed in discovery studio.
RESULTS
The novel series of compound are synthesized2-(9-chloro- 2,3-dimethyl-6,7-dihydro-5H-benzo[7]annulen-8- yl)-3-thiazolidin-4one (9a-i) (10a-i) and its scaffolds were prepared via knoevanagel condensation. All the synthesized compounds are characterized by spectral analysis 1H NMR, 13C NMR, IR, MASS, and TLC. The synthesized compound obtained good yield 70-85%. All the synthesized compounds were screened as in vitro antibacterial and anticancer activity by using MTT assay method. Among the series of compound 9h, 9i, 10h,10i, exhibit more potent against two cancer cell lines breast cancer cell line (MCF7), lung cancer cell line (A549). 9hIC50 μg/ mL (21.16 and 19.26μg/mL), 9i (24.21 and 20.44μg/mL), 10hr (18.32 and 16.32 μg/mL), 10i (17.46 and18.28 μg/mL). With the reference drug doxorubicin (15.29 and 12.26 μg/mL) and also screened antibacterial activity of two-gram positive and two-gram negative bacterial strain by using agar diffusion method. The series of compounds exhibit 9d, 9g, 9h, 9i and 10d,10g,10h,10i were promising active against gram positive and gram negatives bacterial strains compared with reference drug Ciprofloxacin. The molecular docking analysis were performed of the synthesized compounds. The protein is download from PDB 5C5S (human myosin 9b RhoGAP) and it is predicted insilico studies by using Autodock Vina, PyRx (0.8) and all the synthesized compounds are docked with 5C5S protein and it shows a good binding affinity.
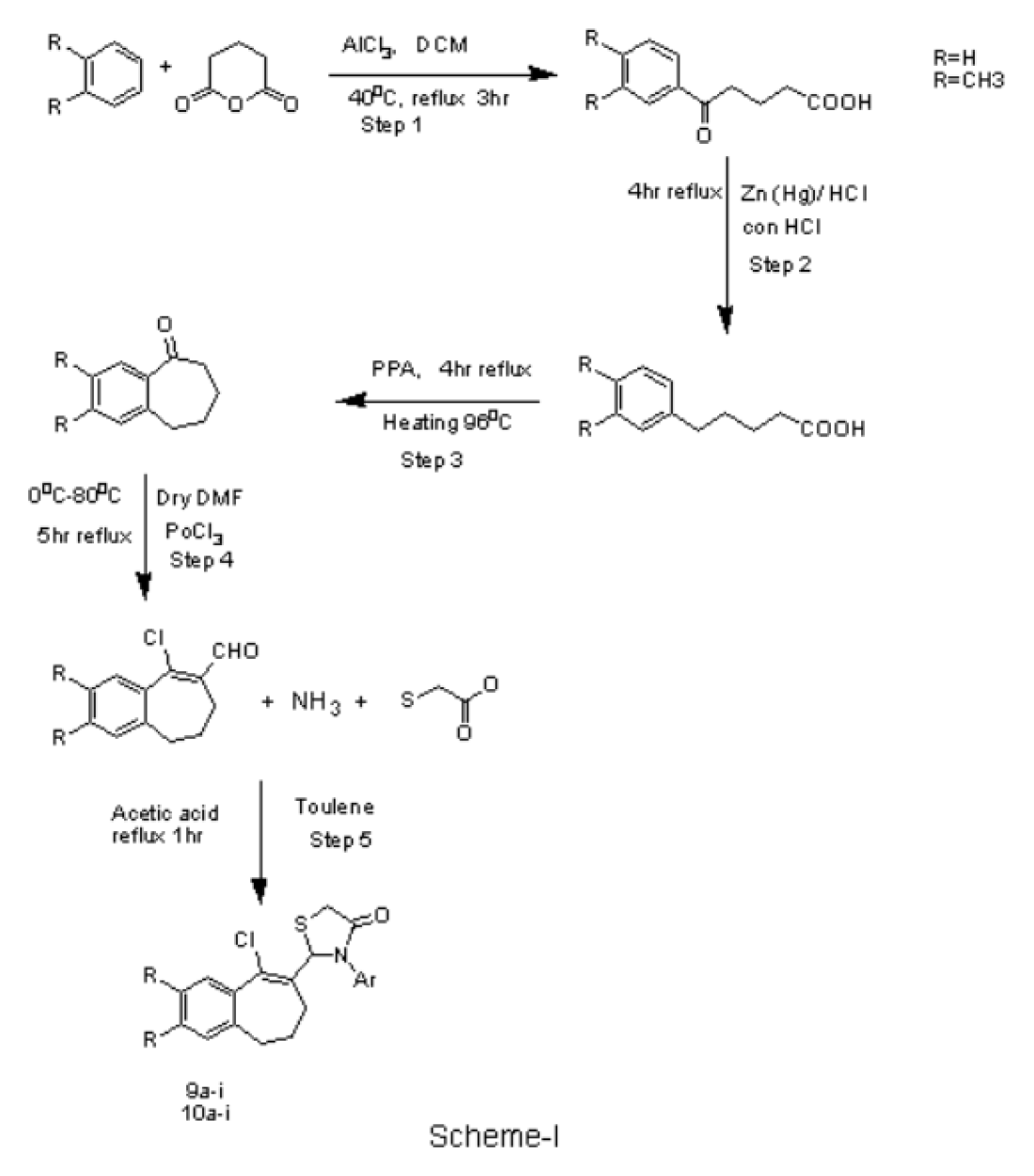
Figure 3.
Scheme-I 2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo[7]annulen-8-yl)-3-thiazolidin-4one. And its derivative (9a-i) (10a-i)
The synthesized scheme is represent in Figure 3 All the synthesized derivatives compound and its physical properties is given in Table 1 and in vitro anti-cancer activity IC50 values are given in Table 2 and anti-cancer morphological screening images are given in Figures 4 anti-cancer activity (% cell viability) graph are shown in Figure 5 antibacterial activity are given in Table 3 and in silico docking binding energy values are given in Table 4 and docking interaction Figures are shown in Figure 6.
Spectral data of newly synthesized compound
9a:-2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H– benzo[7]annulen-8-yl)-3-phenylthiazolidin-4-oneIR (γ/cm-1)2945, 2834 (C-H), 1658 (C=O), 1645 (C=C), 1345 (C-N), 1025(C-C), 769 (C-Cl) 1H-NMRδ = 90-2.08 (m, 4H, CH2), 2.21 (s, 3H, CH), 2.22 (s, 3H, CH3), 2.25-2.38 (m, 2H, CH2), 3.83-3.93 (m, 2H, CH2), 6.85 (s, 1H, CH), 6.86 (s, 1H, Ar-H), 7.21-7.25 (m, 1H, Ar-H), 7.36-7.42 (m, 4H, Ar-H) 13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6 LC-MS m/z 384 [M-H]+.
9b:-3-(4-acetylphenyl)-2-(9-chloro-2,3-dimethyl- 6,7-dihydro-5H-benzo[7]annulen-8-yl) thiazolidin-4-oneIR (γ/ cm-1)2955, 2840(C-H), 1642 (C=O), 1625 (C=C), 1365 (C-N), 1066 (C-C), 775(C-Cl) 1H-NMR δ = 1.83-2.07 (m, 4H, CH2), 2.21 (s, 3H, CH3), 2.23 (s, 3H, CH3), 2.26-2.34 (m, 2H, CH2), 2.58 (s, 3H, CH3), 3.84-3.94 (m, 2H, CH2), 6.85 (s, 1H, CH), 6.89 (s, 1H, Ar-H), 7.20 (s, 1H, Ar-H), 7.55-7.60 (m, 1H, Ar-H), 7.97-8.01 (m, 2H, Ar-H)13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6, 199, 29 LC-MS m/z 426 [M-H]+.
9c:-3-benzyl-2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H– benzo[7]annulen-8-yl)thiazolidin-4-oneIR (γ/ cm-1)2945, 2834 (C-H), 1658 (C=O), 1645 (C=C), 1345 (C-N), 1025(C-C), 769 (C-Cl) 1H-NMR δ = 8.03-7.93 (m, 2H), 7.64 (s, 1H), 7.53-7.40 (m, 4H), 7.28 (s, 1H), 5.65 (brs, 2H) 13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6, 127, 128 (LC-MS) m/z 398 [M+H]+.
9d:- 2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo[7]annulen -8-yl)-3-phenethylthiazolidin-4-oneIR 2857, 2924 (C-H), 1682 (C=O), 1611 (C=C), 1254 (C-N), 1091 (C-C), 761 (C-Cl) cm-1. 1H-NMR δ = 7.94 (d, J = 8.6 Hz, 2H), 7.52 (s, 1H), 7.43 (d, J = 8.4 Hz, 2H), 7.34-7.14 (m, 2H), 5.62 (brs, 2H) 13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6, 126, 128, 127 (LC-MS) m/z 412 [M+H]+.
9e:-2-(9-chloro-2,3-dimethyl6,7dihydro-5H-benzo [7]annulen8yl)-(4methoxyphenyl) thiazolidin-4-oneIR 2930 (C-H), 1714 (C=O), 1641 (C=C), 1385 (C-N), 1028 (C-C), 771 (C-Cl) cm-1. 1H-NMR δ = 7.63-7.52 (m, 1H), 7.49 (s, 1H), 7.11 (s, 1H), 7.05 (d, J = 3.2 Hz, 1H), 6.60-6.45 (m, 1H), 5.55 (brs, 2H)13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6, 114, 122, 55.9 (LC-MS) m/z 414 [M+H]+.
9f:-2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo [7]annulen-8-yl)-3-(4-iodophenyl) thiazolidin-4-oneIR 2924, 2854 (C-H), 1685 (C=O), 1619 (C=C), 1366 (C-N), 1056 (C-C), 750 (C-Cl)1H-NMR δ = 7.57-7.51 (m, 1H), 7.49 (s, 1H), 7.39-7.33 (m, 1H), 7.29 (brs, 1H), 7.13 (brs, 1H), 7.07-6.98 (m, 1H), 5.57 (brs, 2H) 13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6,137.8, 90, 137, 123(LC-MS) m/z 510 [M+H]+.
| Compound | Ar | R | R1 | Molecular formula | Molecular weight (gms) | Yield(%) |
|---|---|---|---|---|---|---|
| 9a | C6H5 | CH3 | CH3 | C22H22ClNOS | 383.93 | 75 |
| 9b | C6H5COCH3 | CH3 | CH3 | C24H24ClNO2S | 425.97 | 73 |
| 9c | C6H5CH2 | CH3 | CH3 | C23H24ClNOS | 397.96 | 74 |
| 9d | C6H5CH2CH2 | CH3 | CH3 | C24H26ClNOS | 411.99 | 75 |
| 9e | C6H5-O-CH3 | CH3 | CH3 | C23H24ClNO2S | 413.96 | 75 |
| 9f | C6H5-I | CH3 | CH3 | C22H21ClNOIS | 509.83 | 77 |
| 9g | CH2CH2-O-CH3 | CH3 | CH3 | C19H24ClNO2S | 365.92 | 76 |
| 9h | CH3CH2C02CH2CH3 | CH3 | CH3 | C21H26ClNO3S | 407.95 | 76 |
| 9i | CH2CH2COOH | CH3 | CH3 | C19H22ClNO3S | 379.90 | 82 |
| 10a | C6H5 | H | H | C20H20ClNOS | 341.90 | 77 |
| 10b | C6H5COCH3 | H | H | C22H20ClNO2S | 397.92 | 75 |
| 10c | C6H5CH2 | H | H | C21H20ClNOS | 369.91 | 76 |
| 10d | C6H5CH2CH2 | H | H | C22H22ClNOS | 383.93 | 78 |
| 10e | C6H5—O — CH3 | H | H | C21H20ClNO2S | 385.91 | 74 |
| 10f | C6H5—1 | H | H | C20H17ClNOIS | 481.78 | 77 |
| 10g | CH2CH2-O-CH3 | H | H | C17H20ClNO2S | 337.86 | 79 |
| 10h | CH3CH2CO2CH2CH3 | H | H | C19H22ClNO3S | 379.90 | 76 |
| 10i | CH2CH2COOH | H | H | C19H22ClNO3S | 351.85 | 83 |
9g:-2-(9-chloro-2,3-dimethyl-6,7-dihydro- 5H-benzo[7]annulen -8-yl)-3–(2methoxyethyl) thiazolidin-4-76one (IR 2954, 2834 (C-H), 1680 (C=O), 1650 (C=C), 1355 (C-N), 1045 (C-C), 759 (C-Cl)1H-NMR δ = 11.63 (brs, 1H), 8.24 (s, 2H), 8.13 (s, 1H), 7.56 (s, 3H), 2.84 (s, 2H), 2.54 (s, 2H), 2.33 (s, 3H), 1.22 (s, 6H) 13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6,44.2, 70.2, 59 (LC-MS) m/z 366 [M+H]+.
9h:-ethyl3-(2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo [7]annulen-8-yl)-4oxothiazolidin -3-yl) propanoate IR 2920, 2852 (C-H), 1684 (C=O), 1625 (C=C), 1360 (C-N), 1052 (C-C), 755 (C-Cl)1H-NMR δ = 11.55 (brs, 1H), 8.19 (d, J = 8.6 Hz, 2H), 8.11 (s, 1H), 7.51 (d, J = 8.6 Hz, 2H), 2.84 (s, 2H), 2.53 (s, 2H), 2.33 (s, 3H), 1.22 (s, 6H) 13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6, 34, 39, 33.1, 173.1, 61, 14.1(LC-MS) m/z 408 [M+H]+.
9i:-3-(2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo [7]annulen-8-yl)-4-oxothiazolidin-3-yl)propanoic acid IR2954, 2834 (C-H), 1680 (C=O), 1650 (C=C), 1355 (C-N), 1045 (C-C), 759 (C-Cl)1H-NMR δ = 11.50 (brs, 1H), 8.12 (s, 1H), 7.67 (m, 1H), 7.55 (d, J = 3.4 Hz, 1H), 6.66-6.61 (m, 1H), 2.83 (s, 2H), 2.52 (s, 2H), 2.32 (s, 3H), 1.21 (s, 6H) 13C NMR (125 MHz, CDCl3) : δ134.1, 136.2,129.8, 133.8, 139.7, 38.7, 23.2, 26.9, 131.9, 125.4, 18.2, 18.1, 50.7, 171.2, 34.4, 141.7, 121.6, 129, 124.4, 129, 121.6, 34.8, 39, 35. 177 (LC-MS) m/z 380 [M+H]+.
10a:-2-(9-chloro-6,7-dihydro-5H-benzo[7]annulen-8- yl)-3-phenylthiazolidin-4-one IR2932, 2875 (C-H), 1625 (C=O), 1620 (C=C), 1342 (C-N), 1045 (C-C), 775 (C-Cl)1H-NMR δ = 11.51 (brs, 1H), 8.01 (s, 1H), 7.91 (d, J = 3.2 Hz, 1H), 7.61 (d, J = 4.5 Hz, 1H), 7.23-7.17 (m, 1H), 2.83 (s, 2H), 2.53 (s, 2H), 2.32 (s, 3H), 1.21 (s, 6H) 13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121 (LC-MS) m/z 356 [M+H]+.
10b:-3-(4-acetylphenyl)-2-(9-chloro-6,7-dihydro -5H-benzo[7]annulen-8-yl)thiazolidin-4-oneIR2920, 2832 (C-H), 1675 (C=O), 1633 (C=C), 1345 (C-N), 1035 (C-C), 770 (C-Cl)1H-NMR δ = 8.31-8.20 (m, 2H), 8.13 (s, 1H), 7.62-7.45 (m, 3H), 2.56 (s, 3H). 13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121, 199, 29(LC-MS) m/z 398 [M+H]+.
10c:- 3-benzyl-2-(9-chloro-6,7-dihydro-5H-benzo[7]annulen- 8-yl)thiazolidin-4-one IR2957 (C-H), 1725 (C=O), 1652 (C=C), 1356 (C-N), 1025 (C-C), 757 (C-Cl)1H-NMR δ = 8.05 (s, 1H), 7.71 (s, 1H), 7.62-7.39 (m, 1H), 6.69-6.61 (m, 1H), 2.53 (s, 3H)13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121, 127.1, 128.6, 128 (LC-MS) m/z 370 [M+H]+.
10d:- 2-(9-chloro-6,7-dihydro-5H-benzo[7]annulen-8-yl)-3- phenethylthiazolidin-4-one IR 2845, 2850 (C-H), 1745 (C=O), 1615 (C=C), 1342 (C-N), 1065 (C-C), 746 (C-Cl)1H-NMR δ = 8.28-8.21 (m, 2H), 8.15 (s, 1H), 7.61-7.45 (m, 3H), 5.55 (s, 1H), 2.93 (s, 2H), 2.32 (s, 3H), 1.14 (s, 6H).13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121, 126, 128.7, 127.8(LC-MS) m/z 384 [M+H]+.
| Compounds | IC50μg/Mi | |
|---|---|---|
| MCF-7 | A-549 | |
| 9a | 39.26 | 34.32 |
| 9b | 45.59 | 58.18 |
| 9c | 95.23 | 112.16 |
| 9d | 32.52 | 29.44 |
| 9e | 134.52 | 122.21 |
| 9f | 110.57 | 116.69 |
| 9g | 33.09 | 31.34 |
| 9h | 21.16 | 19.26 |
| 9i | 24.21 | 20.44 |
| 10a | 42.16 | 36.24 |
| 10b | 50.38 | 66.37 |
| 10c | 156.22 | 142.02 |
| 10d | 26.30 | 24.18 |
| 10e | 218.39 | 195.26 |
| 10f | 95.16 | 90.49 |
| 10g | 25.20 | 23.09 |
| 10h | 18.32 | 16.32 |
| 10i | 17.46 | 18.28 |
| Doxorubicin | 15 29 | 12 26 |
10e:-2-(9-chloro-6,7-dihydro-5H-benzo[7]annulen-8-yl)-3- (4-methoxyphenyl)thiazolidin-4-oneIR 2920, 2832 (C-H), 1675 (C=O), 1633 (C=C), 1345 (C-N), 1035 (C-C), 770 (C-Cl)1H-NMR δ = 1.82-2.08 (m, 4H, CH2), 2.41-2.70 (m, 2H, CH2), 3.79 (s, 3H, CH3), 3.83-3.92 (m, 2H, CH2), 6.79 (s, 1H, CH), 6.87-6.93 (m, 2H, Ar-H), 7.08-7.12 (m, 1H, Ar-H), 7.18-7.24 (m, 2H, Ar-H), 7.27-7.32 (m, 2H, Ar-H), 7.37-7.42 (m, 1H, Ar-H) 13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121, 114.5, 122, 55.9(LC-MS) m/z 386 [M+H]+.
10f:-2-(9-chloro-6,7-dihydro-5H-benzo[7]annulen-8-yl)-3- (4-iodophenyl)thiazolidin-4-oneIR 2932, 2875 (C-H), 1625 (C=O), 1620 (C=C), 1342 (C-N), 1045 (C-C), 775 (C-Cl)1H-NMR δ = 8.21 (d, J = 8.6 Hz, 2H), 8.11 (s, 1H), 7.51 (d, J = 8.5 Hz, 2H), 5.56 (s, 1H), 2.93 (s, 2H), 2.32 (s, 3H), 1.14 (s, 6H) 13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121, 137.8, 90, 137.8, 123.2(LC-MS) m/z 482 [M+H]+.
10g:-2-(9-chloro-6,7-dihydro-5H-benzo[7]annulen-8-yl)- 3-(2-methoxyethyl)thiazolidin-4oneIR 2925, 2855 (C-H), 1677 (C=O), 1617 (C=C), 1349 (C-N), 1109,1036 (C-C), 748 (C-Cl)1H-NMR δ = 1.82-1.99 (m, 2H, CH2), 2.09-2.36 (m, 2H, CH2), 2.50-2.74 (m, 2H, CH2), 2.95-3.01 (m, 1H),3.37 (s, 3H,CH3), 3.55-3.62 (m, 2H, CH2), 3.66-3.77 (m, 2H, CH2), 3.90-3.97 (m, 1H), 6.38 (s, 1H, CH), 7.19 (d, J=7.3 Hz, 1H, Ar-H), 7.27-7.32 (m, 2H, Ar-H), 7.27-7.32 (m, 4H, Ar-H), 7.54 (d, J= 7.6 Hz, 1H,Ar-H)13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121, 34.8, 44.2, 70.2, 59(LC-MS) m/z 338 [M+H]+.
10h:-Ethyl3-(2-(9-chloro-6,7-dihydro-5H-benzo[7]annulen-8- yl)-4-oxothiazolidin-3-yl) propanoate IR 2957 (C-H), 1725 (C=O), 1652 (C=C), 1356 (C-N), 1025 (C-C), 757 (C-Cl)1H-NMR δ = 8.10 (s, 1H), 7.66 (s, 1H), 7.54 (d, J = 3.5 Hz, 1H), 6.66-6.61 (m, 1H), 5.54 (s, 1H), 2.90 (s, 2H), 2.32 (s, 3H), 1.13 (s, 6H) 13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121, 61.3, 14.1(LC-MS) m/z 380 [M+H]+.
10i:-3-(2-(9-chloro-6,7-dihydro-5H-benzo[7]annulen-8- yl)-4-oxothiazolidin-3-yl)propanoic acidIR2845, 2850 (C-H), 1745 (C=O), 1615 (C=C), 1342 (C-N), 1065 (C-C), 746 (C-Cl)1H-NMR δ = 7.99 (s, 1H), 7.89 (d, J = 3.0 Hz, 1H), 7.60 (d, J = 4.5 Hz, 1H), 7.22-7.15 (m, 1H), 5.54 (s, 1H), 2.92 (s, 2H), 2.32 (s, 3H), 1.13 (s, 6H) 13C NMR (125 MHz, CDCl3) : δ126, 128.1, 128, 136, 142, 126, 38.4, 23.2, 26.9, 131.9, 125, 50, 171.2, 34, 141.7, 121.6, 129, 124, 129, 121, 34.8, 39.5, 35, 177.3(LC-MS) m/z 352 [M+H]+.
DISCUSSION
In this research, the novel series of compounds are synthesized 2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo[7]annulen-8- yl)-3-thiazolidin-4one(9a-i) (10a-i) and its scaffold. All the reaction were performed by reflux condenser. In the second position of Thiazolidine ring18 is attach with different types of aromatic compounds and obtained a good yield(%). The synthesized compound is evaluated as in vitro activity anti-cancer and anti-bacterial activity. The anti-cancer activity are tested against two cancer cell lines19 breast cancer cell line(MCF7) Lung cancer cell line (A549)by using MTT assay. Among the series the most potent compounds are 9h, 9i, 10 h,10 i are compared with reference drug Doxorubicin. In vitro Anti-bacterial activity was evaluated against two gram positive and two-gram negative bacterial strain by using agar diffusion method and zone of inhibition is calculated the most of the compound shows promising active compared with reference drug ciprofloxacin. Insilico docking studies are performed Discovery studio software is used for protein stabilization. The protein is download from PDB (protein data bank) website 5C5S (human myosin 9b RhoGAP)20 and the molecular docking analysis predicted in silico studies by using Autodock vina PyRx (0.8) which is academic free version software. The advantage of Autodock vina is stability, whenever we repeat docking, for ligand preparation, The ligands structure were drawn using Chem draw tools and saved as mol format. Energy minimization was done for molecules in OPEN BABEL saved in “.pdb” format. The protein 5C5S and different ligands (18 compounds) are upload in PyRx coordinates of x-axis and y-axis are adjusted. Interaction has been done by docking and excel file is generate and obtained binding affinity values 2D and 3D structure can be viewed by visualization tools like discovery studio. In the present research the molecular docking analysis results shows a good binding affinity with 5C5S protein.
| Compounds | Gram positive bacteria | Gram negative bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Bacillus subtilis | Escherichia coli | Pseudomonas aeruginosa | |||||
| 50μg/ml | 100μg/ml | 50μg/ml | 100μg/ml | 50μg/ml | 100μg/ml | 50μg/ml | 100μg/ml | |
| 9a | 19 | 22 | 20 | 23 | 17 | 20 | 19 | 23 |
| 9b | 13 | 16 | 10 | 12 | 9 | 11 | 11 | 15 |
| 9c | 10 | 16 | 8 | 11 | 12 | 13 | 14 | 17 |
| 9d | 24 | 29 | 23 | 26 | 23 | 27 | 26 | 29 |
| 9e | 12 | 14 | 7 | 12 | 10 | 12 | 14 | 16 |
| 9f | 7 | 10 | 10 | 13 | 15 | 18 | 17 | 20 |
| 9g | 25 | 29 | 23 | 27 | 25 | 29 | 27 | 29 |
| 9h | 26 | 30 | 24 | 28 | 24 | 27 | 27 | 28 |
| 9i | 26 | 31 | 22 | 25 | 25 | 30 | 28 | 30 |
| 10a | 18 | 25 | 18 | 20 | 15 | 17 | 17 | 19 |
| 10b | 11 | 14 | 9 | 13 | 8 | 12 | 9 | 12 |
| 10c | 14 | 15 | 10 | 13 | 9 | 12 | 11 | 14 |
| 10d | 24 | 30 | 23 | 26 | 24 | 28 | 27 | 30 |
| 10e | 8 | 12 | 11 | 13 | 14 | 16 | 15 | 18 |
| 10f | 10 | 15 | 17 | 20 | 15 | 17 | 16 | 20 |
| 10g | 23 | 28 | 23 | 25 | 25 | 30 | 27 | 29 |
| 10h | 25 | 28 | 22 | 24 | 26 | 29 | 26 | 30 |
| 10i | 27 | 31 | 24 | 28 | 24 | 26 | 28 | 31 |
| Ciprofloxacin | 28 | 33 | 25 | 29 | 27 | 32 | 30 | 34 |
| PDBID:5C5S | |||
|---|---|---|---|
| Compounds | Binding Affinity Kcal/mol | Compounds | Binding Affinity Kcal/mol |
| 9a | -7.1 | 10a | -8.1 |
| 9b | -8.7 | 10b | -8.1 |
| 9c | -89 | 10c | -8.4 |
| 9d | -7.9 | 10d | -7.2 |
| 9e | -8.3 | 10e | -8.1 |
| 9f | -8.5 | 10f | -8 |
| 9g | -7.8 | 10g | -6.6 |
| 9h | -10.4 | 10h | -9.4 |
| 9i | -9.2 | 10i | -8.8 |
CONCLUSION
In this research, The novel series of compound are synthesized 2-(9-chloro-2,3-dimethyl-6,7-dihydro-5H-benzo[7]annulen-8- yl)-3-thiazolidin-4one (9a-i) (10a-i) and its scaffold all the reaction were performed by reflux condenser. The synthesized scaffold was evaluated as in vitro anticancer and antibacterial activity. Among the series of compound 9h,9i,10h,10i exhibit more potent against two cancer cell lines MCF7, A549. IC50 μg/ mL9h (21.16 and 19.26 μg/mL), 9i (24.21 and 2O.44μg/mL), 10h (18.32 and 16.32μg/mL), 10i (17.46 and18.28μg/mL). The reference drug Doxorubicin (15.29 and 12.26μg/mL) and in vitro Anti-bacterial activity was evaluated against two gram positive and two-gram negative bacterial strain by using agar diffusion method and zone of inhibition is calculated the most of the compound shows promising active compared with reference drug ciprofloxacin. The synthesized compound were shows more active the carbon chain increases the activity also increases. The in silico molecular docking analysis were predicted and it shows a good binding affinity towards 5C5S protein (human myosin 9b RhoGAP).
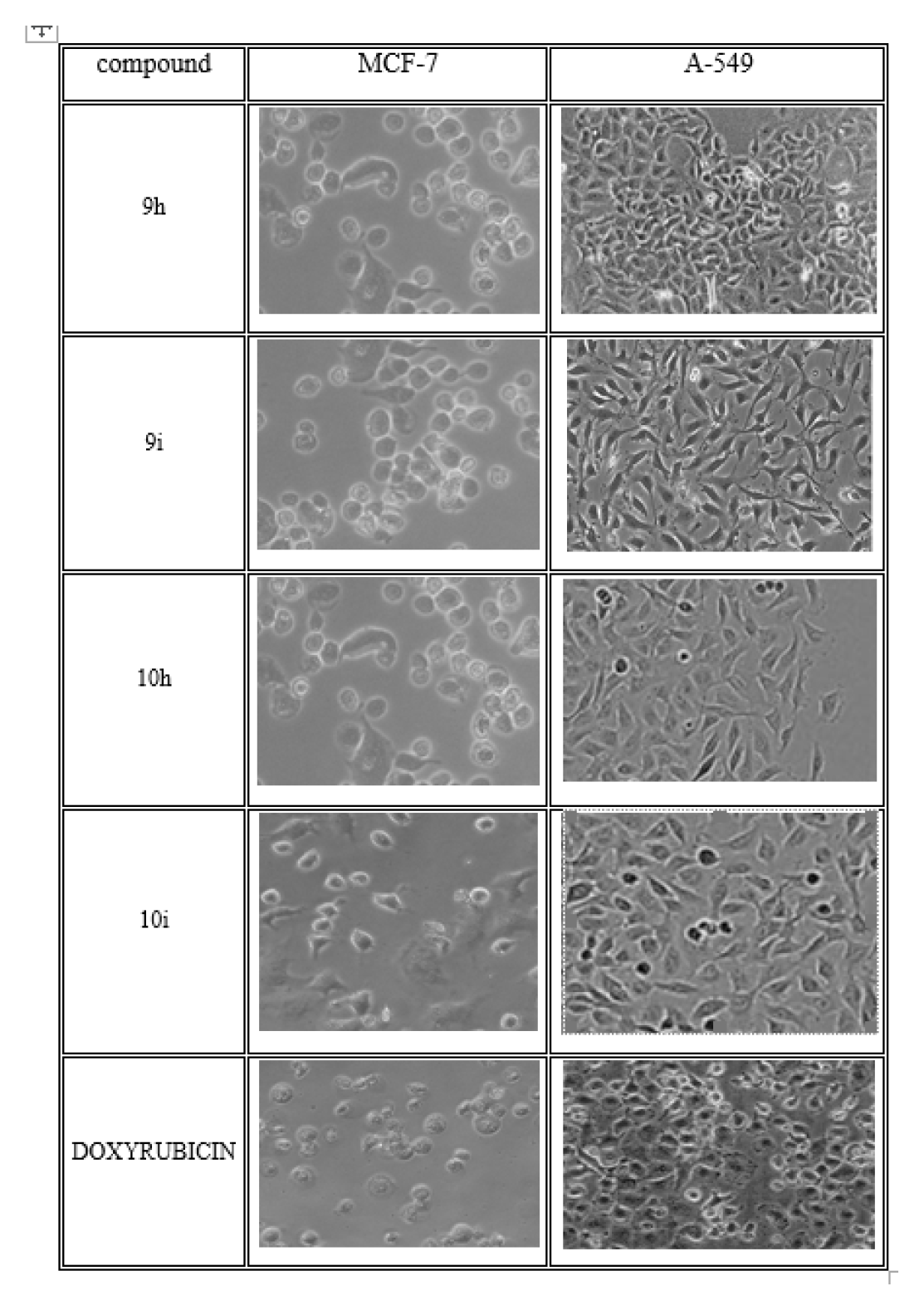
Figure 4.
Anticancer activity of potent synthesized compounds Morphological screening.
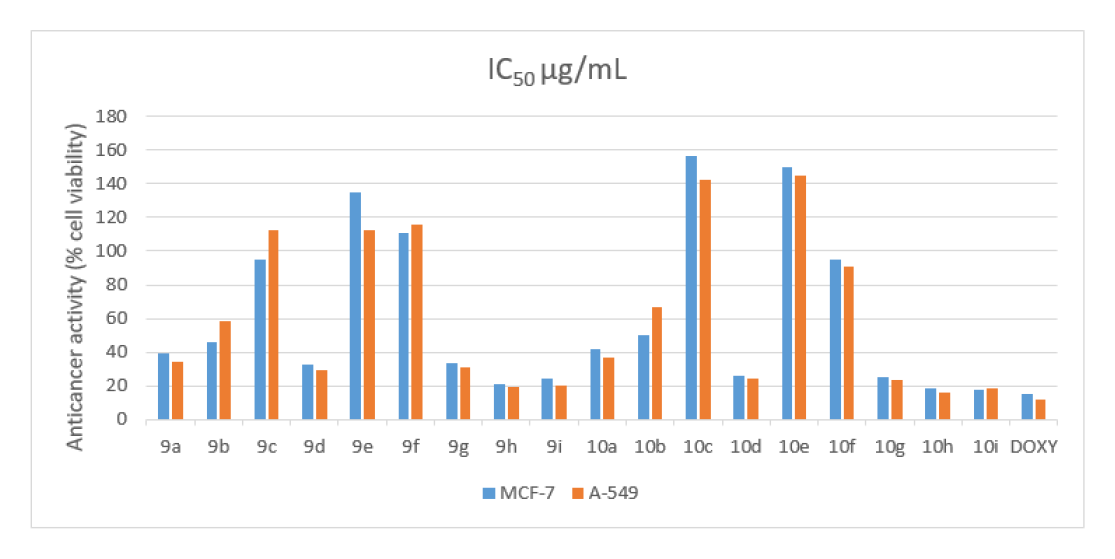
Figure 5.
Anticancer activity (%cell viability).
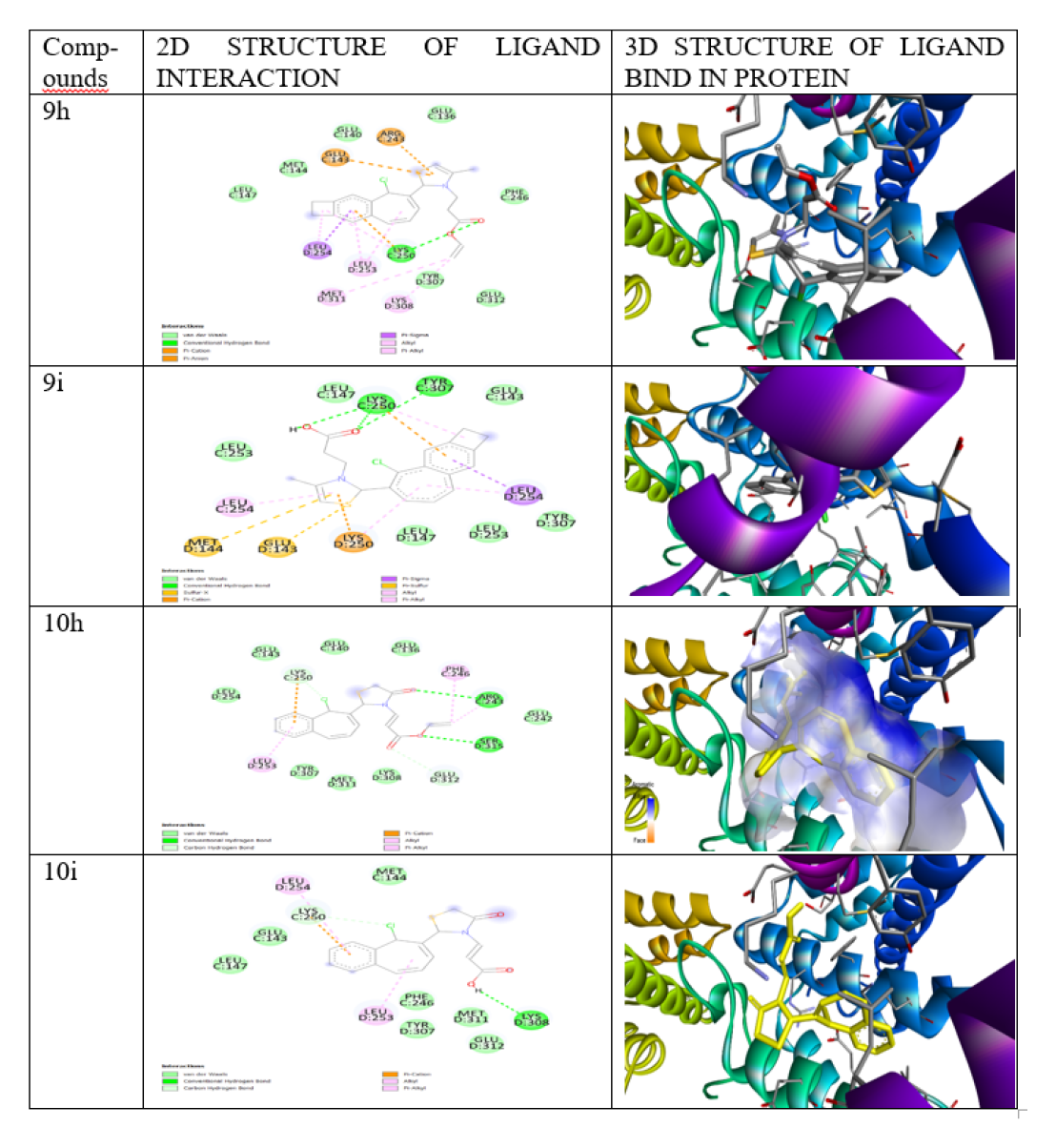
Figure 6.
In silico 5C5S (human myosin 9b RhoGAP) inhibition of potent synthesized compounds and interaction with ligand.
Cite this article
Afroz M, Kumar GS. Design Synthesis, Molecular Docking, and in vitro Anticancer and Antibacterial Evaluation of Novel 2-(9-chloro- 2,3-dimethyl-6,7-dihydro-5H-benzo[7]annulen-8-yl)-3-thiazolidin-4one. J Young Pharm. 2023;15(2):283-91.
ACKNOWLEDGEMENT
The authors are thankful to the director of IICT Hyderabad for providing spectral analysis data and GITAM School of Pharmacy, Hyderabad.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- Tunstall-Pedoe H. Preventing Chronic Diseases. A Vital Investment: WHO Global Report. Geneva: World Health Organization, 2005, 200. CHF 30.00. ISBN 92 4 1563001. Also published onhttp://www.who.int/chp/chronic_disease_report/en
[Google Scholar] - Temme L, Boergel F, Schepmann D, Robaa D, Sippl W, Daniliuc C, et al. Impact of hydroxy moieties at the benzo[7] annulene ring system of GluN2B ligands: Design, synthesis and biological evaluation. Bioorganic and Medicinal Chemistry. 2019;27(23):115146 [Google Scholar]
- Yadagiri B, Holagunda UD, Bantu R, Nagarapu L, Guguloth V, Polepally S, et al. Rational design, synthesis and anti-proliferative evaluation of novel benzosuberone tethered with hydrazide-hydrazones. Bioorganic and medicinal chemistry letters. 2014;24(21):5041-4. [Google Scholar]
- Althagafi I, Farghaly TA, Abbas EM, Harras MF. Benzosuberone as Precursor for Synthesis of Antimicrobial Agents: Synthesis, Antimicrobial Activity, and Molecular Docking. Polycyclic Aromatic Compounds. 2021;41(8):1646-66. [Google Scholar]
- Grimaldi TB, Back DF, Zeni G. Potassium tert-butoxide promoted annulation of 2-alkynylphenyl propargyl ethers: Selective synthesis of benzofuran and 12 H-benzoannulene derivatives. The Journal of organic chemistry. 2013;78(21):11017-31. [Google Scholar]
- Ahmed SK, Haese NN, Cowan JT, Pathak V, Moukha-Chafiq O, Smith VJ, et al. Targeting chikungunya virus replication by benzoannulene inhibitors. Journal of Medicinal Chemistry. 2021;64(8):4762-86. [Google Scholar]
- Oh CH, Kim JH, Oh BK, Park JR, Lee JH. Gold-Catalyzed Sequential Activation of Propargylic Carboxylates: A Facile Route to Benzoannulenes. Chemistry-A European Journal. 2013;19(8):2592-6. [Google Scholar]
- Lazzari P, Distinto R, Manca I, Baillie G, Murineddu G, Pira M, et al. A critical review of both the synthesis approach and the receptor profile of the 8-chloro-1-(2’, 4’-dichlorophenyl)-N-piperidin-1-yl-1, 4, 5, 6-tetra hydro benzo [6, 7] cyclohepta [1, 2-c] pyrazole-3-carboxamide and analogue derivatives. European Journal of Medicinal Chemistry. 2016;121:194-208. [Google Scholar]
- Barros FW, Silva TG, Da Rocha Pitta MG, Bezerra DP, Costa-Lotufo LV, De Moraes MO, et al. Synthesis and cytotoxic activity of new acridine-thiazolidine derivatives. Bioorganic and Medicinal Chemistry. 2012;20(11):3533-9. [Google Scholar]
- Chintakunta R, Subbareddy GV. Synthesis Docking Studies, Characterization and Anti-tubercular Activity of Ofloxacin Containing Thiazolidinone Derivatives. Journal of Young Pharmacists. 2022;14(1):77 [Google Scholar]
- Sahiba N, Sethiya A, Soni J, Agarwal DK, Agarwal S. Saturated five-membered thiazolidines and their derivatives: From synthesis to biological applications. Topics in Current Chemistry. 2020;378(2):1-90. [Google Scholar]
- Osmaniye D, Levent S, Ardıę CM, Atlı Ö, Özkay Y, Kaplancıklı ZA, et al. Synthesis and anticancer activity of some novel benzothiazole-thiazolidine derivatives. Phosphorus, Sulfur, and Silicon and the Related Elements. 2018;193(4):249-56. [Google Scholar]
- Romagnoli R, Baraldi PG, Salvador MK, Camacho ME, Balzarini J, Bermejo J, et al. Anticancer activity of novel hybrid molecules containing 5-benzylidene thiazolidine-2, 4-dione. European Journal of Medicinal Chemistry. 2013;63:544-57. [Google Scholar]
- Asati V, Mahapatra DK, Bharti SK. Thiazolidine-2, 4-diones as multi-targeted scaffold in medicinal chemistry: Potential anticancer agents. European Journal of Medicinal Chemistry. 2014;87:814-33. [Google Scholar]
- Zhang Q, Zhou H, Zhai S, Yan B. Natural product-inspired synthesis of thiazolidine and thiazolidinone compounds and their anticancer activities. Current Pharmaceutical Design. 2010;16(16):1826-42. [Google Scholar]
- Sigalapalli DK, Kiranmai G, Tokala R, Tripura C, Ambatwar R, Nunewar SN, et al. Targeting tubulin polymerization and DNA binding of 4-thiazolidinone-umbelliferone hybrids: Synthesis and cytotoxicity evaluation. New Journal of Chemistry. 2021;45(40):18908-23. [Google Scholar]
- Sahoo M, Jena L, Daf S, Kumar S. Virtual screening for potential inhibitors of NS3 protein of Zika virus. Genomics and Informatics. 2016;14(3):104 [Google Scholar]
- Mahmoodi NO, Mohammadi Zeydi M, Biazar E, Kazeminejad Z. Synthesis of novel thiazolidine-4-one derivatives and their anticancer activity. Phosphorus, Sulfur, and Silicon and the Related Elements. 2017;192(3):344-50. [Google Scholar]
- Bilgicli AT, GencBilgicli H, Hepokur C, Tüzün B, Günsel A, Zengin M, et al. Synthesis of (4R)-2-(3-hydroxyphenyl) thiazolidine-4-carboxylic acid substituted phthalocyanines: Anticancer activity on different cancer cell lines and molecular docking studies. Applied Organometallic Chemistry. 2021;35(7):e6242 [Google Scholar]
- Kong R, Yi F, Wen P, Liu J, Chen X, Ren J, et al. Myo9b is a key player in SLIT/ ROBO-mediated lung tumor suppression. Journal of Clinical Investigation. 2015;125(12):4407-20. [Google Scholar]
