ABSTRACT
Background
Terminalia arjuna is an evergreen tree commonly referred as Arjuna, widely distributed in India and is a prominent member of the genus Terminalia. It has been discovered that the various plant parts, including the root, leaf, bark, fruits, and seeds have unique medicinal property. Among them, bark part is found to have and plethora of bioactive compounds and rich in medicinal property, especially for its cardiovascular properties.
Materials and Methods
In the current investigation we have developed a standardized extract of T arjuna bark called Cardiboost, which contains 1% arjunolic acid. Cardiboost was used to examine in vitro thrombin and ACE enzyme inhibition. Additionally, MTT assay was used to measure cell viability of H9c2 cardiomyocytes, western blot experiments were performed to measure the levels of antioxidative enzyme levels CAT and SOD and the expressions of cell apoptosis regulatory proteins PARP1, Casp antiapoptotic properties to elucidate Cardiboost ability to prevent H9c2 cardiomyocytes cells suffering from oxidative damage induced by H2O2.
Results
Cardiboost, exhibited potent thrombin and ACE inhibition property, with IC50 values of 111.88μg/mL and 196.66 μg/mL respectively, Furthermore, Cardiboost, averted the cell death and significantly increased the levels of antioxidant enzymes CAT and SOD which was subsided in presence of H2O2, and Cardiboost protected the mitochondrial events and reduced the apoptotic effects in H2O2-induced oxidative stress.
Conclusion
Cardiboost could shield H9c2 cardiomyocytes from oxidative stress and may also be used as a medicinal product to prevent oxidative stress in cardiac damage.
INTRODUCTION
Cardiovascular Diseases (CVDs) are the world’s leading cause of mortality, accounting for 17.9 million deaths in 2017 and an estimated 23 million by 2030.1 It has been reported that the term “Cardiovascular Disease” (CVD) describes a group of illnesses which effect blood vessels and the heart, it includes several illnesses, such as heart valve difficulties, arrhythmias, heart failure, and coronary artery disease.2 Several risk factors increase the likelihood of developing cardiovascular disease. These include high blood pressure, high cholesterol levels, smoking, obesity, sedentary lifestyle, diabetes, family history of CVD, age, and certain underlying conditions like chronic kidney disease or autoimmune disorders. Depending on the certain ailment and risk factors, medicines may be recommended to manage high blood pressure, cholesterol levels, and other related conditions. These may include statins, beta-blockers, ACE inhibitors, diuretics, or antiplatelet drugs.3,4
Currently, traditional herbal remedies and extracts from plants are being used as complementary and alternative therapies for the treatment, and prevention of CVDs. Secondary metabolites such as phenolics, flavonoids and terpenes that can scavenge free radicals, strengthen the antioxidant system, and modify redox signalling have been shown to have beneficial pharmacological effects. Terpenophenolics have been implicated in reducing levels of low-density lipoprotein, cholesterol, hyperglycaemia, and hypertension-all of which are key risk factors for CVDs. Herbal extracts and compounds are relatively safe and have low cost, making those attractive candidates for use as nutraceutical agents.5–7
Terminalia arjuna is a tree species native to the Indian subcontinent. It is commonly known as Arjuna or Arjuna tree and belongs to the Combretaceae family. The tree is honoured in Ayurvedic medicine and has a long history of traditional use for various health purposes. Various parts of the tree, including the bark, leaves, and flowers, are utilized for their therapeutic properties. Many therapeutic qualities, including antidyslepimidc, antioxidant, anti-diabetic, antibacterial, and anti-cancer effects, have been shown in T. arjuna bark.8–11According to reports, T. arjuna is used to treat cardiovascular problems such as heart failure, ischemia, cardiopathy, atherosclerosis, and myocardial necrotic disorders. T. arjuna bark includes several bioactive compounds, including triterpene glycosides and aglycones. Arjunolic acid, Arjunoglucoside I, II, and III.12–16
In this study, we developed Cardiboost, a standardised extract from T. arjuna bark containing 3% arjunolic acid and screened for Thrombin and ACE inhibition assay. We also used an H2O2-induced myocardial cell injury model to examine Cardiboost’s potential mechanism of action and ability to act as an antioxidant and antiapoptotic agent.
MATERIALS AND METHODS
Chemicals
Thrombin, Thrombin Substrate III, ACE isolated from rabbit lung, Histidine-L-Hippuryl-L-Leucine-chloride (HHL), Histidine Leucine (His-Leu), dimethyl sulfoxide, pyridineand BSC were bought from Sigma-Aldrich. Antibodies of anti, superoxide dismutase, catalase. PARP1, cleaved caspase 3, Bax and Bcl-2 acquired from Santa Cruz Biotechnology. All remaining supplies and reagents were obtained from HI media.
Standardized extract preparation
Cardiboost, a standardized extract of T. arjuna comprised of 1% arjunolic acid was developed by Vidya Herbs Pvt. Ltd, Bangalore. Briefly, T. arjuna bark part was collected, chopped into small pieces, dried, and processed into a powder. (500gm) which was then extracted with 3 L of 70% ethanol for 3-4 hr at 68-70°C in 5 L round bottom flask, the extraction was repeated two times and under reduced pressure the mother liquor was concentrated till the volume reduces to 500 mL and allow the above concentrated liquid for about 4-5 hr for precipitation, precipitate was washed with water 2-3 times and allow the precipitate to dry in oven under vacuum and the precipitated extract was subjected to HPLC to find out the Arjunolic acid content
Preparation of standard solutions
The stock solution of standard and sample 100 and 800 ppm respectively were prepared by dissolving in methanol. All solutions were kept at 4°C until analysis. Working solutions of the standards were prepared by dilution of stock solution to the appropriate concentrations.
HPLC instrumentation and chromatographic conditions
HPLC analysis was performed using (Shimadzu, Prominence LC2030C), to separate compounds, and a Kinetex C18 column (100Å, 150×4.6 mm) was employed. The mobile phase consists of potassium orthophosphate and ortho phosphoric acid made of water and acetonitrile (Solvent A and solvent B). A linear gradient elution consisting of 30-100% solvent B at 0.01 to 10 min, 100% solvent B continuously up to 10.50 min, 100-30% solvent B from 11.00 to 12.00 min, with a flow rate of 1.5 mL/min and the injection volume is 5μl, spectra were obtained in the region of 210 to 800 nm range. Plots of chromatograms were made at 277 and 254 nm. By injecting the matching standard separately, it was feasible to confirm the retention time of arjunolic acid (Supplementary Figure S1).
Thrombin inhibition assay
The procedure for performing the thrombin inhibition assay was followed by Batra et al., 2004.17 Briefly, a black 96-well plate containing 5-100 μg/mL of Cardiboost was incubated with Tris-buffer at pH 7.5. Next, 0.2 mM of thrombin substrate III was added, and then 1 U/mL, of thrombin. After incubation 96-well plate was read at 450nm of emission and 390nm of excitation in a fluorimeter (Tecan infinite).
Assay for ACE Inhibitory Activity
As previously mentioned, by monitoring the discharge of Hippuric acid from the substrate HHL, the activity of the angiotensin converting enzyme was determined.18 The assay was initiated by mixing sodium borate buffer pH 8.2, 5 mM HHL and ACE enzyme extract. After 30 min of incubation at 37°C, the reaction was stopped by adding 0.2 mL of 1 M HCl. Pyridine (0.4 mL) and BSC (0.2 mL) were added (the sequence in which the chemicals are introduced is crucial), mixed by inversion for a minute, and then allowed to cool on ice. The yellow colour that appeared was detected using a spectrophotometer (Shimadzu UV 1601) at 410 nm. Hippuric acid concentration in the test reaction was lower than in the control.
Cell culture and treatment
Rat H9c2 cardiomyocytes were sourced from NCCS Pune, India. Cells were cultured in 90% Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% foetal bovine serum, 4.5 g/L glucose, 1.5 g/L sodium bicarbonate, and 4 mM L-glutamine. The cells were maintained at 37°C in an environment with 5% carbon dioxide. H9c2 cells were plated in a 60 mm dish at a density of 106 cells/dish and parted into three groups: control, model, and treatment groups and cultured overnight. Following an overnight incubation period, two distinct doses (50 and 100 μg/mL) of Cardiboost were administered for a 24 hr period to the treatment groups. The model group and the control group did not receive any extracts. After that, H2O2 (100 μM) was exposed to the model group and the treatment groups for 24 hr, while the control group received DMEM medium treatment.
Cell viability assay (MTT method)
MTT assay was carried out to determine viability of cells.19 Briefly, in 96-well plates, H9c2 cells were placed at a density of 5×103 cells per well and grown to 80% confluence. Subsequently, the cells were subjected to a 24 hr treatment of 100 μM H2O2 either without any pretreatment or after being pretreated with 100 and 200 μg/mL of Cardiboost. After 24 hr, media was discarded and replaced with 0.5 mg/mL MTT. Following 4 hr of incubation at 37°C, the media was aspirated, and the formazan crystals were dissolved in 100 μL DMSO. Absorbance was measured at 570 nm using (Tecan infinite).
Western Blot Analysis
Western blot analysis was carried out in compliance with the standard procedure. H9c2 cells at the density of 2×105 grown in 6-well plates and subjected to two different concentration 100 and 200 μg/mL of Cardiboost for 24 hr with and without 100μM of H2O2. After incubation, the cells were harvested, collected, and lysed in lysis buffer. Following the determination of the protein concentration, the equal quantities of proteins were separated via 12% SDS-PAGE, and consequently transferred onto a PVDF membrane. The membranes were then blocked for 2 hr at 4°C using 5% (w/v) fat free milk powder, and then membranes were incubated overnight with (SOD, CAT, PARP1, caspase-3, BAX, BCL2) primary antibodies at 4°C. Following washing, the membranes were subjected to incubation for 2 hr at room temperature with a secondary antibody coupled with horseradish peroxidase. In the end, the protein bands were visualized using the ECL chemiluminescence detection system (Immobilon Western Chemiluminescent HRP Substrate, Merck Millipore)
Statistical analysis
GraphPad Prism software was used to analyse the experimental data. On the other hand, the mean and Standard Error (SE) of each measurement value was reported in at least three independent experiments. The one-way analysis of ANOVA and the student’s t-test exhibit statistical significance levels of p<0.01 and p<0.001 were used to identify the significant differences between groups.
RESULTS
Thrombin inhibitory activity of Cardiboost
Thrombin is a serine protease that controls the coagulation cascade with a procoagulant effect and affects haemostasis by catalysing the conversion of fibrinogen to fibrin. Acute coronary syndrome, stroke, and venous thromboembolism are among the CVD-related conditions for which thrombin inhibitors are increasingly recognised as effective therapeutic agents. Cardiboost was tested for their potential to inhibit thrombin. As shown in Figure 1A Cardiboost displayed 76.69%, inhibition at the concentration of 250 μg/mL. The IC50 values of Cardiboost for the inhibition of thrombin is 111.88 μg/mL. This indicates that Cardiboost, standardized extract of Terminalia arjuna is a potential source of Thrombin inhibitor.
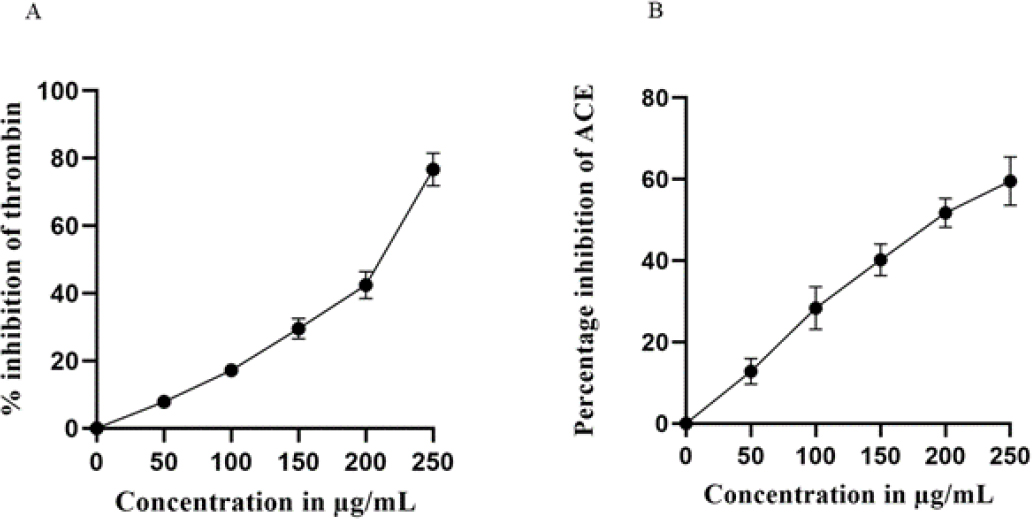
Figure 1:
Effect of Cardiboost on Thrombin Inhibitory (A) and ACE inhibitory activity (B). mean±SEM, n=3
ACE-Inhibitory Activity
Since hypertension is a major risk factor for coronary disease, heart failure, stroke, and other cardiovascular conditions, the in vitro testing of ACE inhibitor activity has proven to be a useful assay method in the development of antihypertensive medications. As shown in Figure 1B Cardiboost demonstrated strong activity with a percent inhibition of 59.59% at a concentration of 250 μg/mL; the IC50 value for ACE inhibition was calculated to be 196.66 μg/ mL.
Effect of Cardiboost on the cell viability
The MTT assay was used to determine the cell viability. As shown in Figure 2. H9c2 cells treated with varying concentrations of Cardiboost, lower concentrations of Cardiboost increased cell viability in comparison to untreated cells, while higher concentrations showed a slight decrease in cell viability. It is evident from the results of the MTT assay that Cardiboost is not cytotoxic to H9c2 cells up to a dose of 400 μg/mL.
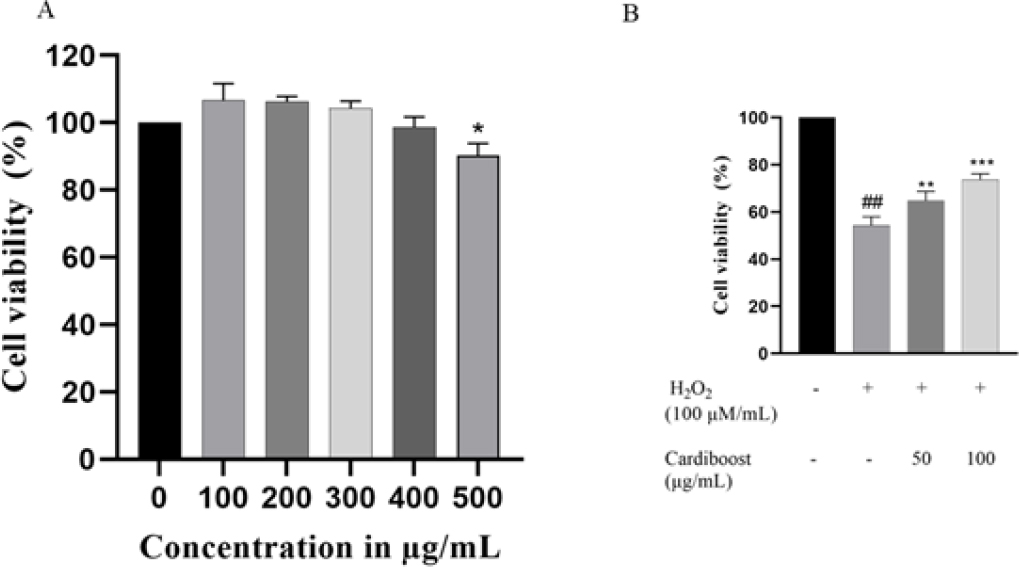
Figure 2:
Effect of different concentration of Cardiboost on cell viability of H9c2 cells (A). Protective effect of Cardiboost against H2O2 induced cell death (B). Data are expressed as mean±SEM, n=3 versus control or untreated cells. Significance was analyzed by a one-way ANOVA followed by the followed by a Tukey test. *p<0 05 versus untreated cells. ##p<0.001 versus control cells or untreated cells, ** p <0 01, and *** p <0 001 versus H2O2 alone-treated cells.
Protective effect of Cardiboost against H2O2 induced cell death
Protective effect of Cardiboost was determined by MTT assay, in this experiment H9c2 cells were pre-treated with two different concentrations (50 and 100 μg/mL) of Cardiboost followed by H2O2 stimulation. As shown Figure 2B, H2O2 stimulation significantly reduced the cell survival rate and increased the number of dead cells. In contrast, cells treated with 50 and 100 μg/ mL of Cardiboost showed a substantial rise in cell viability when compared to cells treated with H2O2 alone. Furthermore, direct microscopic observation of the cell morphology was conducted. When the cells were exposed to H2O2, they lost their spindle-like shape and began to separate. After the cells were pretreated with Cardiboost, particularly at high doses of 50-100 μg/mL, there was a noticeable recovery in the cell morphology. Considering these findings, Cardiboost prevented H2O2-induced alterations in cell shape and cell death.
Cardiboost inhibited H2O2-Induced Oxidative Stress in H9c2 Cells
Antioxidant enzymes form a critical component of the body’s antioxidant defence system and are necessary for preserving cellular homeostasis by scavenging and neutralising reactive oxygen species. When these enzymes aren’t functioning properly, it can cause oxidative stress and a factor number of pathological illnesses, such as cancer, cardiovascular disease, and neurological diseases. In this study we used western blot to detect the expression levels of Catalase (CAT) and Superoxide Dismutase (SOD) protein. As presented in Figure 3, the expression levels of CAT and SOD in the H2O2 treatment group were markedly lower than those in the control group, whereas the course of pre-treatment with Cardiboost (100 μg/mL) significantly increased the expression levels of CAT and SOD (Figures 2B and 2C).
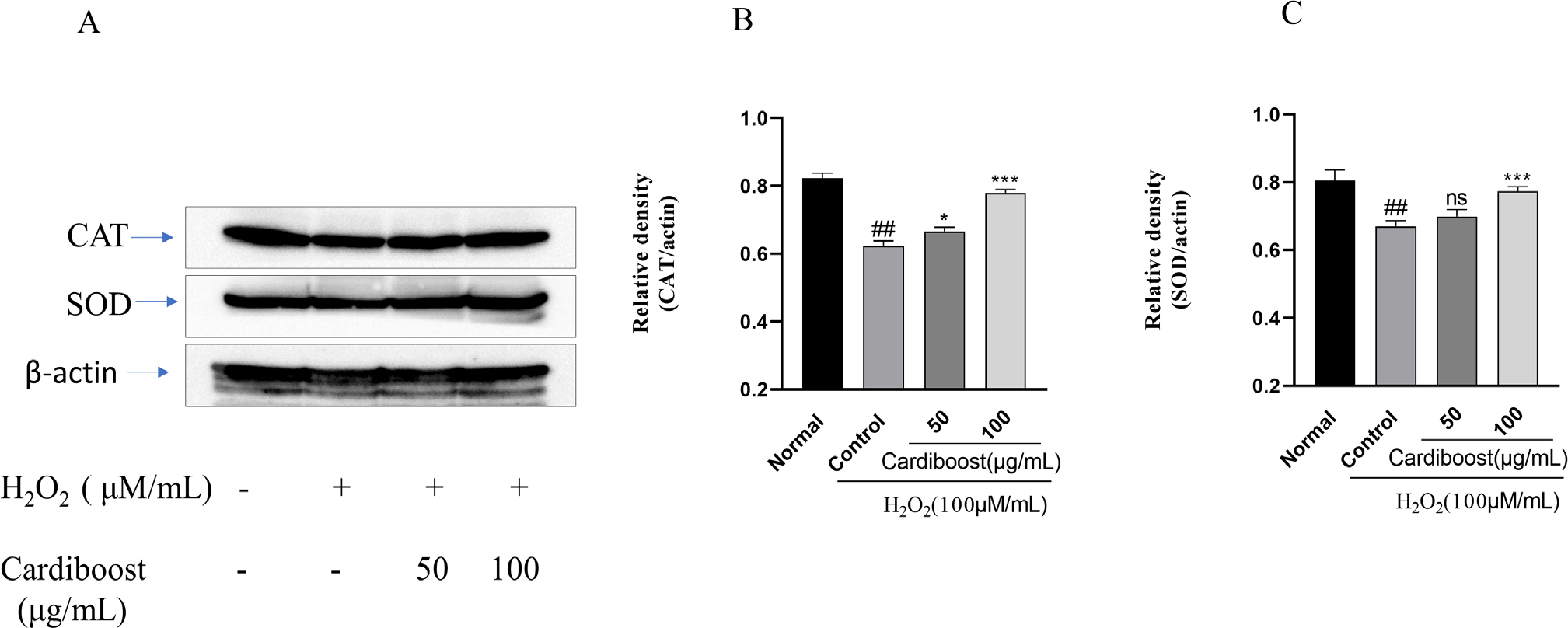
Figure 3:
Western blot analysis of catalase and SOD-1 protein expression levels (A). Protein expression levels were quantified by densitometry. β-Actin was used as the loading control. (B, C). Data are expressed as mean±SEM, n=3. Significance was analyzed by a one-way ANOVA followed by a Tukey test. ##p<0.001 versus untreated normal cells. **p<0 01, and ***p<0 001 versus H2O2 alone-treated cells.
Effect of Cardiboost mitochondrial apoptosis in H9c2 Cardiomyocytes
We investigated probable mechanisms underlying this cardioprotective effect of Cardiboost by evaluating the level of expression of poly [ADP-ribose] polymerase 1 (PARP-1) and caspase-3 using western blot assays. We studied the expression of protein from H9c2 cells exposed to H2O2 with or without Cardiboost. Both cleaved PARP-1 and cleaved caspase-3 (Figure 4A) were significantly subsided by Cardiboost pre-treatment in H2O2 exposed cells. To further support Cardiboost’s antiapoptotic properties, the protein expression levels of apoptosis regulators, including Bax and Bcl-2, were also measured. Figure 4A illustrates a significant increase in Bcl-2 expression in H2O2 group along with a significant increase in BAX. The Bax/Bcl-2 ratio in the H2O2 group was significantly increased in the control group Figure 4D. However, treatment with Cardiboost significantly reduced the Bax/Bcl-2 ratio.
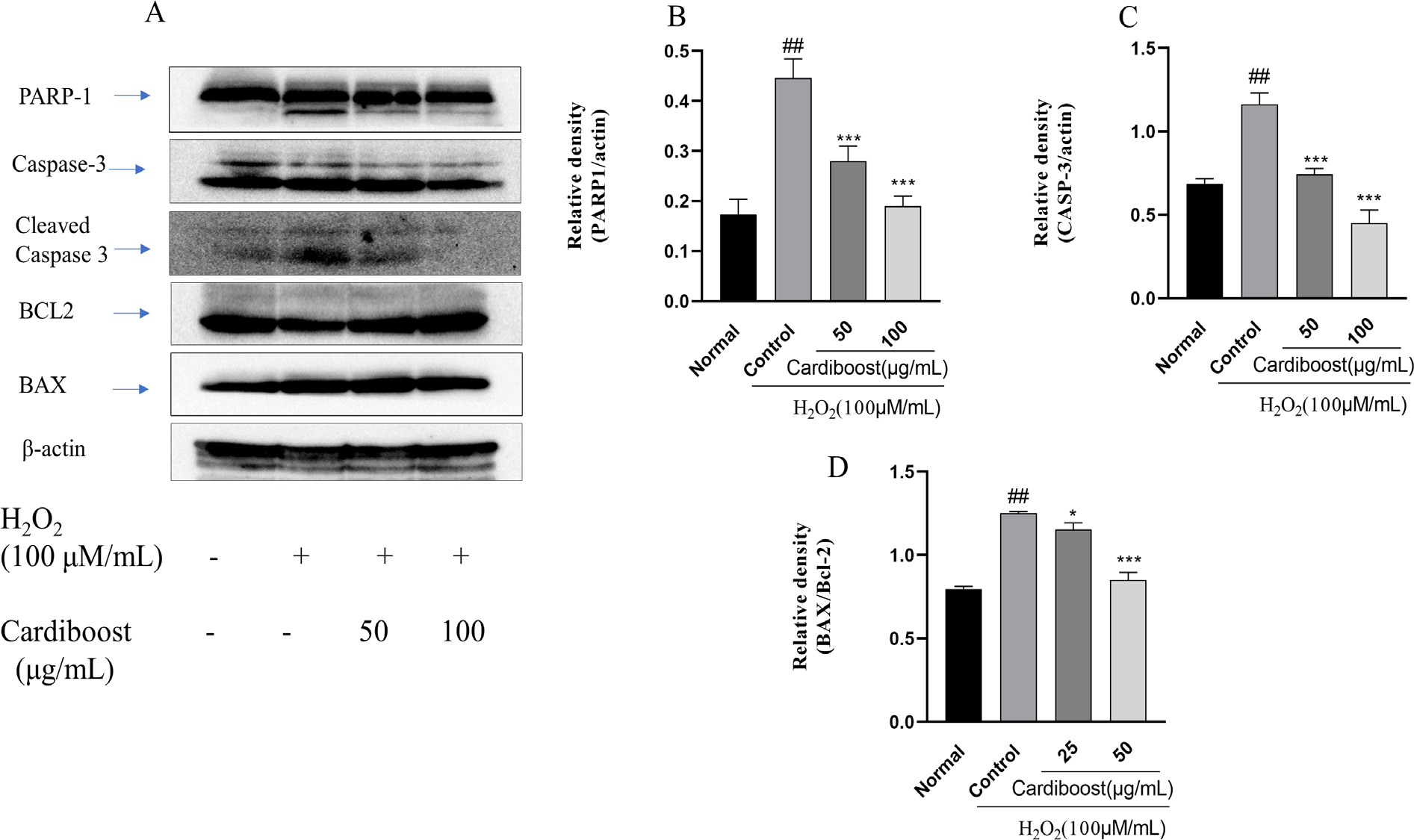
Figure 4:
Effect of Cardiboost against H2O2-induced apoptosis in H9c2 cardiomyocytes. Western blots showing the effect of Cardiboost on regulation of PARP-1, cleaved caspase-3, Bax/Bcl-2(A). Data are expressed as mean±SEM, n=3, significance was analyzed by a one-way ANOVA followed by a Tukey test. ##p<0.001 versus untreated normal cells. **p<0 01, and ***p<0 001 versus H2O2 alone-treated cells.
DISCUSSION
In traditional Indian medicine, Terminalia arjuna is one of the most widely utilised medicinal trees. Previous studies have showed the various medicinal properties of extract obtained from Terminalia arjuna bark.20 In this study, Cardiboost, a standardized bark extract of T. arjuna comprised of 1% Arjunolic acid was used to explore the cardiovascular property. In this study, initially we performed thrombin and ACE inhibition assay. Multifunctional serine proteinase thrombin is involved in several crucial biological functions such as platelet activation, fibrinogen to fibrin conversion, and the reversible coagulation amplification.21 In several clinical conditions, inhibiting thrombin activity has proven helpful, thrombin inhibitors are particularly useful in treating acute myocardial infarction and are mostly utilised as antithrombotic treatment (anticlotting). Recently, it has been demonstrated that extracts of Plumbago zeylanica and E. multiradiatus showed promising thrombin inhibition property.22,23 Similar findings from our investigation indicated that Cardiboost has a sizable thrombin inhibition activity this could be due to the synergic effect of Arjunolic acid and other bioactive components present in Cardiboost.
Angiotensin-converting enzyme inhibitors, or ACEIs, are the medications most frequently recommended for the treatment of cardiovascular and renal conditions, including heart failure, acute coronary syndrome, nephrotic syndrome, diabetes, and hypertension. In the present study Cardiboost exhibited significant ACE inhibition activity, there were several studies on other plants such as Punica granatum and Phaleria macrocarpa that showed significant ACE inhibition activity.24,25 Our findings are consistent with other published reports, it has been reported that Antioxidant medications and Angiotensin-Converting Enzyme (ACE) inhibitors inhibit the onset of oxidative stress and cardiovascular disease.26,27
In this work, we showed that Cardiboost considerably improved cell viability in the face of H2O2-induced cell death. To understand more about the mechanism behind Cardiboost’s protective properties, we also looked at the antioxidant enzyme activity in injured cells that were treated with the Cardiboost. The results show that Cardiboost reduces oxidative stress by increasing the expression of the oxidative stress genes SOD and CAT and decreasing the production of ROS. This is consistent with previous studies that demonstrate how plant extracts boost the body’s production of antioxidant enzymes like SOD and CAT, which improve the elimination of free radicals.28,29 These findings offer further evidence that treating cells with plant extracts increases the activity of antioxidant enzymes, which lowers oxidative stress in cells. Antioxidant effects of T. arjuna may be related to the presence of antioxidant polyphenol compounds, including Arjunolic acid.
Significant number of research have showed that the heart needs a large amount of energy and that mitochondria play a significant role in providing energy and permitting cardiac contractile function. Moreover, an excessive production of ROS due to oxidative stress can harm mitochondria and, in consequence, mitochondrial dysfunction can instigate apoptosis and is a predominant driver of cardiac diseases.30 Consequently, blocking mitochondrial dysfunction might be a therapeutic approach to stop oxidative stress-induced heart damage. Considering this, we aimed to clarify the underlying mechanism of Cardiboost’s protective properties against mitochondrial dysfunction following oxidative stress. Pre-treatment with Cardiboost, as predicted, inhibits apoptosis and preserves mitochondrial activities to protect H9c2 cardiomyoblast from oxidative stress. Our findings are consistent with previous research in that Cardiboost can suppress the expression of proapoptotic proteins (Bax) and increase the expression of antiapoptotic proteins (Bcl-2), both of which are important pathophysiological factors in cardiomyocyte apoptosis after H2O2 injury. Additionally, our study showed that Cardiboost reduced the activity of caspase-3, the primary apoptotic mechanism, caused by H2O2.31,32
CONCLUSION
The present investigation demonstrated that the Cardiboost, exhibited significant thrombin and ACE inhibition. The study also showed that Cardiboost protected H9c2 cardiomyocytes from H2O2 induced cytotoxicity, oxidative stress, and apoptosis. Possible underlying mechanisms include the maintenance of mitochondrial function and reduction of cardiomyocyte death. However, more research is required to determine the exact mechanisms by which Cardiboost exerts its cardioprotective effects. These results provide a scientific basis for its ethnomedical claim in the treatment of cardiovascular diseases.
Cite this article
Kuluvar G. Cardioprotective Potential of Cardiboost, a Standardized Extract from Terminalia arjuna Bark against H2O2 Induced Oxidative Stress in H9c2 Cardiomyocytes. J Young Pharm. 2024;16(1):10-6.
ACKNOWLEDGEMENT
We express our gratefulness to the Department of Phytochemistry and Analytical Development Laboratory at Vidya Herbs Pvt. Ltd., Bangalore, India, specifically to Mr. Chandrappa S., Dr. Deepak, and Dr. Vedamuthy B.M. We also thank the members of our lab for their intuitive recommendations.
ABBREVIATIONS
| CAT | Catalase |
|---|---|
| SOD | Superoxide dismutase |
| MTT | 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide |
| ACE | Angiotensin-converting enzyme |
| H2O2 | Hydrogen Peroxide |
| CVDs | Cardiovascular diseases |
References
- Jagannathan R, Patel SA, Ali MK, Narayan KMV. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Curr Diab Rep. 2019;19(7):44 [PubMed] | [CrossRef] | [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke Statistics-2018 update: A report from the American Heart Association. Circulation. 2018;137(12):e67-e492. [PubMed] | [CrossRef] | [Google Scholar]
- Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-14. [PubMed] | [CrossRef] | [Google Scholar]
- . Cardiovascular, respiratory, and related disorders. 2017 [PubMed] | [CrossRef] | [Google Scholar]
- . 2021:229-54. [PubMed] | [CrossRef] | [Google Scholar]
- Barreto da Silva L, Camargo SB, Moraes RDA, Medeiros CF, Jesus AM, Evangelista A, et al. Antihypertensive effect of carvacrol is improved after incorporation in β-cyclodextrin as a drug delivery system. Clin Exp Pharmacol Physiol. 2020;47(11):1798-807. [PubMed] | [CrossRef] | [Google Scholar]
- Hou N, Mai Y, Qiu X, Yuan W, Li Y, Luo C, et al. Carvacrol attenuates diabetic cardiomyopathy by modulating the PI3K/AKT/GLUT4 pathway in diabetic mice. Front Pharmacol. 2019;10:998 [PubMed] | [CrossRef] | [Google Scholar]
- Chander R, Singh K, Khanna AK, Kaul SM, Puri A, Saxena R, et al. Antidyslipidemic and antioxidant activities of different fractions of Terminalia arjuna stem bark. Indian J Clin Biochem. 2004;19(2):141-8. [PubMed] | [CrossRef] | [Google Scholar]
- Gupta R, Singhal S, Goyle A, Sharma VN. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree-bark powder: a randomised placebo-controlled trial. J Assoc Physicians India. 2001;49:231-5. [PubMed] | [Google Scholar]
- Raghavan B, Kumari SK. Effect of Terminalia arjuna stem bark on antioxidant status in liver and kidney of alloxan diabetic rats. Indian J Physiol Pharmacol. 2006;50(2):133-42. [PubMed] | [Google Scholar]
- Pettit GR, Hoard MS, Doubek DL, Schmidt JM, Pettit RK, Tackett LP, et al. Antineoplastic agents 338. The cancer cell growth inhibitory. Constituents of Terminalia arjuna (Combretaceae). J Ethnopharmacol. 1996;53(2):57-63. [PubMed] | [CrossRef] | [Google Scholar]
- Kumar V, Sharma N, Saini R, Mall S, Zengin G, Sourirajan A, et al. Therapeutic potential and industrial applications of Terminalia arjuna bark. J Ethnopharmacol. 2023;310:116352 [PubMed] | [CrossRef] | [Google Scholar]
- Verma SC, Jain CL, Padhi MM, Devalla RB. Microwave extraction and rapid isolation of arjunic acid from Terminalia arjuna (Roxb. ex DC.) stem bark and quantification of arjunic acid and arjunolic acid using HPLC-PDA technique. J Sep Sci. 2012;35(13):1627-33. [PubMed] | [CrossRef] | [Google Scholar]
- Row LR, Murti PS, Rao GS, Sastry CS, Rao KV. Chemical examination of Terminalia arjuna: Part XIII – Isolation and structure determination of Arjunetin from Terminalia arjuna. Indian J Chem. 1970;8:772-5. [PubMed] | [CrossRef] | [Google Scholar]
- Honda T, Murae T, Tsuyuki T, Takahashi T, Sawai M. Arjungenin, Arjunglucoside, I and Arjunglucoside II. A New Triterpene and New Triterpene Glucosides from Terminalia arjuna. Bull Chem Soc Jpn. 1976;49(11):3213-8. [CrossRef] | [Google Scholar]
- Tsuyuki T, Hamada Y, Honda T, Takahashi T, Matsushita K. A new triterpenes glucoside from Terminalia arjuna. Arjunglucoside III. Bull Chem Soc Jpn. 1979;52(10):3127-8. [CrossRef] | [Google Scholar]
- Batra S, Roy AK, Patra A, Bhaduri AP, Surin WR, Raghavan SA, et al. Baylis-Hillman reaction assisted parallel synthesis of 3,5-disubstituted isoxazoles and their in vivo bio evaluation as antithrombotic agents. Bioorg Med Chem. 2004;12(9):2059-77. [PubMed] | [CrossRef] | [Google Scholar]
- Jimsheena VK, Gowda LR. Colorimetric, high-throughput assay for screening angiotensin I-converting enzyme inhibitors. Anal Chem. 2009;81(22):9388-94. [PubMed] | [CrossRef] | [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55-63. [PubMed] | [CrossRef] | [Google Scholar]
- Anka Zaharaddeen, Singh Vijender, Singh Gunjan, Gimba Saleh. The pharmacological activities of various extracts from Terminalia arjuna Bark: A review. 2020 [CrossRef] | [Google Scholar]
- Fenton JW, Fasco MJ, Stackrow AB. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977;252(11):3587-98. [PubMed] | [CrossRef] | [Google Scholar]
- Sarvan KG, Narender M, Prasanth DSNBK, Anka RA. Evaluation of Thrombolytic and Antioxidant activity of leaf extracts of Plumbago zeylanica. Indian J Pharm Educ Res. 2022;56(4):1181-9. [PubMed] | [CrossRef] | [Google Scholar]
- Luz JRDD, Silva do Nascimento TE, Fernandes de Morais LV, Menezes da Cruz AK, Rezende AA, Neto JB, et al. Thrombin Inhibition: preliminary Assessment of the anticoagulant Potential of Turnera subulata (Passifloraceae). J Med Food. 2019;22(4):384-92. [PubMed] | [CrossRef] | [Google Scholar]
- Mayasankaravalli C, Deepika K, Esther Lydia D, Agada R, Thagriki D, Govindasamy C, et al. Profiling the phyto-constituents of Punica granatum fruits peel extract and accessing it’s in vitro antioxidant, anti-diabetic, anti-obesity, and angiotensin-converting enzyme inhibitory properties. Saudi J Biol Sci. 2020;27(12):3228-34. [PubMed] | [CrossRef] | [Google Scholar]
- Jahan N, Hussian F, Ayub AR, Ilyas M, Khan MA, Manzoor R, et al. Isolation and characterization of flavonoids from roots of Rauvolfia serpentina and evaluation of their hypotensive potential through angiotensin-converting enzyme (ACE) inhibition mode of action. Chemical Papers. 2022;76:1-11. [CrossRef] | [Google Scholar]
- Mikrut K, Kupsz J, Kozlik J, Krauss H, Pruszynska-Oszmałek E, Gibas-Dorna M, et al. Angiotensin-converting enzyme inhibitors reduce oxidative stress intensity in hyperglicemic conditions in rats independently from bradykinin receptor inhibitors. Croat Med J. 2016;57(4):371-80. [PubMed] | [CrossRef] | [Google Scholar]
- Tan M, Yin Y, Ma X, Zhang J, Pan W, Tan M, et al. Glutathione system enhancement for cardiac protection: pharmacological options against oxidative stress and ferroptosis. Cell Death Dis. 2023;14(2):131 [PubMed] | [CrossRef] | [Google Scholar]
- Zhao L, Cheng J, Liu D, Gong H, Bai D, Sun W, et al. Potentilla anserina polysaccharide alleviates cadmium-induced oxidative stress and apoptosis of H9c2 cells by regulating the MG53-mediated RISK pathway. Chin J Nat Med. 2023;21(4):279-91. [PubMed] | [CrossRef] | [Google Scholar]
- Shen Y, Shen Z, Li P, Chen Z, Wei B, Liu D, et al. Protective activity of Malus doumeri leaf extract on H2O2-induced oxidative injury in H9c2 rat cardiomyocytes. Front Cardiovasc Med. 2022;9:1005306 [PubMed] | [CrossRef] | [Google Scholar]
- Aon MA, Tocchetti CG, Bhatt N, Paolocci N, Cortassa S. Protective mechanisms of mitochondria and heart function in diabetes. Antioxid Redox Signal. 2015;22(17):1563-86. [PubMed] | [CrossRef] | [Google Scholar]
- Cao P, Xue Y, Guo M, Jiang X, Lei Z, Gao S, et al. The active ingredient (DSH-20) of Salvia miltiorrhiza flower reduces oxidative damage and apoptosis in cardiomyocytes by regulating miR-1. Mol Biol Rep. 2022;49(5):3675-84. [PubMed] | [CrossRef] | [Google Scholar]
- Yuvaraj S, Ajeeth AK, Puhari SSM, Abhishek A, Ramprasath T, Vasudevan V, et al. Chrysin protects cardiac H9c2 cells against H2O2-induced endoplasmic reticulum stress by up-regulating the Nrf2/PERK pathway. Mol Cell Biochem. 2023;478(3):539-53. [PubMed] | [CrossRef] | [Google Scholar]
