ABSTRACT
Backgroud
Invasive Fungal Infections (IFIs) are on the rise, notably among critical care patients in the Intensive Care Unit (ICU) undergoing treatment with Broad Spectrum Antibiotics (BSAs). These antibiotics, designed to combat a wide range of bacteria, inadvertently disrupt the body’s microbial balance, creating an environment favorable for opportunistic fungi, particularly Candida albicans, leading to severe infections. ICU patients, often with multiple risk factors, are at heightened risk. Aim and Objectives: This study aimed to assess the incidence of fungal infections in ICU patients treated with broad-spectrum antibiotics, highlighting the potential implications for patient outcomes and antibiotic stewardship in healthcare settings.
Materials and Methods
A cross-sectional observational study was conducted in the intensive care unit of Dhiraj General Hospital, Vadodara, Gujarat. Patients aged between 18-65 years who received broad-spectrum antibiotics for more than five days were included in the study. Patients with additional risk factors for developing fungal infections in the ICU, such as diabetes mellitus or corticosteroid therapy, were excluded. Relevant data were collected from patients’ medical records using a predefined proforma. The study aimed to determine if broad-spectrum antibiotics were independently associated with the development of fungal infections in ICU patients.
Results
The study unveiled a significant incidence of fungal infections in patients on broad-spectrum antibiotics. Among the 71 patients, 9.8% (7 individuals) met criteria for fungal infection development, emphasizing the need for vigilant monitoring.
Conclusion
The use of broad-spectrum antibiotics in the ICU is strongly linked to an increased risk of fungal infections. To mitigate this risk, healthcare providers should consider dose adjustments, narrow-spectrum antibiotics and prompt fungal infection diagnosis and treatment. Diagnosing fungal infections remains challenging due to confounding factors, comorbidities and high diagnostic costs. Therefore, it’s advisable to restrict broad-spectrum antibiotic use, such as third-generation cephalosporins and carbapenems, to reduce invasive fungal infection risk and antibiotic-resistant strains. Close monitoring of patients for fungal infection signs during broad-spectrum antibiotic treatment is essential.
INTRODUCTION
Patients in the Intensive Care Unit (ICU) are particularly susceptible to infections due to various factors. Many ICU admissions are a consequence of existing infections, some patients suffer from severe underlying illnesses that weaken their immune systems and the presence of numerous invasive medical devices further increases the risk of infection (Vincentet al., 2020). In the context of hospital care, especially in surgical and ICU settings, antibiotics are among the most commonly prescribed medications (Perveen., et al. 2018). It’s worth noting, however, that a substantial proportion of antibiotic prescriptions, potentially up to 50%, may be unnecessary (da Fonseca Pestana Ribeiro and Park. 2020). Notably, antibiotics such as Ceftriaxone, Meropenem and Cefoperazone+Sulbactam are frequently administered in the ICU (Joshi., et al. 2023). Beta-Lactam Antibiotics (BLAs) are the predominant class of antibiotics used in the ICU. ICU patients display various pathophysiological characteristics that give rise to specific Pharmacokinetic (PK) and Pharmacodynamic (PD) considerations, increasing the risk of underdosage and the need for tailored antibiotic therapy (Guilhaumou et al., 2019). Invasive Fungal Infections (IFIs) have seen an increasing incidence in recent times (Borjian Boroujeniet al., 2021). The most common nosocomial fungal infection among humans, invasive candidiasis, is closely linked to antibiotic use as a modifiable iatrogenic risk factor, although the underlying mechanisms remain elusive (Drummondet al., 2022). IFIs, which are a significant cause of nosocomial bloodstream infections, can result in severe conditions in patients with various underlying health issues and host factors (Liet al., 2020). Candida albicans, like numerous other microbial species, resides in the human gastrointestinal system. The gut microbiota’s substantial impact on fungal growth is increasingly evident, dating back to the discovery in the 1960s that human antibiotic use could trigger Candida overgrowth (Pérez 2021). Early diagnosis of invasive fungal infections is vital due to the associated morbidity and mortality. Nonetheless, making an early diagnosis is challenging because of the nonspecific symptoms and radiographic findings (Haydouret al., 2019). Effective utilization of laboratory testing, including antigen testing, serological assays and PCR-based methods, plays a pivotal role in swiftly and accurately identifying fungal infections (Hageet al., 2019). The human microbiota harbors organisms such as Candida spp., capable of causing opportunistic infections in healthy individuals and life-threatening conditions like invasive candidiasis (Drummondet al., 2022). To enhance patient outcomes, the judicious use of antibiotics is imperative (Kayambankadzanjaet al., 2020). This study is conducted at Dhiraj General Hospital, Vadodara, which houses a well-established critical care unit, including an ICU where broad-spectrum antibiotics are regularly administered to a significant patient population. This unique context provides an ideal opportunity to explore the potential link between broad-spectrum antibiotics and invasive fungal infections. By focusing on this specific hospital, we can gather local data and assess how broad-spectrum antibiotic use influences fungal infection rates among the hospital’s patients. The insights gained from this research have the potential to inform clinical practices and interventions, aiming to reduce fungal infections in critical care settings, ultimately improving patient safety and outcomes, particularly within the Dhiraj General Hospital.
Invasive
Fungal Infections (IFIs) have been increasingly documented within healthcare facilities, with a particular prevalence among critical care patients, notably those in the Intensive Care Unit (ICU) receiving Broad Spectrum Antibiotics. Despite this emerging concern, there is a paucity of comprehensive literary evidence addressing this issue. This study seeks to bridge this knowledge gap by investigating the incidence of fungal infections in the specific context of broad-spectrum antibiotic use. By delving into this underexplored area, our research aims to contribute to the understanding of how the incidence of fungal infections can be mitigated. This includes optimizing antibiotic usage, with a particular focus on the prudent application of broad-spectrum antibiotics. The overarching goal of this study
is to provide healthcare professionals with data-driven insights that can lead to more judicious antibiotic practices and ultimately decrease the occurrence of fungal infections in the critical care setting. The research endeavors to enhance patient safety and care outcomes within the ICU, reinforcing the imperative need for a comprehensive examination of this critical issue.
MATERIALS AND METHODS
In this cross-sectional observational study conducted over six months in the Intensive Care Unit (ICU) of Dhiraj General Hospital, Vadodara, Gujarat, we investigated the incidence of fungal infections among patients aged 18 to 65 years who had received broad-spectrum antibiotics. Ethical approval was obtained from the Sumandeep Vidyapeeth Institutional Ethics Committee (SVIEC/ON/PHAR/BNPG21/NOV/22/23). A total of 71 ICU patients were included and informed consent was obtained from them and, where necessary, from their relatives. Patients with pre-existing risk factors such as diabetes mellitus, corticosteroid therapy, or those already on broad-spectrum antibiotics before admission were excluded. Data collection involved recording details of antibiotic duration, dose and frequency. After five days of antibiotic therapy, fungal infection diagnosis was initiated through laboratory cultures, with subsequent identification of fungal species and administration of appropriate antifungal treatment. The data were analyzed using Microsoft Excel, representing quantitative data as percentages and mean±standard deviation. The incidence of fungal infection was calculated as part of the analysis, contributing to our understanding of the impact of broad-spectrum antibiotics on fungal infections in ICU patients (Figure 1).

Figure 1:
Schedule of the study.
RESULTS
The study was carried out in the Medical Intensive Care Unit (MICU) at Dhiraj Hospital. In our study, the total sample size comprised 71 patients. Among them, 7 patients developed fungal infections after the administration of broad-spectrum antibiotics, accounting for 0.098% of all the patients included in our study. Patients were diligently screened on a daily basis for a period of four months until we reached our desired sample size. We continued to monitor patients until we achieved the designated sample size. The majority of patients in the study (38%) were in the 51-60 age groups, followed by the 41-50 age group (28%), with the 31-40 age group accounting for 9.8% and the 21-30 age group at 5.6%. The mean age of the patients was 50.23±10.79, indicating that the average age of the study population is approximately 50 years. Out of the total patients, 60.56% were male, while 39.43% were female, suggesting that fungal infections in ICU patients receiving broad-spectrum antibiotics do not exhibit significant gender bias. The data also reveals the presence of various comorbidities in the patient population. The most prevalent comorbidity was hypertension, observed in 32.39% of the patients. Other comorbidities included alcoholic conditions (7.04%), aortic stenosis (2.81%), asthma (2.81%), COPD (2.81%), Cerebrovascular Accident (CVA) (1.40%), filariasis (1.40%), anemia (1.40%), Ischemic Heart Disease (IHD) (1.40%), liver disease (1.40%), tuberculosis (1.40%) and Rheumatic Heart Disease (RHD) (4.22%). It’s noteworthy that a significant proportion of patients (38.02%) had no reported underlying diseases (Table 1).
| Total No. 71 | |
|---|---|
| Age | No. of Patients (%) |
| ≤20 years | 01 (1.4) |
| 21-30 years | 04 (5.6) |
| 31-40 years | 07 (9.8) |
| 41-50 years | 20 (28) |
| 51-60 years | 27 (38) |
| ≥60 years | 12 (16.9) |
| Mean age (Mean±SD) | 50.23±10.79 |
| Gender | |
| Male | 43 (60.56) |
| Female | 28 (39.43) |
| Comorbidities | |
| Alcoholic | 05 (7.04) |
| Anemia | 01 (1.40) |
| Aortic Stenosis | 02 (2.81) |
| Asthmatic | 02 (2.81) |
| COPD | 02 (2.81) |
| CVA | 01 (1.40) |
| Filariasis | 01 (1.40) |
| Hypertension | 23 (32.39) |
| IHD | 01 (1.40) |
| Liver disease | 01 (1.40) |
| Tuberculosis | 01 (1.40) |
| RHD | 03 (4.22) |
| No Disease | 27 (38.02) |
Out of the 71 patients included in the study, 7 (9.85%) developed a fungal infection. Notably, none of the patients who received Salbactum, Augmentin, Cefotaxime, Ceftriaxone, Ciprofloxacin, Meropenem, Tazobactum, or Clindamycin developed a fungal infection. However, when patients were prescribed two or three antibiotics, the percentage of patients who developed a fungal infection increased to 4.22%. Furthermore, one patient who received four antibiotics developed a fungal infection, representing a 1.41% rate. These findings collectively suggest that patients who are administered multiple antibiotics may be at an elevated risk of developing fungal infections when compared to those receiving a single antibiotic (Figure 2 and Table 2).
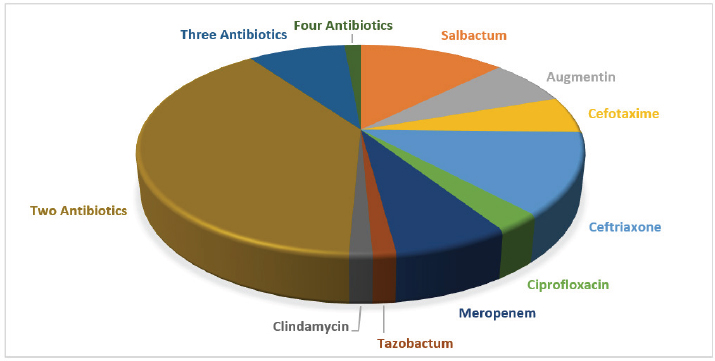
Figure 2:
Prescribed Antibiotics.
| Drugs (Single and combination) | No. of patients received | No. of patient developed fungal infection | Drugs | No. of patients received | No. of patient developed fungal infection |
|---|---|---|---|---|---|
| Salbactum | 08 | Meropenem Clindamycin | 01 | ||
| Augmentin | 05 | Salbactum Meropenem | 01 | ||
| Cefotaxime | 04 | Tazobactum Levofloxacin | 01 | ||
| Ceftriaxone | 09 | Meropenem Augmentin | 01 | ||
| Ciprofloxacin | 02 | Azithromycin Ceftriaxone | 01 | ||
| Meropenem | 05 | Meropenem Ceftriaxone | 01 | ||
| Tazobactum | 01 | Meropenem Linezolid | 01 | ||
| Clindamycin | 01 | Azithromycin Augmentin | 01 | ||
| Salbactum Azithromycin | 03 | Clindamycin Ceftriaxone | 01 | ||
| Azithromycin Meropenem | 04 | 02 | Meropenem Ciprofloxacin | 01 | |
| Augmentin Levofloxacin | 02 | Clindamycin Augmentin | 01 | ||
| Salbactum Clindamycin | 02 | Tazobactum Clindamycin Augmentin | 02 | 01 | |
| Augmentin Linezolid | 02 | 01 | Azithromycin Clindamycin Augmentin | 01 | |
| Tazobactum Clindamycin | 05 | 01 | Meropenem Clindamycin Ciprofloxacin | 01 | 01 |
| Levofloxacin Ceftriaxone | 02 | Meropenem Tazobactum Clindamycin Augmentin | 01 | 01 |
In the total population under study, 30% were addicted to smoking, 31% were addicted to smokeless tobacco and 34% were addicted to alcohol. Out of the 71 samples tested, 7 samples (approximately 10%) showed the presence of fungal elements, while 64 samples (about 90%) did not exhibit any fungal elements. Regarding the specific fungal components, the first component, Candida Non Albicans, was observed in 5 patients. The second component, Yeast budding cells, was identified in 2 patients. The remaining patients, who did not exhibit a fungal infection, accounted for a frequency of 64. Among these findings, 5 patients who tested positive for fungal infection received a daily dose of 200 mg of fluconazole administered intravenously. Additionally, two patients who tested positive for fungal infection were administered a dose of 50 mg of caspofungin twice daily (Table 3).
| Age group (years) | Gender | Total | Female | Male |
|---|---|---|---|---|
| ≤ 20 | 1 | 0 | 1 | |
| 21-30 | 3 | 1 | 4 | |
| 31-40 | 2 | 5 | 7 | |
| 41-50 | 7 | 13 | 20 | |
| 51-60 | 8 | 19 | 27 | |
| ≥ 61 | 7 | 5 | 12 | |
| Total | 28 | 43 | 71 | |
| Data according to addiction | ||||
| Addiction | Frequency (%) | |||
| Smoking | 30 | |||
| Tobacco | 31 | |||
| Alcohol | 34 | |||
| Data according to the diagnosis of fungal infection (culture test) | ||||
| Result | Frequency | |||
| Fungal elements seen | 07 | |||
| No fungal elements seen | 64 | |||
| Total | 71 | |||
| Data according to the diagnosis of fungal infection (culture test) | ||||
| Result | Frequency | |||
| Fungal elements seen | 07 | |||
| No fungal elements seen | 64 | |||
| Total | 71 | |||
| Data according to the diagnosis of fungal infection (culture test) | ||||
| Name (isolated species) | Frequency (%) | |||
| Candida Non Albicana | 05 | |||
| Yeast budding cells | 02 | |||
| NA | 64 | |||
| Total | 71 | |||
| Data according to treatment of fungal infection | ||||
| Name of drug | Dose | No. of patient received among the positive for fungal infection | Frequency | ROA |
| Fluconazole | 200 mg | 05 | OD | IV |
| Caspofungin | 50 mg | 02 | BD | IV |
DISCUSSION
In our study conducted within the Medical Intensive Care Unit (MICU) of Dhiraj Hospital, a total of 71 patients met the inclusion and exclusion criteria. Among these, 60% were male and 38% were female. Following the initiation of broad-spectrum antibiotic therapy, seven patients developed fungal infections in conjunction with various broad-spectrum antibiotics. A separate study involving 105 patients found that 82.9% received antibiotics known to impact anaerobic gut flora, such as imipenem, vancomycin, ceftazidime, metronidazole, clindamycin, or ampicillin-sulbactam. Additionally, 44.5% used prophylactic ofloxacin. Patients on ofloxacin, aminoglycosides, or azithromycin (antibiotics not affecting anaerobic flora) displayed higher rates of C. albicans infection (58.9% vs. 33.7%, p=0.04). Conversely, those on third-generation cephalosporins, carbapenems, glycopeptides and broad-spectrum penicillin were at a greater risk of developing invasive Aspergillus spp. Infections (Soysaet al., 2008). Bajpai et al.’s review underscores the growing significance of fungi as clinical pathogens, particularly in critically ill patients, including those who are immunocompromised. Candida, Cryptococcus and filamentous fungi are frequently encountered in clinical settings (Bajpaiet al., 2019). Another study suggests that broad-spectrum antibiotic exposure in preterm neonates without underlying illness is associated with an increased risk of Invasive Fungal Infections (IFIs) (Esaiassenet al., 2017). This study also concluded that both Carbapenem monotherapy and its combination with amikacin resulted in a significant increase in Candida albicans concentration (Samoniset al., 2013). In a study encompassing 71 critically ill patients, all of whom were administered various broad-spectrum antibiotics such as meropenem, salbactam, ceftriaxone, clindamycin, augmentin and ciprofloxacin, those who received these antibiotics as monotherapy did not develop Candida spp. infections. However, patients who received combinations of these broad-spectrum antibiotics exhibited a potential risk for such infections. Peres Bota et al.’s prospective study among critically ill patients (28 infected patients) unveiled that those with an ICU stay exceeding 24 hr predominantly suffered from bacterial infections (89%), while 11% met the criteria for Candida infection. This study further underscored the association between broad-spectrum antibiotic therapy and increased fungal growth in patients previously colonized, suggesting that curtailing antibiotic usage may serve as a preventive measure against fungal infections (Peres-Botaet al., 2004; Tianet al., 2018). Typically, the likelihood of developing a fungal infection increases in patients exposed to broad-spectrum antibiotics for more than 5 to 15 days. However, our study revealed that patients who developed fungal infections had received antibiotics for a duration ranging from 5 to 9 days. In a prospective study conducted in New Delhi, the focus was on children with acute leukemia and persistent febrile neutropenia. The study revealed a concerning prevalence of Invasive Fungal Infection (IFI) at 22.97% among those who were not on antifungal prophylaxis. Predictors of IFI included abnormal chest X-rays and clinical sinusitis and these factors played a significant role in influencing patient outcomes, including mortality or discharge (Kumaret al., 2018). A retrospective analysis in Germany spanning from 2002 to 2016 delved into fungal infections in patients with pancreatic necrosis and pseudocysts, involving 113 patients and 187 Fine-needle aspirations. Notably, approximately 46% of these patients were found to have fungal pancreatic infections, primarily caused by Candida species. This risk was significantly associated with pre-FNA antibiotic use (p=0.003) and the duration of treatment (Reukenet al., 2018). In a study, focusing on patients with severe acute pancreatitis, fungal infections, predominantly Candida albicans, were identified in 36% of cases. Prolonged hospital stays (exceeding 4 weeks) and extended courses of antibiotics were found to increase the risk of fungal infections (Eggimannet al., 2015). Considering the insights from these studies, the initial hypothesis put forward to explain the occurrence of fungal infections in the current study is that modifications in antibiotic treatment, which led to a reduction in early deaths caused by bacterial infections, might have rendered the patients more susceptible to secondary events like fungal infections. In simpler terms, the new antibiotic regimen indirectly contributed to the emergence of fungal infections. Consequently, it is imperative to closely monitor patients receiving broad-spectrum antibiotics for the development of fungal infections.
CONCLUSION
In conclusion, our study highlights a strong link between the use of broad-spectrum antibiotics in ICU settings and a heightened risk of fungal infections. These findings reinforce the need for
prudent antibiotic stewardship to protect patient health and minimize complications. Limiting the use of broad-spectrum antibiotics, such as third-generation cephalosporins and carbapenems, when narrower-spectrum options are available, may reduce the incidence of Invasive Fungal Infections (IFI) and help curb antibiotic resistance. Early identification and intervention for fungal infections are vital, particularly given the complex health profiles of ICU patients. The substantial costs and diagnostic complexities of fungal infections further stress the importance of careful antibiotic selection. By encouraging a restrained approach to antibiotic use, healthcare providers can improve patient outcomes, lower healthcare costs and support broader public health efforts against antibiotic resistance. An evidence-based, vigilant strategy in ICUs can ultimately reduce the societal burden of these infections and enhance patient care in critical care settings.
Cite this article:
Hadia R, Chauhan A, Thakkar B, Patel D, Bagban M, Rajput HS. Assessing the Impact of Broad-Spectrum Antibiotics on Fungal Infection Rates in ICU Patients: Implications for Patient Safety. J Young Pharm. 2025;17(2):387-93.
ACKNOWLEDGEMENT
We would like to express our sincere appreciation for the support and resources provided by the Department of Pharmacy at Sumandeep Vidyapeeth (Deemed to be University) in Vadodara. Your assistance has been invaluable in the preparation and completion of our recent article. We are grateful for your commitment to fostering research and academic excellence, which has greatly contributed to the success of our work.
ABBREVIATIONS
| IFIs | Invasive Fungal Infections |
|---|---|
| BSAs | Broad Spectrum Antibioticsl |
| BLAs | Beta:lactam antibiotics |
| PK | Pharmacokinetic |
| PD | Pharmacodynamic |
| C. albicans | Candida albicans |
| MICU | Medical Intensive Care Unit |
| ROA | Route of Administration |
| FNA | Fine needle aspiration |
| ICU | Intensive Care Unit |
References
- Bajpai VK, Khan I, Shukla S, Kumar P, Rather IA, Park Y-H, Huh YS, Han Y-K, et al. (2019) Invasive fungal infections and their epidemiology: Measures in the clinical scenario. Biotechnology and Bioprocess Engineering 24: 436-444 https://doi.org/10.1007/s12257-018-0477-0 | Google Scholar
- Borjian Boroujeni ZB, Shamsaei S, Yarahmadi M, Getso MI, Salimi Khorashad A, Haghighi L, Raissi V, Zareei M, Saleh Mohammadzade A, Moqarabzadeh V, Soleimani A, Raeisi F, Mohseni M, Mohseni MS, Raiesi O, et al. (2021) Distribution of invasive fungal infections: Molecular epidemiology, etiology, clinical conditions, diagnosis and risk factors: A 3-year experience with 490 patients under intensive care. Microbial Pathogenesis 152 https://doi.org/10.1016/j.micpat h.2020.104616 | Google Scholar
- da Fonseca Pestana Ribeiro JM, Park M. (2020) Less empiric broad-spectrum antibiotics are more in the ICU. Intensive Care Medicine 46: 783-786 https://doi.org/10.1007/s00134-019-05863-z | Google Scholar
- Drummond RA, Desai JV, Ricotta EE, Swamydas M, Deming C, Conlan S, Quinones M, Matei-Rascu V, Sherif L, Lecky D, Lee CR, Green NM, Collins N, Zelazny AM, Prevots DR, Bending D, Withers D, Belkaid Y, Segre JA, Lionakis MS, et al. (2022) Long-term antibiotic exposure promotes mortality after systemic fungal infection by driving lymphocyte dysfunction and systemic escape of commensal bacteria. Cell Host and Microbe 30: 1020-1033e6 https://doi.org/10.1016/j.chom.2022.04.013 | Google Scholar
- Eggimann P, Que Y-A, Revelly J-P, Pagani J-L. (2015) Preventing invasive Candida infections: Where could we do better?. The Journal of Hospital Infection 89: 302-308 https://doi.org/10.1016/j.jhin.2014.11.006 | Google Scholar
- Esaiassen E, Fjalstad JW, Juvet LK, van den Anker JN, Klingenberg C. (2017) Antibiotic exposure in neonates and early adverse outcomes: A systematic review and meta-analysis. The Journal of Antimicrobial Chemotherapy 72: 1858-1870 https://doi.org/10.1093/jac/dkx088 | Google Scholar
- Guilhaumou R. (2019) Optimization of the treatment with beta-lactam antibiotics in critically ill patients-Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’anesthésie et Réanimation-SFAR). Critical Care 23: 1-20 https://doi.org/10.1093/jac/dkx088 | Google Scholar
- Hage CA, Carmona EM, Epelbaum O, Evans SE, Gabe LM, Haydour Q, Knox KS, Kolls JK, Murad MH, Wengenack NL, Limper AH, et al. (2019) Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice: An official American Thoracic Society clinical practice guideline. American Journal of Respiratory and Critical Care Medicine 200: 535-550 https://doi.org/10.1164/rccm.201906-1185ST | Google Scholar
- Haydour Q, Hage CA, Carmona EM, Epelbaum O, Evans SE, Gabe LM, Knox KS, Kolls JK, Wengenack NL, Prokop LJ, Limper AH, Murad MH, et al. (2019) Diagnosis of fungal infections: A systematic review and meta-analysis supporting American Thoracic Society Practice Guideline. Annals of the American Thoracic Society 16: 1179-1188 https://doi.org/10.1513/AnnalsATS.201811-766OC | Google Scholar
- Joshi ID. (2023) Prospective observational study on prescription pattern of antibiotics in general ward and intensive care unit in a tertiary care hospital. World Journal of Biological and Pharmaceutical Health Sciences 14: 42-52 https://doi.org/10.1513/AnnalsATS.201811-766OC | Google Scholar
- Kayambankadzanja RK, Lihaka M, Barratt-Due A, Kachingwe M, Kumwenda W, Lester R, Bilima S, Eriksen J, Baker T, et al. (2020) The use of antibiotics in the intensive care unit of a tertiary hospital in Malawi. BMC Infectious Diseases 20: 20 https://doi.org/10.1186/s12879-020-05505-6 | Google Scholar
- Kumar J, Singh A, Seth R, Xess I, Jana M, Kabra SK, et al. (2018) Prevalence and predictors of invasive fungal infections in children with persistent febrile neutropenia treated for acute leukemia-A prospective study. Indian Journal of Pediatrics 85: 1090-1095 https://doi.org/10.1007/s12098-018-2722-0 | Google Scholar
- Li Y, Gao Y, Niu X, Wu Y, Du Y, Yang Y, Qi R, Chen H, Gao X, Song B, Guan X, et al. (2020) A 5-year review of invasive fungal infection at an academic medical center. Frontiers in Cellular and Infection Microbiology 10 https://doi.org/10.3389/fcimb.2020.553648 | Google Scholar
- Peres-Bota D, Rodriguez-Villalobos H, Dimopoulos G, Melot C, Vincent JL. (2004) Potential risk factors for infection with Candida spp. in critically ill patients. Clinical Microbiology and Infection 10: 550-555 https://doi.org/10.1111/j.1469-0691.2004.00873.x | Google Scholar
- Pérez JC. (2021) Fungi of the human gut microbiota: Roles and significance. International Journal of Medical Microbiology 311 https://doi.org/10.1016/j.ijmm.2021.151490 | Google Scholar
- Perveen RA. (2018) Antibiotics in ICU: The challenges of use, cost and response in a tertiary care hospital. International Journal of Medical Research and Health Sciences 7: 94-99 https://doi.org/10.1016/j.ijmm.2021.151490 | Google Scholar
- Reuken PA, Albig H, Rödel J, Hocke M, Will U, Stallmach A, Bruns T, et al. (2018) Fungal infections in patients with infected pancreatic necrosis and pseudocysts: Risk factors and outcome. Pancreas 47: 92-98 https://doi.org/10.1097/MPA.0000000000000965 | Google Scholar
- Samonis G, Galanakis E, Ntaoukakis M, Sarchianaki E, Spathopoulou T, Dimopoulou D, Kofteridis DP, Maraki S, et al. (2013) Effects of carbapenems and their combination with amikacin on murine gut colonisation by Candida albicans. Mycoses 56: 105-109 https://doi.org/10.1111/j.1439-0507.2012.02212.x | Google Scholar
- Soysa NS, Samaranayake LP, Ellepola AN. (2008) Antimicrobials as a contributory factor in oral candidosis-A brief overview. Oral Diseases 14: 138-143 https://doi.org/10.1111/j.1601-0825.2006.01357.x | Google Scholar
- Tian S, Rong C, Nian H, Li F, Chu Y, Cheng S, Shang H, et al. (2018) First cases and risk factors of super yeast Candida auris infection or colonization from Shenyang, China. Emerging Microbes and Infections 7: 7 https://doi.org/10.1038/ s41426-018-0131-0 | Google Scholar
- Vincent J-L, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, Finfer S, Pelosi P, Brazzi L, Aditianingsih D, Timsit J-F, Du B, Wittebole X, Máca J, Kannan S, Gorordo-Delsol LA, De Waele JJ, Mehta Y, Bonten MJM, et al. (2020) Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 323: 1478-1487 https://doi.org/10.1001/jama.2020.2717 | Google Scholar
