ABSTRACT
Background
Amid the pressing global concern of Antimicrobial Resistance (AMR), where Antimicrobial Stewardship (AMS) stands as a promising solution in relation to this, critical priority has been assigned to AMR pathogens in the Indian Pathogen Priority List to steer research focused on antibiotic-resistant bacteria. The study’s particular objective is to assess how an AMS intervention affects these pathogens in adult patients.
Materials and Methods
Over a dedicated two-year period from January 2021 to November 2022 research focused on adult patients harboring critical priority pathogens, adhering to ICMR directives. The primary goal was to comprehend antimicrobial drug usage in hospital’s medicine and surgery unit. Employing a qualitative approach, study conducted a Prospective Audit with Feedback (PAF), implementing deliberate constraints on antimicrobial drug usage to gain insights.
Results
The analysis encompassed 314 participants: 96 in control phase, and 115 and 103 in the intervention phases 2 and 3. Comparable demographics and service scope existed between intervention and control groups. All arms exhibited the presence of culture-positive organisms from the critical priority pathogen list defined by ICMR. Impressively, length of therapy per 1000 patient days notably dropped from 908.50 to 758.33 (p=0.001) post-intervention.
Conclusion
The study’s conclusion highlights responsible antimicrobial use in a tertiary care setting, showcasing promising progress. Noteworthy impacts on the dependent variable (Log_LOT) emerged across study phases, emphasizing intervention significance. Statistically significant Ward and Phase variables further enriched the overall insights.
INTRODUCTION
In the relentless battle against Antimicrobial Resistance (AMR), the critical role of antimicrobial stewardship comes into focus, fortifying the global fight for effective solutions.1 Acknowledged widely as a vital tool, its effectiveness in managing antimicrobial resistance has garnered considerable attention.2 However, the existing evidence supporting intervention studies largely originates from Developed Nations.3 To facilitate widespread antimicrobial stewardship programs, developing countries must take proactive steps by either initiating or strengthening existing wits in this critical domain.4,5 While intervention studies have demonstrated significant improvements in antimicrobial use, it remains imperative to expand this evidence base to include Developing Nations as well.6,7 By taking a comprehensive approach, we can effectively tackle the global challenge of antimicrobial resistance.
The Leading Role of ICMR Guidelines in India to regularly updating and refining the guidelines based on emerging research and clinical data, they also ensure that healthcare professionals remain updated with the recent evident advancements in disease management.8,9 Despite persistent challenges and limited resources in healthcare settings, the remarkable adaptability of antimicrobial stewardship in such environments has proven to be highly effective. This adaptability has led to tangible and positive outcomes, notably a substantial reduction in antimicrobial consumption. In a proactive stance, countries boldly address challenges and craft innovative strategies, advancing global antimicrobial stewardship initiatives toward a promising future.10 India, in collaboration with WHO, established the Indian Priority Pathogen List (IPPL) in 2019 to prioritize pathogens with a high AMR risk and outbreak potential.11
The concept of AMS not only applies to human healthcare but also extends to include the responsible use of these drugs in the animal and agronomy, underscoring the significance of judicious and responsible usage of this agent.10 Healthcare practitioner’s stand at the frontline, defending against antimicrobial resistance. Equipped with responsible prescribing practices and patient education, they play a pivotal role in safeguarding current and future patients from the dangers of declining antimicrobial effectiveness. United as essential stewards, healthcare practitioners confront this emerging health and economic challenge, paving the way for a safer tomorrow. AMS interventions aim to cultivate lasting behavioral changes in antibiotic prescriptions, ensuring the optimal use of antimicrobials and preserving effective treatment options for infectious agents.12
Infection control measures include promoting hand hygiene, patient isolation, implementing contact restriction and precautions, maintaining environmental cleanliness, conducting active prospective surveillance, and employing antibiotic stewardship programs.13 In the AI era, individualized care is on the rise, and this personalized approach is crucial for effective antimicrobial stewardship and combating antimicrobial resistance. Harnessing AI and genomics allow us to optimize treatments and preserve the efficacy of antibiotics.14 Significant advancements in this field can be expected through the effective integration of bacterial genomics, biochemistry, bioinformatics, and physiology.13
The central objective of this study is to emphasize responsible antimicrobial practices, focusing on crucial metrics like Length of Treatment per 1000 patient days.15 Our goal is to ensure the judicious use of this invaluable medical resource for patients with positive cultures of critical priority pathogens. To achieve this objective, we address pivotal issues that influence our targeted interventions. These include prospective audits and feedback, utilization restrictions, and comprehensive training in alignment with ICMR and AMS guidelines. By evaluating Length of Treatment and conducting a thorough segmentation analysis within Medicine and Surgical units.
MATERIALS AND METHODS
Study Design
This study employed an antimicrobial stewardship intervention to comprehensively examine the patients admitted to medicine and surgery wards, guided by specific selection criteria. The study was conducted over the period from January 2021 to November 2022 and received ethical approval from the Institutional Ethical Committee at the research site, MM Institute of Medical Sciences and Research, Mullana, under registration number 1861. The researcher conducting the study had received training and possessed prior experience in the field of Antimicrobial Stewardship.
Inclusion and Exclusion criteria
The selection criteria focused on all gender adult or elderly subject in medicine and surgery ward of study site with positive cultures of critical priority pathogens as defined in the Indian Pathogen Priority List, encompassing Enterobacteriaceae (Klebsiella pneumoniae and Escherichia coli), Acinetobacter baumannii, and Pseudomonas aeruginosa.11,16 Exclusion Criteria of this study is patients not meeting inclusion criteria, including pediatric patients, those admitted outside medicine and surgery wards without a diagnosed condition necessitating antimicrobial treatment, and individuals with communication barriers.
Experimental Plan
The study was structured into three distinct phases. The first phase consisted of a control group, while the second phase involved the Prospective Audit and Feedback group. During the third phase, we implemented an expanded policy intervention that included the introduction of preauthorization for the prescription of certain reserved drugs. This approach was adopted due to the challenges in enforcing abrupt restrictions, especially for specific reserved and several watch group drugs defined by WHO’s AWaRe category.17 Additionally, comprehensive training was provided with hands-on guidance from experts in this phase, while the Prospective Audit and Feedback process continued seamlessly. To improve compliance and integration of this intervention into daily practice, it was initially introduced in phase 2, and subsequently, the restriction of antimicrobial usage was added in phase 3. This approach allowed for a comprehensive evaluation within the hospital environment, emphasizing the effective implementation of drug restrictions.17,18
Utilized antibiotics Categorization
The WHO AWaRe categorization classifies antibiotics into three groups-Access, Watch, and Reserve-based on their importance in human health, in an effort to promote rational and responsible antibiotic use.19 To gather data, trained researchers in AMS adhered to a methodical process. This encompassed a comprehensive examination of existing information, engaging in profound discussions and feedback sessions with experts, and utilizing validated forms to collect data during individual interactions with selected subjects. The composed data was investigated using a deductive thematic analysis framework, incorporating various standards like WHO, Infectious Diseases Society of America (IDSA), and ICMR.20
Intervention Implementation
The critical execution of the study entailed a thorough prevalence survey, centering on the examination of antimicrobial utilization and protocols. A clinical pharmacist, who also held the role of a researcher, assumed a significant role in implementing an intervention that encompassed prospective auditing and feedback, in addition to antibiotic usage restrictions, all under the strong leadership of the hospital management.21 This intervention aimed to assess the appropriateness of prescriptions, considering factors like dosage, therapeutic duplication, double anaerobic coverage, and prescription rationality.22
The resulting findings, feedback, and implications had the potential to provide valuable guidance among healthcare providers, nursing, pharmacist and patients.23 Considerate these factors is crucial in promoting precise antibiotic use and effectively countering the challenge of AMR in an Indian hospital.17
Process Analysis
The analysis involved utilizing AMS metrics-Length of Treatment per 1000 patients’ days with antimicrobial drug utilization. This approach facilitated a systematic and comprehensive examination of the data collected from the study site.20 Further, this has gained insights into the specific infection challenges within the hospital environment. This entailed exploring various aspects, such as critical pathogen infected patient demographics, resource accessed, and healthcare practices.21
Statistical Analysis
The length of therapy is standardized as an AMS Matrix in each phase, calculated as LOT per 1000 patient days.24 The statistical analysis was conducted at a significance level of 95% using ANOVA with Log_LOT as the dependent variable. The analysis included factors such as phase, ward, and method (SStype), with POSTHOC analysis applied to the Phase-Wards interaction. The data was determined to be homogenous following the Levene test. Descriptive parameters for homogeneity were assessed with a criteria alpha of 0.05, and the design included phase, ward, and intercept as factors.
RESULTS
The results of the study indicate a significant reduction in the prevalence of the targeted pathogens following the implementation of the Antimicrobial Stewardship (AMS) intervention in the adult patient population. The data reveals a noteworthy decrease in the Length of Treatment (LOT) per 1000 patient days, suggesting a positive impact on the management of these pathogens.
Furthermore, a sub-analysis of specific pathogen types demonstrates varying response to the AMS intervention. Participants were recruited based on inclusion criteria, specifically targeting positive culture pathogens aligned with the Critical Pathogen Priority outlined in the Indian Pathogen Priority List, as depicted in Figure 1.

Figure 1:
Flowchart of Research and Subject Enrollment with Outcome Measures.
Segregation of subjects based on according to Indian Pathogen Priority List (IPPL) across different phases, aimed at assessing Length of Therapy (LOT) as antimicrobial stewardship matrices at the study site of the tertiary care hospital. Sensitivity pattern of Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa observed increasing in different phases. Additionally, the data provides LOT per 1000 Patient Days (PD) for each phase, allowing for comparisons across different phases with varying numbers of patient days.
The study had also identified differences in the length of antimicrobial therapy per 1000 Patient Days. The study found that the length of antimicrobial therapy varied across different phases. However, when normalized for patient days, LOT per 1000 PD was highest in Phase 1, followed by Phase 2, and then Phase 3. These results offer valuable insights of different wards at study site for assessing antimicrobial stewardship practices in the hospital and enhancing patient care.
In the data provided in Figure 2, the “Reserve drugs” have the lowest percentage in each phase. During Phase 1, they account for 11.15% of the total. In Phase 2, their percentage decreases to 8.08%, and in Phase 3, it further declines to 7.05%. This decline in utilization of “Reserve drugs” across the phases suggests that they are sparingly used and considered as a last resort or reserved for specific situations. Therefore, in the context of the AWaRe classification, “Reserve drugs” play a critical role in maintaining the effectiveness of antimicrobial treatment by preserving their use for situations where they are most needed, contributing to the overall strategy for responsible antimicrobial stewardship.

Figure 2:
Distribution of AWaRe drugs across different phases.
The provided Table 1 presents descriptive statistics for the dependent variable “Log_LOT” across different phases and wards. In Phase 1, the mean Log_LOT values for the Medicine ward and Surgery ward were 0.8202 and 0.9658, respectively, with corresponding standard deviations (SD) of 0.24967 and 0.28093. The total mean Log_LOT for Phase 1, considering both wards, was 0.8566, with an SD of 0.26403 and a total sample size (N) of 96. Moving to Phase 2, the mean Log_LOT values for the Medicine ward and Surgery ward were 0.8878 and 0.7584, respectively, with SD of 0.19039 and 0.20804. The total mean Log_LOT for Phase 2 was 0.8439, with an SD of 0.20509 and a total sample size (N) of 115.
| Descriptive Statistics | ||||
|---|---|---|---|---|
| Dependent Variable: | Log_LOT | |||
| Phase | Ward | Mean | Std. Deviation | N |
| Phase 1 | Medicine ward | 0.8202 | 0.24967 | 72 |
| Surgical ward | 0.9658 | 0.28093 | 24 | |
| Total | 0.8566 | 0.26403 | 96 | |
| Phase 2 | Medicine ward | 0.8878 | 0.19039 | 76 |
| Surgical ward | 0.7584 | 0.20804 | 39 | |
| Total | 0.8439 | 0.20509 | 115 | |
| Phase 3 | Medicine ward | 0.8040 | 0.17037 | 72 |
| Surgical ward | 0.6400 | 0.11379 | 31 | |
| Total | 0.7547 | 0.17242 | 103 | |
| Total | Medicine ward | 0.8383 | 0.20827 | 220 |
| Surgical ward | 0.7723 | 0.23866 | 94 | |
| Total | 0.8185 | 0.21952 | 314 | |
Finally, in Phase 3, the mean Log_LOT values for the medicine ward and surgery ward were 0.8040 and 0.6400, respectively, with SD of 0.17037 and 0.11379. The total mean Log_LOT for Phase 3 was 0.7547, with an SD of 0.17242 and a total subject sample size (N) of 103.
Further post-hoc tests (Table 2) were conducted to compare mean LOT per 1000 PD for each phase pairwise. The results showed that Phase 1 had the highest mean LOT (972.830), followed by Phase 2 (888.230), and Phase 3 (878.510). These findings further support the rejection of the null hypothesis, confirming the significance between the data and the differences among the three phases.
| (I) Phase | (J) Phase | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Phase 1 | Phase 2 | 0.0127 | 0.02956 | 0.668 | -0.0455 | 0.0709 |
| Phase 3 | .1020* | 0.03033 | 0.001 | 0.0423 | 0.1616 | |
| Phase 2 | Phase 1 | -0.0127 | 0.02956 | 0.668 | -0.0709 | 0.0455 |
| Phase 3 | .0893* | 0.02901 | 0.002 | 0.0322 | 0.1463 | |
| Phase 3 | Phase 1 | -.1020* | 0.03033 | 0.001 | -0.1616 | -0.0423 |
| Phase 2 | -.0893* | 0.02901 | 0.002 | -0.1463 | -0.0322 | |
These statistics offer valuable insights into the variations in Log_LOT across different phases and wards, providing a basis for further analysis and interpretation in this study as represented in Table 3.
| Dependent Variable: | Log_LOT | ||||
|---|---|---|---|---|---|
| Source | Type III Sum of Squares | Df | Mean Square | F | Sig. |
| Corrected Model | .912a | 3 | 0.304 | 6.651 | 0.000 |
| Intercept | 169.094 | 1 | 169.094 | 3699.027 | 0.000 |
| Phase | 0.626 | 2 | 0.313 | 6.842 | 0.001 |
| Ward | 0.278 | 1 | 0.278 | 6.091 | 0.014 |
| Error | 14.171 | 310 | 0.046 | ||
| Total | 225.469 | 314 | |||
| Corrected Total | 15.083 | 313 |
The resulting p-value was smaller than the chosen significance level, leading to rejection of H0. This indicates a significant difference in mean LOT per 1000 PD among the three phases.
The descriptive statistics provided valuable insights into the central tendency and dispersion of “Log_LOT” variable across different phases and wards as illustrated in Figure 3. The mean Log_LOT value across all phases is 0.8383 for the Medicine ward and 0.7723 for the Surgery ward, with total sample sizes of 220 and 94, respectively. Overall, the mean Log_LOT value across all phases and wards is 0.8185, with a SD of 0.21952 and a total sample size of 314.
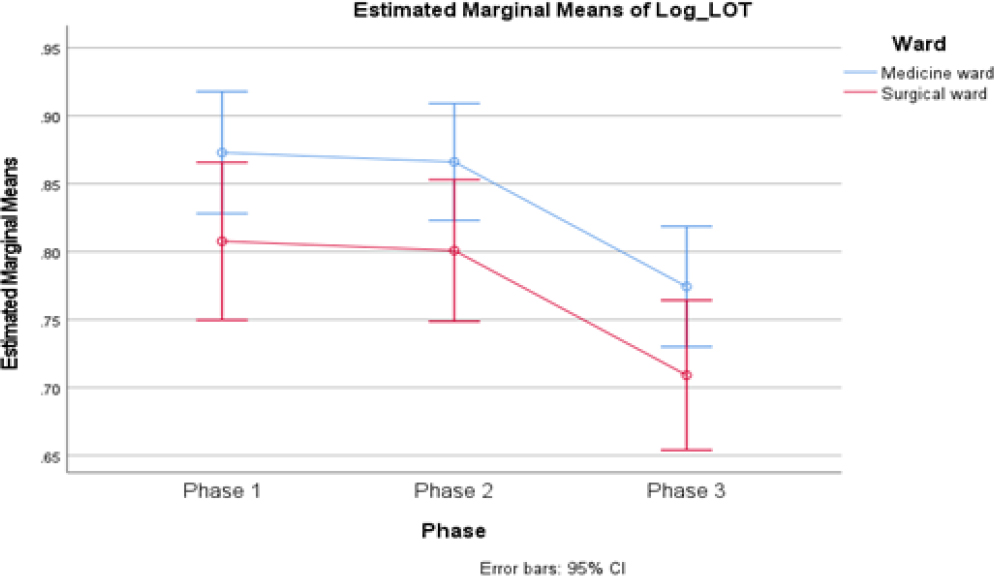
Figure 3:
Estimated marginal means of Log Length of therapy.
Conclusively, the Ward variable shows a significant effect on Log_LOT, explaining 1.9% of, with a Type III Sum of Squares of 0.278 and a significant F-statistic of 6.091 (p=0.014) and 1 degree of freedom.
Analysis indicted in Figure 4 shows that both the Phase and Ward variables have a significant impact on Log_LOT, providing valuable insights into the factors influencing treatment duration. The Intercept accounts for the variation in the dependent variable at alpha=0.05. The AMS Matrix offers extensive potential for assessing the utilization of antimicrobial drugs and monitoring antimicrobial resistance.

Figure 4:
Significance representation of LOT in different phases.
DISCUSSION
Interpreting the results, it is evident that the AMS intervention has played a crucial role in addressing the challenge of Antimicrobial Resistance (AMR) posed by these pathogens. The observed reduction in LOT per 1000 patient days indicates a more efficient and targeted use of antibiotics, contributing to the overall goal of AMS.
The differential responses among specific pathogens underscore the importance of tailoring interventions to the unique characteristics of each pathogen. Indeed, the implementation of Antimicrobial Stewardship (AMS) programs in medical institutions proved to be a formidable task, filled with challenges and hurdles during our study. In the initial phase, we encountered numerous obstacles that made the process particularly arduous. Securing agreement from consultants, a pivotal step in the success of AMS initiative emerged as a primary challenge. Furthermore, the act of defining and addressing the terminology within the core element of the Antimicrobial Stewardship (AMS) intervention, referred to as “Prospective Audit,” posed substantial challenges during implementation. Convincing stakeholders to support antibiotic restriction policies demanded persistent effort, ongoing discussions, brainstorming sessions, and expert consultations. Fortunately, COVID-19 pandemic and its associated opportunistic infections have garnered significant interest in the fight against pathogens and AMR. The journey to draft and implement these policies stood as a testament to our team’s dedication and determination.25,26
This study examines the effectiveness of an Antimicrobial Stewardship (AMS) strategy in three phases: Phase 1 is the Control group, and Phases 2 and 3 are the intervention group. Phase 2 focuses on the Prospective Audit and Feedback approach to assess antimicrobial prescriptions and improve their quality. Phase 3 adds antimicrobial restrictions and expert-led training for stakeholders to enhance AMS execution. Throughout the intervention, assessment relies on three metrics: demographic evaluation, and AWaRe Categorization and AMS Matrix-LOT. These assessments collectively gauge the intervention’s impact on antimicrobial utilization in the Medicine and Surgery Units.27,28
Demographic analysis discerned patient profile variations across intervention phases, contextualizing subsequent antimicrobial utilization trends and implications. The AMS Matrix provided an overall view of antimicrobial usage patterns and its key impact on Length of treatment into strategy efficacy.29
While the results of our study demonstrated a modest reduction in Length of Treatment (LOT) per 1000 patient days, it was imperative to acknowledge the structural limitations of our tertiary care hospital. These limitations encompassed the absence of electronic medical record systems, limited pharmacy facilities for all admitted patients, and the participation of patients’ family members in delivering medical care and procuring medical supplies from the pharmacy, leading to occasional lapses in adherence to strict infection control practices. Furthermore, a significant proportion of our patients had been transferred from other hospitals, potentially complicating our infection control measures.
Critics could argue that the reduction in LOT might lead to an increase in the use of broad-spectrum antibiotics. Nevertheless, a more thorough analysis of particular antibiotics, including Linezolid, Colistin, Imepenem+Cilastatin, Faropenem, piperacillin+tazobactam, and carbapenem, indicated a reduction in their utilization. This is evident in the decrease in Defined Daily Doses (DDD) per 1000 patient days across various segments, aligned with AWaRE categorization. Notably, the increased use of cefoperazone+sulbactam as a first-line empirical agent for infections during the intervention phase suggested that our efforts became more adept at differentiating between Community Acquired Infections (CAI) and Hospital Acquired Infections (HAI), resulting in more appropriate treatment choices. The categorization of subjects according to the Indian Pathogen Priority List (IPPL) during various phases notably, we observed an escalating sensitivity pattern in Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa across different phases of the study.11
Our multifaceted approach to antimicrobial stewardship showed promise in optimizing antimicrobial agent use during the study. However, it was crucial to recognize that ongoing efforts were essential to address structural limitations and the emerging challenge of multidrug-resistant infections. Our study emphasized the significance of perseverance, collaboration, and adaptability in achieving improved healthcare outcomes. These factors, in turn, contributed to enhanced patient care and played a vital role in addressing the global challenge of antimicrobial resistance. While our findings indicate a positive impact, further research is warranted to delve deeper into this crucial area.
CONCLUSION
Study offers significant insights into the variations of the “Log_ LOT” variable across different phases and wards. Significantly, the p-values of 0.001 and 0.014 emphasize the statistical significance of our findings in relation to both the phases and wards. The distinct average Log_LOT values of 0.8383 and 0.7723 for the Medicine and Surgery wards, respectively, reinforce the impact of the ward context on Log_LOT patterns. In our study, we emphasized the significance of persistence, collaboration, and adaptability in achieving improved healthcare outcomes. As a result, these factors raised the bar for patient care standards and assumed a pivotal role in tackling the worldwide issue of antimicrobial resistance through intervention in antimicrobial stewardship.
Our findings also underscored the importance of tailoring antimicrobial stewardship strategies to specific hospital wards, a practice that promotes more effective management as well as its application to critical priority pathogens in accordance with the IPPL. Considering these implications, we strongly suggest conducting further research in this field to further enhance and expand our comprehension of AMS interventions. Extending this practice to the other categorized priority pathogens and enhancing the dedicated involvement of clinical pharmacists according to the AMS guidelines will contribute to shaping a future marked by more effective antimicrobial usage and fortified healthcare practices.
Cite this article
Singh T, Gupta S, Malik A, Mothsara C, Pandey A, Kaur N, et al. Effectively Combating Antimicrobial Resistance: Assessing the Clinical Outcomes of Antimicrobial Stewardship Intervention in Patients with Critical Priority Pathogens. J Young Pharm. 2024;16(1):50-7.
ACKNOWLEDGEMENT
We gratefully acknowledge the invaluable support and guidance of the Department of Pharmacology, PGIMER, Chandigarh, which was instrumental in shaping this research. Additionally, we extend heartfelt appreciation to the Institutional Ethical Committee at MM Institute of Medical Science and Research, Mullana, for granting ethical clearance, ensuring adherence to the highest ethical standards. Finally, we thank all the participants and individuals whose cooperation made this research possible and meaningful.
References
- Ismaeil R, Fata Nahas AR, Kamarudin NB, Mat Nor MB, Abubakar U, Mohamed MHN, et al. Healthcare-associated infections: A ten-year bibliometric analysis. J Young Pharm. 2023;15(3):397-405. [CrossRef] | [Google Scholar]
- Spellberg B, Blaser M, Guidos RJ. Infectious Diseases Society of America (IDSA); Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(Suppl 5):S397-428. [CrossRef] | [Google Scholar]
- Leuthner KD, Doern GV. Antimicrobial stewardship programs. J Clin Microbiol. 2013;51(12):3916-20. [PubMed] | [CrossRef] | [Google Scholar]
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13(12):1057-1098. [published correction appears in Lancet Infect Dis. 2014; 14(1): 11-10.1016/S1473-3099(13)70322-0] [published correction appears in Lancet Infect Dis, 2014; 14(3), 182, doi: 10.1016/S1473-3099(13 )70344-X, PMID 24571968 [PubMed] | [CrossRef] | [Google Scholar]
- Howard P, Pulcini C, Levy Hara G, West RM, Gould IM, Harbarth S, et al. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother. 2015;70(4):1245-55. [PubMed] | [CrossRef] | [Google Scholar]
- Sarkar S, Srivastava V, Samajhdar SS, Pattanayak C, Tripathi S. Rational use of antibiotics: an area of concern. J Young Pharm. 2022;14(2):165-8. [CrossRef] | [Google Scholar]
- Brouwers MC, Florez ID, McNair SA, Vella ET, Yao X. Clinical practice guidelines: tools to support high quality Patient Care. Semin Nucl Med. 2019;49(2):145-52. [PubMed] | [CrossRef] | [Google Scholar]
- Behera SK, Das S, Xavier AS, Selvarajan S, Anandabaskar N. Indian Council of Medical Research’s National Ethical Guidelines for biomedical and health research involving human participants: the way forward from 2006 to 2017. Perspect Clin Res. 2019;10(3):108-14. [PubMed] | [CrossRef] | [Google Scholar]
- Mathur R, Swaminathan S. National ethical guidelines for biomedical and health research involving human participants, 2017: A commentary. Indian J Med Res. 2018;148(3):279-83. [PubMed] | [CrossRef] | [Google Scholar]
- Indian Priority Pathogen List: to guide research, discovery, and development of new antibiotics in India: Developed by WHO Country Office for India in collaboration with Department of Biotechnology, Government of India site; Published. 2019 [accessed on 2020]. Available fromhttps://dbtindia.gov.in/sites/default/files/IPPL_final.pdf
- Villanueva-Cabezas JP. One health: A brief appraisal of the Tripartite – UNEP definition. Transbound Emerg Dis. 2022;69(4):1663-5. [PubMed] | [CrossRef] | [Google Scholar]
- Bennett N, Schulz L, Boyd S, Newland JG. Understanding inpatient antimicrobial stewardship metrics. Am J Health Syst Pharm. 2018;75(4):230-8. [PubMed] | [CrossRef] | [Google Scholar]
- Walia K, Ohri VC, Mathai D. Antimicrobial stewardship programme (AMSP) practices in India. Indian J Med Res. 2015;142(2):130-8. [PubMed] | [CrossRef] | [Google Scholar]
- McKay F, Williams BJ, Prestwich G, Bansal D, Treanor D, Hallowell N, et al. Artificial intelligence and medical research databases: ethical review by data access committees. BMC Med Ethics. 2023;24(1):49 [PubMed] | [CrossRef] | [Google Scholar]
- Shafiq N, Gautam V, Pandey AK, Kaur N, Garg S, Negi H, et al. A meta-analysis to assess usefulness of procalcitonin-guided antibiotic usage for decision making. Indian J Med Res. 2017;146(5):576-84. [PubMed] | [CrossRef] | [Google Scholar]
- Panditrao A, Shafiq N, Kumar-M P, Sekhon AK, Biswal M, Singh G, et al. Impact of an antimicrobial stewardship and monitoring of infection control bundle in a surgical intensive care unit of a tertiary-care hospital in India. J Glob Antimicrob Resist. 2021;24:260-5. [PubMed] | [CrossRef] | [Google Scholar]
- Nampoothiri V, Sudhir AS, Joseph MV, Mohamed Z, Menon V, Charani E, et al. Mapping the implementation of a clinical pharmacist-driven antimicrobial stewardship programme at a tertiary Care Centre in South India. Antibiotics (Basel). 2021;10(2):220 [PubMed] | [CrossRef] | [Google Scholar]
- Broom A, Doron A. Antimicrobial resistance, politics, and practice in India. Qual Health Res. 2020;30(11):1684-96. [PubMed] | [CrossRef] | [Google Scholar]
- Dikkatwar MS, Vaghasiya J. Antibiogram and Antimicrobial Stewardship Program: Fighting global antimicrobial resistance and rationalizing the antibiotic treatment. J Young Pharm. 2023;15(1):41-8. [CrossRef] | [Google Scholar]
- Walia K, Ohri VC, Madhumathi J, Ramasubramanian V. Policy document on antimicrobial stewardship practices in India. Indian J Med Res. 2019;149(2):180-4. [PubMed] | [CrossRef] | [Google Scholar]
- Morris AM. Antimicrobial stewardship programs: appropriate measures and metrics to study their impact. Curr Treat Options Infect Dis. 2014;6(2):101-12. [PubMed] | [CrossRef] | [Google Scholar]
- Pauwels I, Versporten A, Vermeulen H, Vlieghe E, Goossens H. Assessing the impact of the Global Point Prevalence Survey of antimicrobial Consumption and Resistance (Global-PPS) on hospital antimicrobial stewardship programmes: results of a worldwide survey. Antimicrob Resist Infect Control. 2021;10(1):138 [PubMed] | [CrossRef] | [Google Scholar]
- Saha SK, Thursky K, Kong DCM, Mazza D. A novel GPPAS model: guiding the implementation of antimicrobial stewardship in primary care utilising collaboration between general practitioners and community pharmacists. Antibiotics (Basel). 2022;11(9):1158 [PubMed] | [CrossRef] | [Google Scholar]
- Shafiq N, Praveen Kumar M, Gautam V, Negi H, Roat R, Malhotra S, et al. Antibiotic stewardship in a tertiary care hospital of a developing country: establishment of a system and its application in a unit-GASP Initiative. Infection. 2016;44(5):651-9. [PubMed] | [CrossRef] | [Google Scholar]
- Ranjalkar J, Chandy SJ. India’s National Action Plan for antimicrobial resistance – an overview of the context, status, and way ahead. J Fam Med Prim Care. 2019;8(6):1828-34. [PubMed] | [CrossRef] | [Google Scholar]
- Kakkar AK, Shafiq N, Sahni N, Mohindra R, Kaur N, Gamad N, et al. Assessment of appropriateness of Antimicrobial Therapy in resource-constrained settings: development and piloting of a novel tool-AmRAT. Antibiotics (Basel). 2021;10(2):200 [PubMed] | [CrossRef] | [Google Scholar]
- Yoon YK, Kwon KT, Jeong SJ, Moon C, Kim B, Kiem S, et al. Guidelines on implementing antimicrobial stewardship programs in Korea. Infect Chemother. 2021;53(3):617-59. [PubMed] | [CrossRef] | [Google Scholar]
- Pauwels I, Versporten A, Drapier N, Vlieghe E, Goossens H, Global-PPS network, et al. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): results from a worldwide point prevalence survey in 69 countries. J Antimicrob Chemother. 2021;76(6):1614-24. [PubMed] | [CrossRef] | [Google Scholar]
