ABSTRACT
Background
Albendazole is a bitter drug and a common antihelmintic drug with a wide range of effects due to its first-pass metabolism and limited bioavailability. It is available in oral dosage form to treat various intestinal illnesses. The goal of this study is to develop a new oral dosage form, a gelatin-based chewable, for pediatrics and devise a dosage form that is easier to take, tastes better, and does not smell strange.
Materials and Methods
Gelatin (natural polymer used as gelling agent), sucrose and sorbitol, albendazole (active ingredient), citric acid and sodium benzoate, flavorant, and colorant were used. The study involves the formation of two separate solutions according to standard protocols. The first solution contained sorbitol, sucrose, and water 1:1, with a water-to-sorbitol ratio of 2:1. In order to make the second solution, water and gelatin were mixed and heated at 60°C. Both solutions were mixed, followed by the addition of other excipients.
Results
Preformulation studies involved bulk characterization and solubility analysis. Solubility analysis (pKa determination and partition coefficient) was carried out. Post-formulation studies were carried out to characterize the formulation, including in vitro disintegration and dissolution. A release kinetics study of the formulation revealed that these gummies followed first-order kinetics because it is an immediate-release formulation.
Conclusion
The developed formulation of albendazole gummies was found to be similar to other formulations of albendazole available in the market in terms of physiochemical parameters and therefore can be produced as a generic formulation by an interested local pharmaceutical industry in the country.
INTRODUCTION
The burgeoning domain of clinical pharmaceutics has compelled formulation development to concentrate on patient demands. This is unquestionably pertinent to pediatrics, as evolving regulatory reforms have increased the focus on making it easier to authorize and gain access to high-quality, secure children’s pharmaceuticals globally. A major problem is the creation of age-appropriate dosage form for the diverse pediatric population. Licensed pediatric dosage form are still not as readily distributed as those for adult patients, despite the growing realization of the need for age-appropriate dosages. Children are referred to as “therapeutic orphans” because the majority of research is typically conducted on diseases that affect adults. The phrase indicates the necessity of paediatric research and product development. In order to have a better effect and greater safety, children have the right to use medications that are appropriate for their age.1,2
Oral medications can be used for localized therapy by targeting particular GI regions.3 Children require different oral dosage forms than adults due to differences in swallowing issues, taste preferences, and dosage requirements. Typically, drugs are manufactured in capsules and pills for adults, which are inappropriate to be used frequently in children.4
Albendazole, also known as methylcarbamate, is a broad-spectrum anthelmintic medicine. When administered in recommended quantities for 1-3 days, albendazole is typically considered safe with no major side effects.5,6
Albendazole functions by interfering with the cytoskeletal system of parasite cells, preventing glucose uptake and transport and, eventually, cell death.7 Albendazole is available in tablet form, which dissolves swiftly and provides an instant mechanism of action.8 The majority of these efforts have focused on making drugs more accessible while maintaining quality of life. Albendazole is quite bitter in taste and has reduced absorption.9,10 The aim of this study is to develop albendazole in a dosage form with improved patient compliance, improved taste, convenience of administration, and greater palatability.
A novel dosage form, a gelatin-based chew, is being developed to make it easier for paediatrics to consume. This dosage form is easier to swallow, tastes better, and has no off-putting odor. The dosage form is designed with the perception of improving adherence of children in mind, and its structural qualities make it simple to handle and consume. The enhancement of flavour, fragrance, and texture can stimulate salivation, making swallowing easier. Furthermore, gummies are an appealing dosage form for people of all ages, not just children.5
MATERIALS AND METHODS
Materials
Different substances have been utilized in this formulation. As sweeteners, sucrose and sorbitol are utilized. Gelatin is a gelling agent, colourant, and flavouring agent that can be purchased from the local market. This preservation and flavouring ingredient, acquired from Merck Pharma, is known as citric acid. Albendazole from Sigma Aldrich is the main ingredient in antihelmintic formulations. Water that has been distilled in a laboratory is the vehicle. All of the ingredients purchased are of pharmaceutical grade and should be used as they were purchased.
Preformulation Studies
The powder mixture was analyzed prior to the gummy synthesis. The quality of the powder that produces high-grade gummies was assessed using a variety of parameters, such as hygroscopicity, bulk density, tapped density, Carr’s index, Hausner’s ratio, and angle of repose.
Hygroscopicity
The amount of water present in the material was determined using the quantitative reaction of water with iodine and sulphur dioxide in the presence of a low molecular weight alcohol and an organic base.11
Bulk Characterization
To understanding the micrometric characteristics of powder blends; bulk density, tapped densities, hausner’s ratio, and carr’s index were determined.12
Bulk density
A granule is a particle gas combination containing both inter-particle gaps and intra-particle voids, it may be calculated using the formula below.12
Tapped density
The tapped density of a powder represents its random dense packing that can be calculated by:12
Hausner ratio
This ratio can be applied to provide an index of the flow characteristics of a granule. Hausner ratio is being an indicator of the flowability of bulk solids.12
It is calculated by:
Carr’s ratio
Carr’s ratio is applied assuming that the compressibility of a solid is related to its flowability, it is supposed to measure the bulk and tapped density of bulk materials and calculate a ratio to estimate the flow of material.12 It is measured by following formula,
Angle of repose
A funnel was used to accurately pour a powder mixture so that the maximum cone height (h) could be achieved. The funnel’s height should not exceed 1 cm above the cone height (h) and the Angle of repose was calculated by using the following formula.12
Where “h” is the height and “r” radius
Inclusion/exclusion criteria
Because albenzaole is not recommended for children under the age of two, and there is a risk of choking, gummies are intended to be used for children over the age of two.
Method of gummies preparation
The method of gummy preparation includes the preparation of two solutions, i.e., a solution of water and sugar and a water and gelatin solution. As in Table 1, the ratio of water to sorbitol was kept at 2:1, and the ratio of water to sugar was kept at 1:1. Add 12.60 g sugar and 4.06 g sorbitol to 10 mL of water in a beaker. This solution was heated on medium-high while being stirred with a glass stirrer. Adjust the temperature to a point where the temperature of the solution doesn’t rise above 130°C. Keep heating until viscous sugar syrup is formed. Avoid overheating to prevent the caramelization of sugars. Once a thick, consistent syrup is obtained, turn off the flame. Lower its temperature to 60°C. In a solution of sugar and sorbitol, add 4.06 g of citric acid to the solution. In another beaker, 3.22 g of gelatin was admixed with 5 mL of water and heated for a few minutes on low heat. The ratio of gelatin to water was kept at nearly 1:2. Then pour the gelatin solution into the warm sugar solution and mix thoroughly, and then heat the mixture for a few seconds to 70°C. Add 2.8 g of albendazole to the above mixture and stir to form a uniform solution. Now add 5.04 mL of flavorant and 0.035 g of sodium benzoate, one by one, to the solution and mix it thoroughly. Lubricate the silicone mould with glycerin to avoid sticking. Pour the warm mixture into the mould and tap them on the shelf to even up the level of fillings. Remove and wipe any excess solution from the mould. Place the mould in a safe place at room temperature for 60 min. Seven formulations (F1–F9) were synthesized by varying the concentrations of sucrose, sorbitol, and gelatin as shown in Table 2.
| Water: Sucrose | 1:1 |
| Water: Sorbitol | 2:1 |
| Water: Gelatin | 2:1 |
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| Sucrose | 0.8g | 0.8g | 0.9g | 0.9g | 0.9g | 0.9g | 0.9g |
| Sorbitol | 0.45g | 0.44g | 0.33g | 0.29g | 0.28g | 0.27g | 0.26g |
| Gelatin | 0.14g | 0.15g | 0.16g | 0.20g | 0.21g | 0.22g | 0.23g |
| Citric acid | 0.29g | 0.29g | 0.29g | 0.29g | 0.29g | 0.29g | 0.29g |
| Colorant | 0.007g | 0.007g | 0.007g | 0.007g | 0.007g | 0.007g | 0.007g |
| Albendazole | 200mg | 200mg | 200mg | 200mg | 200mg | 200mg | 200mg |
| Flavourant | 0.36mL | 0.36mL | 0.36mL | 0.36mL | 0.36mL | 0.36mL | 0.36mL |
| Na benzoate | 0.0025g | 0.0025g | 0.0025g | 0.0025g | 0.0025g | 0.0025g | 0.0025g |
| Total weight | 2.24g | 2.24g | 2.24g | 2.24g | 2.24g | 2.24g | 2.24g |
Characterization (Post Formulation Studies) Organoleptic evaluation
By using our senses, it is utilized to assess the flavour of the dosage forms. 20 healthy volunteers with an appropriate sense of taste were recruited, and after being asked to chew the gummies, they were questioned about their evaluation according to the organoleptic evaluation scale as described in Table 3.14
| Category | Scale |
|---|---|
| Very sweet | 5 |
| Sweet | 4 |
| Neutral | 3 |
| Bitter | 2 |
| Very bitter | 1 |
Weight variation/Uniformity of mass
Each of the 20 gummies were weighed individually, the average weight was calculated and the individual gummies weights were compared to it. If no more than two gummies fall outside the allowed % range as shown in Table 4. and no gummies deviates by more than twice the allowed range, the gummies pass the test. The following formulas are used:14
| Average Weight (mg) | Percentage Deviation (%) |
|---|---|
| 130 or less | 10 |
| 130-324 | 7.5 |
| More than 324 | 7 |
Hardness Test
Friability
A sample of 10 gummies at random and placing them in the plastic chamber of the Rosch Friabilator, the friability of gummies was examined. For 4 min, the friabilator drum was circulated at 25 rpm. The formula given below was used to compute the percentage drop in gummies weight. Friability should be under 1%.17
Moisture content
Drying finely ground samples (10 g) in an air oven at 105°C overnight to create a constant weight was carried out.18
pH determination
A micro pH meter with a glass combination electrode was used to monitor the pH. The materials were divided into thin slices, added to boiling water (1:3, w: w), and mixed continuously until completely dissolved. The pH was measured after the heated solution was tempered at 25°C. Each measurement was taken thrice times. The acceptable pH range of chewable gummies is 5.69 to 6.34.19
In vitro disintegration Test
The USP disintegration apparatus is made up of six glass tubes that are 3 inches long, open at the top, and positioned at the bottom end of the basket rack assembly against the 10-mesh screen. Plastic discs with perforations can also be utilized in the tests. These are put on top of the gummies and have a negative impact on them. Use the tool for a predetermined period of time. If all particles pass through the 10-mesh screen at the designated time while the gummies are unplugged, the gummies complies with the test. Any remaining material must have a soft bulk and no visible solid core. Gummies was found to disintegrate within 15 min.20
In vitro dissolution testing
Gummies are tested for dissolution to determine rate at which it produces a solution. British and US Pharmacopoeia dissolution apparatus (paddle/basket apparatus) are made of a cylindrical vessel. Using a water bath or heating apparatus, the test vessel’s internal temperature can be maintained at 37 ± 0.5°C while maintaining a consistent bath fluid level. Withdraw a sample not less than 1 cm from the vessel wall, from the area centered between the dissolving medium’s surface and the top of the spinning basket, within a certain time frame or at each interval provided. Most formulations in dissolution tests released 85% of the drug after 15 min.20
Kinetics of drug release: Various statistical methods have been used to determine the release kinetics of formulations. The models were the Kosmeyers Peppas model, the first-order model and the zero-order model.21
RESULTS
Albendazole gummies were successfully synthesized. Different testing was performed on gummies, and the results obtained from various tests ensure that albendazole gummies remain stable, showing no physiochemical changes. All the formulations show acceptable results with reference to hardness, weight variation, friability, disintegration time, water activity, dissolution, and release kinetics.
Preformulation Studies
Bulk characterization
Several variables, including hygroscopicity, bulk density, tapped density, Carr’s index, Hausner’s ratio, and angle of repose, were used to evaluate the powder’s quality, and their results are summarized and given in Table 5.12
| Formulation | Bulk Density (g/cm3) | Tapped Density (g/cm3) | Carr’s Index (%) | Hausner’s Ratio | Angle Of Repose (θ) | Flowability |
|---|---|---|---|---|---|---|
| F1 | 0.72±0.0360 | 0.77± 0.014 | 4.2±0.974 | 1.07 ± 0.097 | 27.6 ± 0.635 | Excellent |
| F2 | 0.7 ± 0.013 | 0.75± 0.033 | 4.5±0.706 | 1.04 ± 0.013 | 28.1 ± 0.870 | Excellent |
| F3 | 0.66 ± 0.021 | 0.69 ±0.058 | 4.3±0.161 | 1.04 ± 0.013 | 26.1 ± 0.920 | Excellent |
| F4 | 0.76 ± 0.010 | 0.8 ± 0.013 | 5 ± 0.894 | 1.05 ± 0.028 | 28.5 ± 1.022 | Excellent |
| F5 | 0.71 ± 0.012 | 0.73 ±0.024 | 2.7±0.828 | 1.02 ± 0.016 | 27.3 ± 0.372 | Excellent |
| F6 | 0.68 ± 0.012 | 0.79 ±0.007 | 4.9±0.501 | 1.06 ± 0.094 | 28.9 ± 0.375 | Excellent |
| F7 | 0.73 ± 0.047 | 0.71 ±0.060 | 4.8±0.859 | 1.05 ± 0.011 | 29 ± 0.534 | Excellent |
Post Formulation Studies
Organoleptic evaluation
In organoleptic studies, it was found that gummies are sweet in taste and elastic and chewy in nature. So the formulations (F1-F7) were graded according to an organoleptic evaluation scale of 4, which is sweet.14
Weight variation
All the formulations show adequate percentage variations, according to USP specifications. The weight variation of F1 to F7 is shown in Table 6. 20
| Formulation | Hardness (kg/cm2) | Weight Variation | Friability (%) | Moisture Content (%) | pH | In vitro Disintegration (minutes) |
|---|---|---|---|---|---|---|
| F1 | 0.81±0.028 | 2.04±0.5 | 0.23 | 18 | 6.1 | 6:50 |
| F2 | 0.81±0.035 | 1.98±0.4 | 0.21 | 18 | 6.3 | 10:42 |
| F3 | 0.81±0.01 | 2.00±0.8 | 0.25 | 18 | 5.8 | 6:05 |
| F4 | 0.81±0.044 | 2.14±0.6 | 0.21 | 18 | 5.4 | 5:50 |
| F5 | 0.80±0.005 | 2.05±0.4 | 0.26 | 18 | 5.6 | 5:50 |
| F6 | 0.82±0.029 | 1.94±0.5 | 0.25 | 19 | 6.0 | 3:00 |
| F7 | 0.82±0.013 | 1.85±0.6 | 0.22 | 20 | 6.2 | 6:55 |
Hardness
Friability
Moisture content
Results for the moisture content of gummies are given in Table 6. All formulations had moisture content values that were less than 20%, which is the ideal level for this kind of product. Our findings were consistent with earlier studies, which found that jelly candies had moisture levels in the range of 18–21%.18
pH determination
It was found that chewable gummies have a pH. All the formulations F1 to F7 showed satisfactory results, and their pH values reside within the recommended range (5.69 to 6.34).19
In vitro disintegration
In vitro dissolution
The percentage of drug release was observed for a period of 30 minutes, as demonstrated in Table 7. In the kinetic investigation, the drug release percentage versus time graph plot was used to determine the order of release for all formulations. Most of the formulations tested in dissolution tests released 85% of the drug after 25 min. At time 0, there is no drug release. The initial release of the drug was relatively slow. All the formulations except F7 have released 50% of the drug within 15 min. For F7, more than 50% of the drug was released earlier, within 10 min. At the end of 30 min, F1–F7 have released the maximum amount of the drug. The formulation F7 showed the highest drug release within 30 min; that’s why it is considered to be the best formulation. The pattern of drug release is shown in Figure 1.
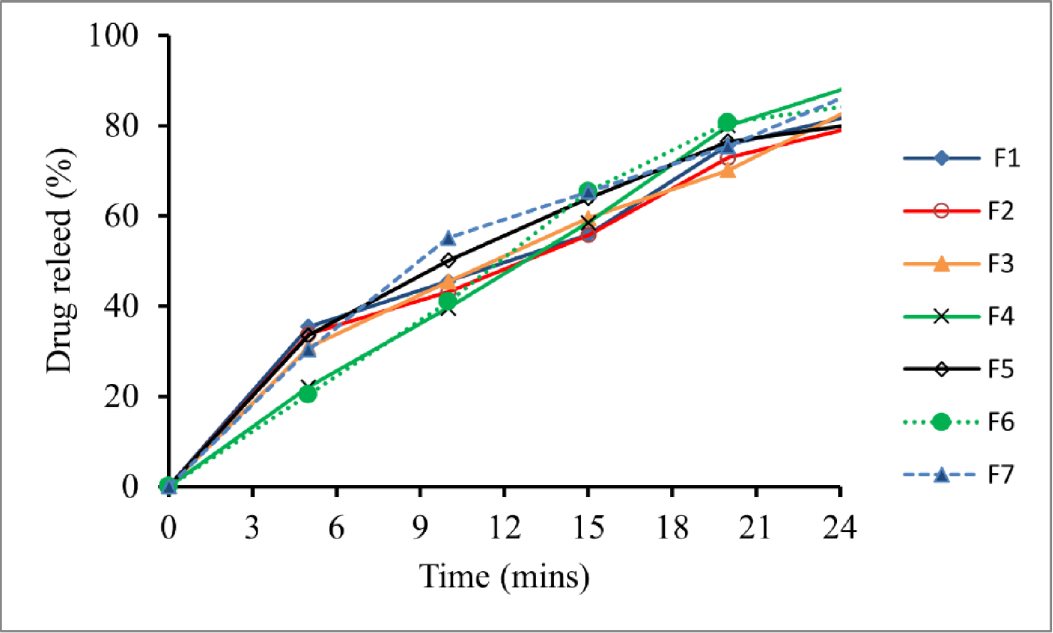
Figure 1:
Percentage Drug Release.
| Time (mins) | Percentage (%) Drug Release | ||||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 35.4 | 33.8 | 30.9 | 22.1 | 33.5 | 20.4 | 30.3 |
| 10 | 45.5 | 43.1 | 45.4 | 39.6 | 50.1 | 40.9 | 55.1 |
| 15 | 55.9 | 55.7 | 59.5 | 58.5 | 63.9 | 65.3 | 65.3 |
| 20 | 75.7 | 72.9 | 70.1 | 80.0 | 76.5 | 80.6 | 75.4 |
| 25 | 83.0 | 80.4 | 85.5 | 89.9 | 80.7 | 85.0 | 88.5 |
| 30 | 91.8 | 95.4 | 94.8 | 93.2 | 96.1 | 95.2 | 97.3 |
Release Kinetics
During formulation kinetic data modelling, we concluded that the F1-F7 formulations were following first order because their R2 value is near 1. So, it was shown that the release of drugs is dependent on concentration, which explained the first-order behaviour of the formulation and immediate release kinetics. The n value in Korsmeyer and Peppa’s release kinetics model describes the formulation’s specified drug release through Fick’s law of diffusion. In this case, a transport mechanism that is Fickian corresponds to an n value less than 0.45. The release kinetics of seven different formulations are described in the Table 8.13,14,20
| Formulation | Zero Order | First Order | Korsmeyer | |||
|---|---|---|---|---|---|---|
| K0 | R (Square) | K1 | R (Square) | kKP | n | |
| F1 | 3.501 | 0.9227 | 0.068 | 0.9603 | 10.36 | 0.32 |
| F2 | 3.389 | 0.9281 | 0.064 | 0.9695 | 9.873 | 0.30 |
| F3 | 3.508 | 0.9501 | 0.068 | 0.9681 | 1.247 | 0.28 |
| F4 | 3.549 | 0.9689 | 0.067 | 0.9549 | 6.711 | 0.37 |
| F5 | 3.547 | 0.8902 | 0.072 | 0.9813 | 12.681 | 0.42 |
| F6 | 3.538 | 0.9493 | 0.068 | 0.9645 | 7.657 | 0.44 |
| F7 | 3.681 | 0.9038 | 0.078 | 0.9811 | 12.292 | 0.43 |
DISCUSSION
Albendazole is very bitter in taste, due to which it used to have very low acceptability in children, which provoked and made realize the necessity to develop a novel drug delivery form for those children or paediatrics in order to increase compliance among them and increase the palatability. So, after immense effort, this research finally resulted in the formulation of the flavouring for Albendazole gummies for children and paediatrics. The pre-formulation studies were carried out for the powder, such as hygroscopicity, bulk density, tapped density, Carr’s index, Hausner’s ratio, and angle of repose, to produce high-quality powder for the gummies. The results of all of these were in accordance with international standards. A number of post-formulation studies were performed, including organoleptic evaluation, weight variation, hardness test, friability, moisture content, pH, in vitro disintegration, dissolution, and release kinetics. The results for hardness, disintegration time, friability, and weight variation were identical across all trials, indicating an efficient and organized formulation process. Hardness, chewability, and compliance are significantly influenced by the gelatin content. The formulation contains the right levels of pH and moisture, indicating that the product has the potential to remain stable at room temperature. In dissolution testing, the majority of the formulations were found to release almost 85% of the medication after 25 min. At first, the medication was released relatively slowly. In less than 15 min, every formulation released almost 50% of the medication, demonstrating that every trial passed the quality control checks. The water and gelatin concentrations were varied in all these formulations to give it a good gummy texture after all. At 0.23 g of gelatin per gummy, the F7 formulation was found to be the best of all. As a result, formulation 7 (F7) was identified as an optimal formulation and was shown to have an optimized characteristic. With the help of this research, it is possible to enhance the compliance and acceptance of the paediatric population by providing a suitable level of sweetness and flavour.
CONCLUSION
For the first time, chewable albendazole gummies for paediatrics were prepared from a natural polymer, gelatin. The aim of these gummies was enhanced compliance in paediatrics, better taste, ease of administration, and increased palatability. Seven formulations of gummies were made by adjusting the water and gelatin contents. The F7 formulation was found to be the best of all, with 0.23 g of gelatin per gummy. Organoleptic evaluation revealed that the texture of gummy bears was elastic and chewy. All formulations revealed satisfactory percentage weight variation, hardness, moisture content and friability results according to USP standards. All formulation having a disintegration time with in a slandered (6 min), disintegration and dissolution yielded satisfactory results. As a result, the newly developed albendazole gummies dosage form was found to be satisfactory and suitable for anthelminthic activity.
References
- Preis M, Breitkreutz J. Pediatric drug development and dosage form design, Editor Editors. AAPS PharmSciTech. 2017;18(2):239-40. [PubMed] | [CrossRef] | [Google Scholar]
- Ranmal SR, Cram A, Tuleu C. Age-appropriate and acceptable paediatric dosage forms: insights into end-user perceptions, preferences and practices from the Children’s Acceptability of Oral Formulations (CALF) Study. Int J Pharm. 2016;514(1):296-307. [PubMed] | [CrossRef] | [Google Scholar]
- Davies P. Oral solid dosage forms. Pharm Pre Formula. 2016:379-442. [PubMed] | [CrossRef] | [Google Scholar]
- Zajicek A, Fossler MJ, Barrett JS, Worthington JH, Ternik R, Charkoftaki G, et al. A report from the pediatric formulations task force: perspectives on the state of child-friendly oral dosage forms. AAPS J. 2013;15(4):1072-81. [PubMed] | [CrossRef] | [Google Scholar]
- Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. 2000;121(Suppl 1):S113-32. [PubMed] | [CrossRef] | [Google Scholar]
- Movahedi F, Li L, Gu W, Xu ZP. Nanoformulations of albendazole as effective anticancer and antiparasite agents. Nanomedicine (Lond). 2017;12(20):2555-74. [PubMed] | [CrossRef] | [Google Scholar]
- Kim HJ, Lee YJ, Back SO, Cho SH, Lee HI, Lee MR, et al. Treatment with extracellular vesicles from alleviates dextran sulfate sodium-induced colitis in C57BL/6 mice. Korean J Parasitol. 2022;60(5):309-15. [PubMed] | [CrossRef] | [Google Scholar]
- Koradia KD, Parikh RH, Koradia HD. Albendazole nanocrystals: optimization, spectroscopic, thermal and anthelmintic studies. J Drug Deliv Sci Technol. 2018;43:369-78. [CrossRef] | [Google Scholar]
- Vasani C, Shah K. Preparation and evaluation of chocolate drug delivery system of albendazole. Res J Pharm Technol. 2016;9(11):1994-8. [CrossRef] | [Google Scholar]
- Schulz JD, Neodo A, Coulibaly JT, Keiser J. Pharmacokinetics of albendazole, albendazole sulfoxide, and albendazole sulfone determined from plasma, blood, dried-blood spots, and Mitra samples of hookworm-infected adolescents. Antimicrob Agents Chemother. 2019;63(4):e02489-18. [PubMed] | [CrossRef] | [Google Scholar]
- Arigo A, Jawahar N, Nikhitha K, Jubie S. Effect of Hygroscopicity on pharmaceutical ingredients, methods to determine and overcome: an Overview. J Pharm Sci. 2019;11(1):6-10. [PubMed] | [CrossRef] | [Google Scholar]
- Sharma KSC, Kumar YK, Reddy KR. Effect of drug release on albendazole chewable tablets by using different formulation techniques. Int J Pharm Sci. 2014;5(10):4543 [PubMed] | [CrossRef] | [Google Scholar]
- Zhou X-y, Yu J-h, Yu H. Effect of gelatin content and oral processing ability on vitamin C release in gummy jelly. JFST. 2021:1-9. [PubMed] | [CrossRef] | [Google Scholar]
- Abbas M, Abbas M, Tariq F, Yasin R, Nabeel M. Formulation and evaluation of chewable tablets of desloratadine prepared by aqueous and non-aqueous techniques. J Drug Delivery Ther. 2020;10(1):5-10. [CrossRef] | [Google Scholar]
- Kimaro E, Tibalinda P, Shedafa R, Temu M, Kaale E. Formulation development of chewable albendazole tablets with improved dissolution rate. Heliyon. 2019;5(12):e02911 [PubMed] | [CrossRef] | [Google Scholar]
- Nyamweya N, Kimani S. Chewable tablets: a review of formulation considerations. Pharm Technol. 2020;44(11):38-44-38–44 [PubMed] | [CrossRef] | [Google Scholar]
- . Wahid, a comparative study on quality analysis on marketed vitamin C (ascorbic acid) chewable tablets of different brands available in Bangladesh. World J Pharm Res.. 2019;9(5):146-57. [PubMed] | [CrossRef] | [Google Scholar]
- Teixeira-Lemos E, Almeida AR, Vouga B, Morais C, Correia I, Pereira P, et al. Development and characterization of healthy gummy jellies containing natural fruits. Open Agric J. 2021;6(1):466-78. [CrossRef] | [Google Scholar]
- Delgado P, Bañón S. Determining the minimum drying time of gummy confections based on their mechanical properties. CyTA J Food CyTA-J Food.. 2015;13(3):329-35. [CrossRef] | [Google Scholar]
- SONY , AHMED M, ULLAH B, BISWAS SK, AZAD MAS, HOSSAIN MS, et al. A review article on pharmaceutical analysis of pharmaceutical industry according to pharmacopoeias. Orient J Chem. 2020;36(1):1-19. [CrossRef] | [Google Scholar]
- Paarakh MP, Jose PA, Setty C, Christoper G. Release kinetics–concepts and applications. Int J Pharm Res Technol. 2018;8(1):12-20. [CrossRef] | [Google Scholar]
