ABSTRACT
Background
In this present work, methanol extracts of the leaves and flowers of Salvia officinalis Linn. (MES) and Rosmarinus officinalis Linn. (MER) were examined for their potential anti-aging and antioxidant properties using in vitro methods.
Materials and Methods
Several in vitro methods and techniques, such as estimation of reducing power, DPPH radical assay, ABTS decolorising assay, total antioxidant activity, superoxide radical (O2• -), metal chelating activity, hydrogen peroxide and hydroxyl radical scavenging assay and assay of scavenging of nitric oxide were employed to appraise the scavenging of free radicals and antioxidant action of MES and MER. The anti-aging activity of the extracts was evaluated using the enzyme inhibition assays involving elastase inhibitory assay, collagenase inhibitory assay, and hyaluronidase inhibitory activity models. Using the elastase inhibitory assay, collagenase inhibitory assay, and hyaluronidase inhibitory assay models, the mechanistic antiaging potential of the extracts (MES and MER) had been appraised.
Results
Because of their lower IC50 values, the extracts (MES and MER) showed significant antioxidant activity. It was discovered that they had poor metal chelating activity. By inhibiting elastase, collagenase, and hyaluronidase activity, both extracts showed significant anti-aging properties.
Conclusion
The conclusions of this present research work thus imply that both the plant extracts possess the potential to act as antioxidant and may be used in herbal treatment for both normal and photoaging.
INTRODUCTION
Ageing is a foreseeable and natural process which affects entirely living things including humans. Skin aging can be categorised into two kinds: chronological ageing brought on by aging and premature ageing brought on by photoaging.1 A leathery appearance, changes in pigmentation, and deep furrows are just a few signs of photoaging, which is brought on by outside factors. Photoaging is a complicated and multifactorial process that is affected by a variety of extrinsic and intrinsic factors, together with UVB radiation. Long-term skin exposure to UVB rays can also cause erythema, sunburn, edema, hyperplasia, melanoma, carcinogenesis, and hyperplasia.1
Naturally ageing causes the skin to start wrinkling. The top layer of skin is made up of fibroblasts, collagen, elastin, and other proteins; this layer is known as the Extracellular Matrix (ECM). The ECM offers a structural foundation that is vital for the skin’s elasticity and expansion. The degradation of the ECM has been connected to increased action of a few enzymes intricated in skin-ageing process, together with collagenase, hyaluronidase and elastase, which is directly related to skin ageing.1 An upsurge in the making of ROS and oxidative stress can consequence from the skin absorbing UV radiation. It can also result in mitochondrial and DNA damage. Increased ROS levels can cause hyaluronidase, collagenase, and elastase to be activated, which can hasten the process of aging of the skin tissue. Modern photoprotection techniques use organic entities or composites which can absorb UV light and guard the skin from UVA and UVB radiation. Owing to structural resemblances and absorbance range profiles with those of marketed organic UV filters, polyphenolic compounds have been recommended as potential photoprotective agents in the literature.2 They are promising candidates because of these qualities for reducing skin damage from UV radiation.3
Numerous studies have examined the topical use of formulations containing natural ingredients to lessen UV-related skin damage, including green tea, marigold, wild Chrysanthemum, and Pimenta pseudocaryophyllus extracts. The biological effects of these extracts in defending the skin from UV-related deterioration have been very positive.4,5 Plants have been used as skin lighteners and sunblock for a very long time in the cosmetics industry. Plants can reduce oxidative stress and prevent the activity of enzymes like collagenase, hyaluronidase and elastase, according to in vitro research.6,7 Researchers have reported that Salvia officinalis Linn. and Rosmarinus officinalis L., the plants from the Lamiaceae family have high concentrations of antioxidants and may be useful in anti-aging and photoprotection. Sage and rosemary were chosen for extraction and assessment of their anti-aging and antioxidant properties in this present study. The selection of these plants was made in accordance with their traditional, historical and indigenous use in cosmetics or their prior reports of antioxidant activity in research studies or the researchers’ own lab, though these findings have not yet been published. A review of the literature revealed that these two plants have numerous traditional uses, several of that have already been pharmacologically proven. The anti-aging and antioxidant properties of the leaves and flowers of these plants haven’t been studied, though. The purpose of the current research is to evaluate the anti-radical, anti-aging and antioxidant characteristics of a methanol extract of leaves and flowers of Salvia officinalis L. and Rosmarinus officinalis Linn. We investigated the preliminary phytochemical composition, anti-ageing activity, and antioxidant capacity using various in vitro techniques.
MATERIALS AND METHODS
Assortment, identification, and extraction of the plants
The flowers and leaves of Salvia officinalis and Rosmarinus officinalis were collected from the Dehradun region in the late spring of the year. The plants were identified and authenticated by a botanist. Two voucher specimens (AKG/SO/2022/11 and AKG/RO/2022/12) have been preserved for potential use. The flowers and leaves were dried, cut and extracted with methanol using standard method. The extracts obtained were dark green brownish concentrated methanol extracts of leaves and flowers of Salvia officinalis (MES) and Rosmarinus officinalis (MER). Following that, the finished products were kept at 4°C until they were used.
Drugs and chemicals
The following chemicals and reagents were used in the study: Collagenase; FALGPA [N-[3-(2-Furyl)acryloyl]-L-leucy l-glycyl-L-prolyl-L-alanine]; HEPES (4-(2-hydroxyethyl)-1-p iperazineethanesulfonic acid), N-Methoxysuccinyl-Ala-Al a-Pro-Val p-nitroanilide; p-Dimethylaminobenzaldehyde (DMAB; Ehrlich’s Reagent); 1,1-diphenyl-2-picrylhydrazyl hydrate (DPPH) and 2,2′-00azinobis-3-ethylbenzothia zoline-6-sulfonic acid (ABTS) were obtained from Sigma-Aldrich (St. Louis, MO, USA). As a gift sample from a trustworthy source, Quercetin was received. All other chemicals and reagents used in the study were of reagent grade and were commercially available (SRL Mumbai, E. Merck India).
Preliminary phytochemical screening
The extracts were subjected to standard phytochemical testing through a number of standard chemical tests to identify the chemical components they contain.8
Estimation of total phenolic compounds
The soluble phenolics as Total Phenolic Compounds (TPC) in the methanol extracts had been assessed employing the Folin-Ciocalteu technique, with quercetin serving as the assay’s reference phenolic compound.9 Employing the linear regression analysis equation of the quercetin standard graph, the amount of TPC in the methanol extracts was articulated in grams of Quercetin Equivalent (QE).
Where, x was the concentration and y were the absorbance.
Reducing power and total antioxidant activity
Using a previously described method, the reducing power of MES and MER was assessed. Utilising the thiocyanate technique, the total antioxidant activity of both extracts had been evaluated.10
Appraisal of DPPH (1 – 1 – diphenyl – 2 – picryl hydrazyl) radical scavenging activity
Using DPPH•, as explained in a prior method,11 the scavenging activity of MES and MER for the free radicals was assessed. The succeeding equation was used to determine the percent DPPH scavenging outcome:
Where A0 denoted the control absorbance and At denoted the absorbance in the occurrence of the extract or standard,
ABTS (2, 2′ – azinobis – 3 – ethylbenzothiazoline – 6 – sulfonic acid) radical decolorization assay
To evaluate MES and MER’s ability to scavenge ABTS radicals, we employed the ABTS assay method, which was previously described elsewhere.12 The following formula was used to calculate the percentage of scavenging of ABTS:
Where A0 denoted the control absorbance and At denoted the absorbance in the occurrence of the extract or standard.
Evaluation of superoxide radical (O2• -) scavenging activity
The ability of MES and MER to obstruct blue formazan development served as the foundation for the assay. According to a process previously described,13 superoxide radicals were produced in the occurrence of light-NBT (Nitro-blue Tetrazolium)-riboflavin system. To calculate the level of superoxide anion production inhibition, we used the equation below:
Where, A0 = Control optical density/absorbance (without extract) and At = optical density/absorbance in the existence of the extract/standard.
Evaluation of nitric oxide scavenging activity
The Greiss reaction, as previously described, serves as the foundation for the methodology used in this procedure.14 The below formula was employed to determine the
Where, A0 = Control absorbance (without extract) and At = absorbance in the presence of the extract or standard.
Appraisal of hydrogen peroxide and hydroxyl radical scavenging activity
Using a previously published method, as previously described,15 we assessed the capacity of MES and MER to scavenge Hydrogen Peroxide (H2O2). The scavenging capacity of hydroxyl radical of the extracts (MES and MER) was assessed utilising the previously described 2-deoxy-d-ribose assay method.16 The below formula was employed to determine the inhibition percentage.
Where, A0 = Control optical density/absorbance (without extract) and At = optical density/absorbance in the presence of the extract or standard.
Metal chelating activity
Measurements of the chelation of Ferrous (Fe++) ions by MES and MER were made using a technique that has previously been discussed.14 The succeeding formula was utilised to compute the inhibition percentage of ferrous-ferrozine complex fabrication:
In the above formula, Where, A0 = Control absorbance (without extract) and At = optical density/absorbance in the existence of the extract/standard. As the typical chelating compound, Ethylenediaminetetraacetic Acid (EDTA) had been utilised.
Anti-ageing activity
Determination of anti-elastase activity
An improved technique based on Kraunsoe et al.17 was employed to estimate the elastase inhibitory action of the test sample.
Estimation of collagenase inhibitory activity
Hyaluronidase inhibitory activity
Statistical analysis
The study’s findings had been represented using the mean and SD (standard deviation; n = 6 and n = 3). One-way ANOVA (Analysis of Variance) and a post hoc test “Dunnett’s Multiple Comparison Test” were two statistical tests that had been performed employing the GraphPad prism software. A 0.05, “p” value or less was regarded as significant statistically.
RESULTS
Preliminary phytochemical screening
Both plant extracts contained a variety of classes of phytoconstituents, including flavonoids, alkaloids, phenols, phytosterols, saponins, etc., according to the results of the qualitative preliminary phytochemical screening. Borntrager’s test outcomes for both extracts were favourable as well.
Estimation of Total Phenolic Compounds
The Total Phenolic Compounds (TPC) in the MES and MER were calculated to be 172.18 ± 2.45 mg and 155.90 ± 2.71 mg, respectively, in terms of quercetin equivalents per g of extract (Figure 1).
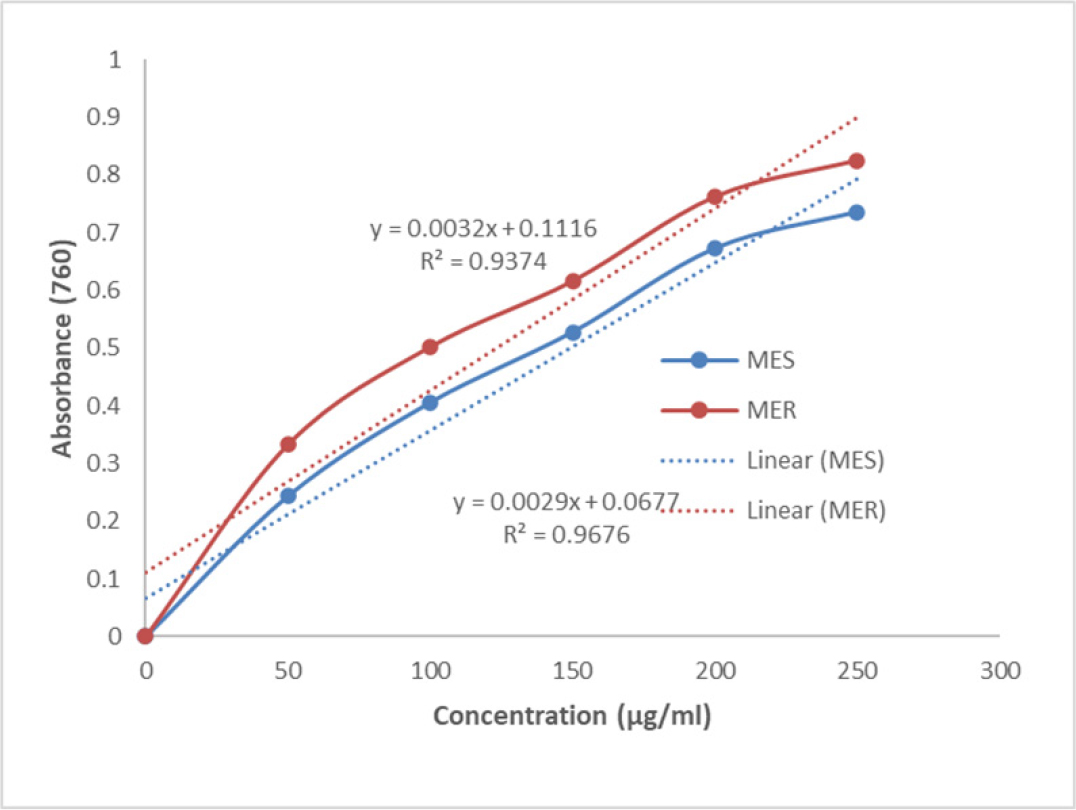
Figure 1:
Showing the total phenolic compounds in MES and MER.
Reducing power assay
The assay of the reducing ability results demonstrated that MES and MER were potential antioxidants when compared to quercetin, sodium metabisulphite, and ascorbic acid (Figure 2). It was found that the MES and MER’s reducing power depended on concentration and increased as concentration increased.
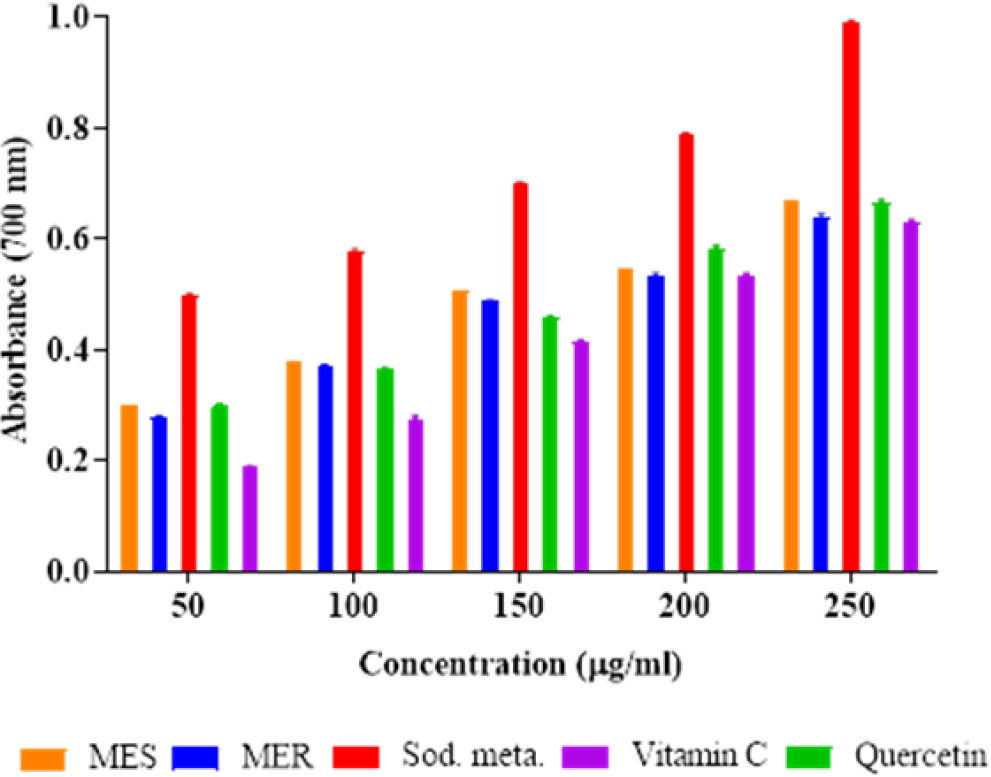
Figure 2:
Showing the reducing power assay of MES and MER.
Total antioxidant activity
The significant antioxidant action of MES and MER primarily augmented over longer incubation times (up to 36 hr). The research discovered that the antioxidant activity of MES and MER was inferior than that of Butylated Hydroxyanisole (BHA) at the identical concentration but higher than that of 250 g/mL of α-tocopherol. It was discovered that MES, MER, BHA, and α-Tocopherol had percentage inhibitions of 61.99 ± 0.68%, 60.94 ± 0.59%, 85.65 ± 0.65% and 42.59 ± 0.67%, respectively (Figure 3).
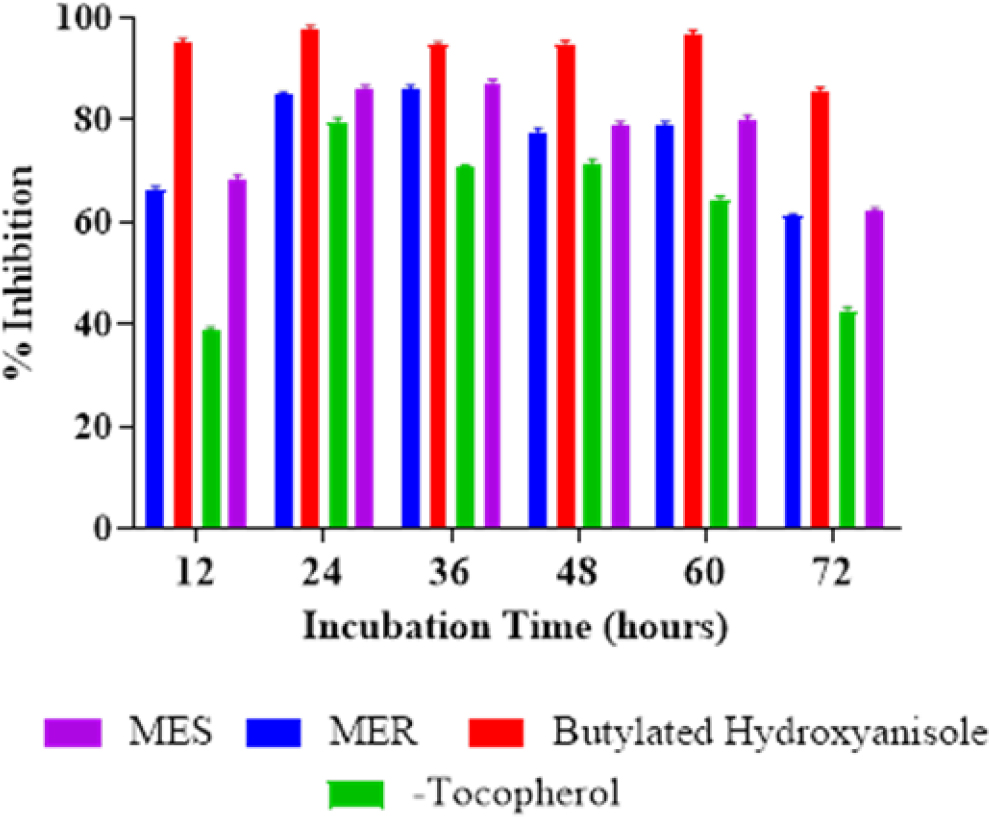
Figure 3:
Depicting the total antioxidant activity of MES and MER.
Estimation of DPPH radical scavenging activity
The DPPH scavenging capacities of MES, MER, quercetin, and ascorbic acid as percentage were determined to be 98.99%, 98.98%, 98.99%, and 97.13%, correspondingly, at 250 g/mL. concentration. The research found that the plant had DPPH radical scavenging capacities that were comparable to standards. MES, MER, quercetin, and ascorbic acid each had IC50 values (Table 1 and Figure 4) of 83.72, 81.24, 77.36, and 86.19 µg/mL, correspondingly.
| Sl. No. | Antioxidant Assay | IC50 (µg/mL) | ||||
|---|---|---|---|---|---|---|
| MES | MER | Ascorbic acid | EDTA | Quercetin | ||
| 1. | Determination of DPPH radical scavenging activity. | 83.72 | 81.24 | 86.19 | – | 77.35 |
| 2. | ABTS radical decolorization assay. | 106.83 | 108.47 | 127.07 | – | 78.17 |
| 3. | Assay of superoxide radical (O2•-) scavenging activity. | 138.57 | 147.83 | 87.24 | – | – |
| 4. | Assay of nitric oxide scavenging activity. | 140.18 | 165.14 | 105.07 | – | – |
| 5. | Hydrogen peroxide scavenging activity. | 106.59 | 112.22 | 104.86 | – | – |
| 6. | Hydroxyl radical scavenging. | 131.56 | 139.32 | 109.79 | 74.23 | |
| 7. | Metal chelating activity. | 318.49 | 347.64 | – | 111.59 | – |

Figure 4:
Depicted the antiradical activity of MES and MER (DPPH; ABTS and Superoxide radical).
ABTS radical decolorization assay
The research revealed that MES and MER, like the standard antioxidants, had a significant and concentration-dependent capacity to scavenge the ABTS+ radical. For MES, MER, ascorbic acid, and quercetin, the calculated IC50 values were 106.83, 108.47, 127.07, and 78.17 µg/mL, correspondingly (Table 1 and Figure 4).
Assay of superoxide radical (O2• -) scavenging activity
According to the study, as MES, MER, and the standard compound concentrations were increased, so did the percentage inhibitions of superoxide radical production. Even though MES and MER were not as effective as quercetin and ascorbic acid, they still showed a sizable amount of superoxide radical scavenging ability. The IC50 values (Table 1 and Figure 4) for MES, MER, and ascorbic acid were 138.57 ± 0.57 µg/mL, 147.83 ± 0.83 µg/mL, and 87.24 ± 0.91 µg/mL, correspondingly.
Nitric oxide scavenging activity assay
Equated to ascorbic acid, MES and MER had less scavenging activity. For MES, MER, and the standard compound, the percentage inhibitions had been calculated to be 66.92 ± 0.97%, 62.72 ± 0.93%, and 73.92 ± 0.53%, respectively, at 250 µg/mL concentration Table 1 and Figure 5. Following a linear regression analysis, the IC50 values (Table 1) for MES, MER, and ascorbic acid were determined to be 140.18±0.81, 165.14±0.88, and 105.07±0.65 µg/mL, correspondingly.
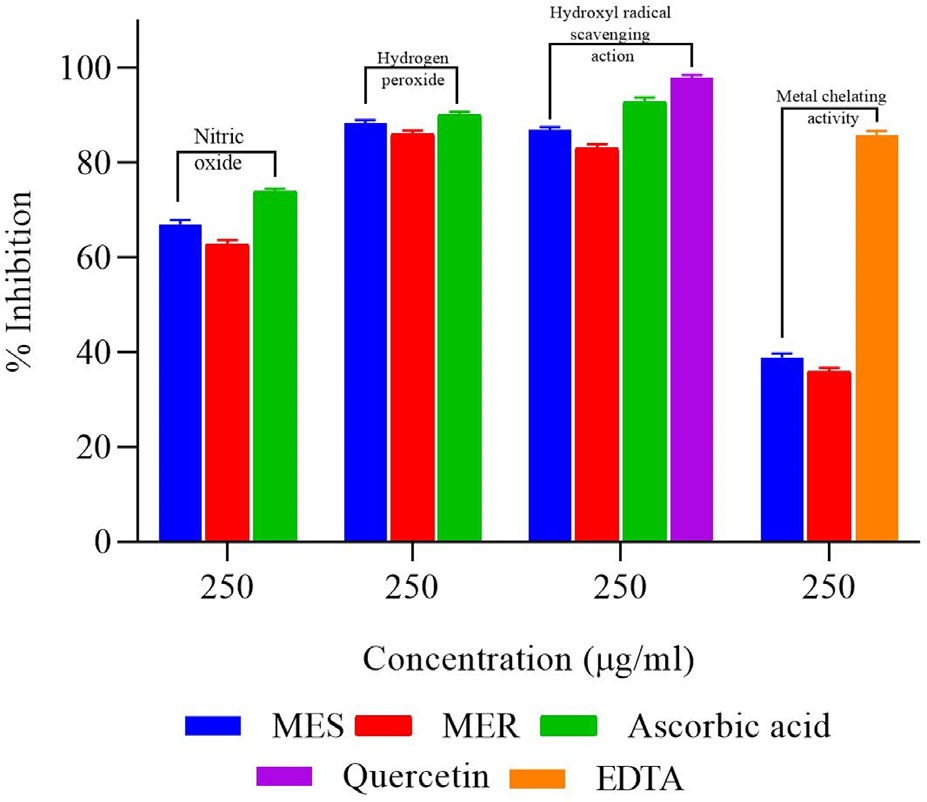
Figure 5:
Antioxidant activity of MES and MER at 250 μg/mL concentration (Nitric oxide; Hydrogen peroxide; Hydroxyl radical scavenging action and Metal chelating activity).
Appraisal of hydrogen peroxide and hydroxyl radical scavenging activity
The percentage H2O2 scavenging activity of MES, MER, and ascorbic acid were found to be 88.31 ± 0.61%, 85.96 ± 0.79% and 89.98 ± 0.72%, correspondingly, at a 250 g/mL concentration. The values of IC50 for MES, MER, and vitamin C were computed to be 106.59 ± 0.91 µg/mL, 112.22 ± 0.48 and 104.85 ± 0.43 µg/ mL, correspondingly (Table 1 and Figure 5). Between 50 and 250 g/mL, MES and MER demonstrated concentration-dependent hydroxyl radical scavenging activity in vitro. The values of IC50 were computed to be 131.56 ± 0.54 µg/mL, 139.31 ± 0.74, 74.24 ± 0.81 and 109.78 ± 0.59 µg/mL for MES, MER, quercetin and ascorbic acid correspondingly (Table 1).
Appraisal of metal chelating activity
Higher concentrations of MES, MER, and EDTA were found to consequence in an upsurge in the percentage of metal chelating activity. Using a linear regression analysis, the IC50 values for MES, MER, and EDTA were determined to be 347.64 ± 0.79 µg/ mL and 111.59 ± 1.14 µg/mL, respectively (Table 1 and Figure 5).
Anti-Ageing activity
Anti-elastase assay
Elastase was significantly (>80%) inhibited by MES and MERS (Table 2). MES (93.83 ± 3.73%) demonstrated superior action than N-Methoxysuccinyl-Ala-Ala-Pro-Chloro (92.06 ± 5.26%), the substrate but comparable activity to elafin (94.27± 5.21%). MER, on the other hand, had less activity than N-Methoxysucciny l-Ala-Ala-Pro-Chloro (89.83±2.38%) the substrate. The amount of elastase activity was barely affected by the solvents used.
| Plants (Code) | Plant parts used | Extract | Inhibitory activity (IC50) µg/mL | ||
|---|---|---|---|---|---|
| Elastase | Collagenase | Hyaluronidase | |||
| Salvia (MES) | Leaves and Flowers | Methanol | 93.83 ± 3.73 | 86.63 ± 4.76 | 78.67 ± 4.62 |
| Rosemary (MER) | Leaves and Flowers | Methanol | 89.83 ± 2.38 | 84.34 ± 3.64 | 75.45 ± 4.49 |
| Controls (10 µg/mL) | |||||
| Elafin | 94.27± 5.21 | – | – | ||
| N-Methoxysuccinyl-Ala-Ala- Pro-Chloro | 92.06 ± 5.26 | – | – | ||
| Ethylenediaminetetraacetic Acid (EDTA) | – | 84.65 ± 3.78 | – | ||
| Sodium salt of aurothiomalate | – | – | 100 ± 0.04 | ||
Collagenase inhibitory activity
When equated to the positive control, EDTA, MES action was higher (86.63 ± 4.76%), whereas MER activity was lower (84.34 ± 3.64%). The studied plants have never before been associated with any anti-collagenase activity (Table 2).
Hyaluronidase inhibitory activity
The enzyme action of hyaluronidase had been most significantly inhibited by the MES, at 78.67±4.62%. MER demonstrated potential inhibitory action (Table 2). Sodium aurothiomalate as the positive control, entirely inhibited the enzyme activity of hyaluronidase. These extracts (MES and MER) have not yet been publicized to have anti-hyaluronidase activity.
DISCUSSION
The physiological system is particularly susceptible to the harmful effects of oxidative stress, which can result from an excess of Reactive Oxygen Species (ROS) and/or a deficiency in the antioxidant defense systems. Numerous diseases, including cardiovascular, cancer, inflammatory, and neurodegenerative disorders, have been linked to oxidative stress, according to a growing body of research.22 Numerous studies have shown that Reactive Oxygen Species (ROS) overproduction causes oxidative stress. An important pathway that results in the death of both neuronal and vascular cells is oxidative stress. In the current study, antioxidant activity and potential antioxidant mechanisms of MES and MER were investigated by evaluating a variety of in vitro mechanistic antioxidant assays, including reducing power, DPPH radical assay, ABTS decolorization assay, total antioxidant activity, superoxide radical (O2• -), metal chelating activity, hydrogen peroxide and hydroxyl radical scavenging assay, and assay of nitric oxide scavenging. The total antioxidant activity and the reducing power assay are two of the most studied and notable methods for evaluating antioxidant potential. Bioactive compounds’ ability to produce hydrogen and electrons is what most strongly suggests that they have a reducing effect.23 MES and MER revealed a sufficient and significant concentration-dependent rise in reducing power. DPPH and ABTS radical are very ubiquitous and entrenched free radical’s pragmatic to explore free radical scavenging potential in vitro.24,25 MES and MER also demonstrated significant DPPH and ABTS radicals scavenging activity. There was also observed a significant inhibition of degree of lipid peroxidation by MES and MER as advised by the results of the thiocyanate method in the total antioxidant activity assay. In addition, MES and MER also demonstrated significant antioxidant activity in terms of superoxide radical (O2•-) scavenging activity, metal chelating activity, hydrogen peroxide and hydroxyl radical scavenging assay and nitric oxide scavenging action.
The anti-aging activity of the extracts was evaluated using the enzyme inhibition assays involving elastase inhibitory assay, collagenase inhibitory assay, and hyaluronidase inhibitory activity models. Using the elastase inhibitory assay, collagenase inhibitory assay, and hyaluronidase inhibitory assay models, the mechanistic assessment and appraisal of the extracts (MES and MER) were performed. The inhibitory action of elastase of these two extracts has not been previously published, as far as the authors are aware. However, several plant species from various families have been said to have anti-elastase properties including Coffea arabica (Fabaceae) leaf extracts and extracts from Hedyotis diffusa, also called Oldenlandia diffusa (Rubiaceae).26 It was discovered that two compounds, Pterolinus L and Pterolinus K, from Pterocarpus santalinus (Fabaceae), repressed the creation of superoxide anion and the elastase release.27 Additionally, elastase release was described to be repressed by glycoside ester phytocompounds isolated from Ixora coccinea (Rubiaceae) ariel methanol extract.28 However, it has been noted that collagenase-1 activity can be inhibited by extracts made from the leaves of Coffea arabica, a plant in the Fabaceae family. This inhibition is reportedly dose-dependent. The essential component of the extracellular matrix, collagen, is broken down by this enzyme in a crucial way. Skin aging and arthritis are two conditions that can be brought on by excessive collagen degradation in addition to tissue damage. Collagen deterioration can be stopped and the mechanical integrity of the ECM can be preserved due to the repressing effect of MES and MER on collagenase-1 activity. To achieve the greatest therapeutic benefits, additional investigation is essential to estimate the ideal dose and course of treatment.29 A literature review exposed, however, that several other plants, including Caesalpinia paraguariensis (Fabaceae) extracts, had an inhibitory effect on hyaluronidase.30 Additionally, it has been noted that extracts from Astragalus membranaceus’ leaves can raise the concentration of hyaluronic acid in fibroblasts and keratinocytes that have been cultured by enhancing the mRNA expression for hyaluronan synthase-2 and synthase-3.7 In the long run, it has been concluded that methanol extracts from the leaves and flowers of Salvia officinalis (MES) and Rosmarinus officinalis (MER) demonstrated clearly the powerful antioxidant, anti-radical as well as antiaging activity as evident by the findings of the deployed mechanistic assays. These findings may support the use of an adjuvant prophylactic for postponing the onset of natural aging of the skin as well as an alternative herbal therapy for the treatment of skin damage and photoaging. The inhibition of the by-products of oxidative damage and other related pathways may be the cause of the activity that has been observed. Due to their ability to scavenge free radicals and inhibit enzyme activity, plant extracts may help restore skin elasticity, slow down the aging process, and offer protection against skin damage and photo-aging.
CONCLUSION
Methanol extracts from the leaves and flowers of Salvia officinalis (MES) and Rosmarinus officinalis (MER) showed significant antiradical and free radical scavenging activity, anti-ageing activity, as well as potent antioxidant activity. These results may point to an alternative herbal therapy for the management of skin damage and photoaging, as well as an adjuvant prophylactic for delaying normal skin aging. This can act as the basis for further investigation. The reason for the observed activity could be due to the inhibition of oxidative damage products and other associated pathways.
References
- Takayama KS, Monteiro MC, Saito P, Pinto IC, Nakano CT, Martinez RM, et al. Array. An Acad Bras Cienc. 2022;94(4):e20201058 [PubMed] | [CrossRef]
- Velasco MVR, Sarruf FD, Salgado-Santos IMN, Haroutiounian-Filho CA, Kaneko TM, Baby AR, et al. Broad spectrum bioactive sunscreens. Int J Pharm. 2008;363(1-2):50-7. [PubMed] | [CrossRef] | [Google Scholar]
- Dinkova-Kostova AT. Phytochemicals as protectors against ultraviolet radiation: versatility of effects and mechanisms. Planta Med.. 2008;74(13):1548-59. [PubMed] | [CrossRef] | [Google Scholar]
- Campanini MZ, Custódio DL, Ivan AL, Martins SM, Paranzini MJ, Martinez RM, et al. Topical formulations containing extract: antioxidant activity and efficacy against UV-B-induced oxidative stress. AAPS PharmSciTech. 2014;15(1):86-95. [PubMed] | [CrossRef] | [Google Scholar]
- Sun S, Jiang P, Su W, Xiang Y, Li J, Zeng L, et al. Wild chrysanthemum extract prevents UVB radiation-induced acute cell death and photoaging. Cytotechnology. 2016;68(2):229-40. [PubMed] | [CrossRef] | [Google Scholar]
- Sumantran VN, Kulkarni AA, Harsulkar A, Wele A, Koppikar SJ, Chandwaskar R, et al. Hyaluronidase and collagenase inhibitory activities of the herbal formulation . J Biosci. 2007;32(4):755-61. [PubMed] | [CrossRef] | [Google Scholar]
- Hsu MF, Chiang BH. Stimulating effects of natto-fermented on hyaluronic acid production in human skin cells. J Ethnopharmacol. 2009;125(3):474-81. [PubMed] | [CrossRef] | [Google Scholar]
- [PubMed] | [CrossRef] | [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49-55. [PubMed] | [CrossRef] | [Google Scholar]
- Mitsuda H, Yuasumoto K, Iwami K. Antioxidation action of indole compounds during the autoxidation of linoleic acid. Eiyo Shokuryo. 1996;19:210-4. [PubMed] | [CrossRef] | [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthin on autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40(6):945-8. [CrossRef] | [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med.. 1998;72:1231-7. [CrossRef] | [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276-87. [PubMed] | [CrossRef] | [Google Scholar]
- Kumaran A, Joel Karunakaran R, Kumaran A, Joel Karunakaran R. Antioxidant activities of the methanol extract of . Pharm Biol.. 2006;44(2):146-51. [CrossRef] | [Google Scholar]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003-8. [PubMed] | [CrossRef] | [Google Scholar]
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple b test-tube Q assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165(1):215-9. [PubMed] | [CrossRef] | [Google Scholar]
- Kraunsoe JA, Claridge TD, Lowe G. Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry. 1996;35(28):9090-6. [PubMed] | [CrossRef] | [Google Scholar]
- Moore S, Stein WH. Photometric Ninhydrin method for use in the chromatography of amino acids. J Biol Chem. 1948;176(1):367-88. [PubMed] | [CrossRef] | [Google Scholar]
- Mandl I, Maclennan JD, Howes EL. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J Clin Invest. 1953;32(12):1323-9. [PubMed] | [CrossRef] | [Google Scholar]
- Reissig JL, Storminger JL, Leloir LF. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955;217(2):959-66. [PubMed] | [CrossRef] | [Google Scholar]
- Takahashi T, Ikegami-Kawai M, Okuda R, Suzuki K. A fluorimetric Morgan-Elson assay method for hyaluronidase activity. Anal Biochem. 2003;322(2):257-63. [PubMed] | [CrossRef] | [Google Scholar]
- Serra JA, Marschoff ER, Domínguez RO, Guareschi EM, Famulari AL, Pagano MA, et al. Oxidative stress in Alzheimer’s and vascular dementias: masking of the antioxidant profiles by a concomitant type II diabetes mellitus condition. J Neurol Sci. 2004;218(1-2):17-24. [PubMed] | [CrossRef] | [Google Scholar]
- Ak T, Gülcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact.. 2008;174(1):27-37. [PubMed] | [CrossRef] | [Google Scholar]
- Villaño D, Fernández-Pachón MS, Moyá ML, Troncoso AM, García-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71(1):230-5. [PubMed] | [CrossRef] | [Google Scholar]
- Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113(4):1202-5. [CrossRef] | [Google Scholar]
- Xu GH, Kim YH, Chi SW, Choo SJ, Ryoo IJ, Ahn JS, et al. Evaluation of human neutrophil elastase inhibitory effect of iridoid glycosides from . Bioorg Med Chem Lett. 2010;20(2):513-5. [PubMed] | [CrossRef] | [Google Scholar]
- Wu SF, Hwang TL, Chen SL, Wu CC, Ohkoshi E, Lee KH, et al. Bioactive components from the heartwood of . Bioorg Med Chem Lett. 2011;21(18):5630-2. [PubMed] | [CrossRef] | [Google Scholar]
- Lee CL, Liao YC, Hwang TL, Wu CC, Chang FR, Wu YC, et al. Ixorapeptide I and ixorapeptide II, bioactive peptides isolated from . Bioorg Med Chem Lett. 2010;20(24):7354-7. [PubMed] | [CrossRef] | [Google Scholar]
- Chiang HM, Lin TJ, Chiu CY, Chang CW, Hsu KC, Fan PC, et al. Array. Food Chem Toxicol. 2011;49(1):309-18. [PubMed] | [CrossRef] | [Google Scholar]
- Sgariglia MA, Soberón JR, Cabanes AP, Sampietro DA, Vattuone MA. Anti-inflammatory properties of phenolic lactones isolated from stem bark. J Ethnopharmacol. 2013;147(1):63-73. [PubMed] | [CrossRef] | [Google Scholar]
