ABSTRACT
Background: Dexmedetomidine is a promising candidate for pain relief and is being evaluated as an alternative or adjuvant to other treatment modalities. The intraarticular administration of such drugs is expected to give localised effect and the release of drug from drug delivery systems can be programmed to lengthen the duration of action. Purpose: The aim of the present investigation was to develop dispersion of lipospheres for the Dexmedetomidine for intraarticular administration that releases drug over a period of one week. Materials and Methods: DEX-LPS were formulated using the multiple emulsion technique. The particle size of lipospheres, drug entrapment, and percentage of drug release qualified as critical quality attributes. Risk assessment was done for the material attributes and process parameters based on the quality by design principles. The amount of cholesterol, triglyceride and the speed of homogenization during the primary emulsion formulation were identified as critical variables and therefore studied systematically using a Box-Behnken Design. Mathematical models generated for each critical quality attribute and their relationship with predictors was explored, using RStudio, to get a product that had the largest particle size, highest entrapment and desirable drug release profile. Results: Overlay plots were generated and the formulation at the center point settings had the required desirability. The acceptance range for the in-process and end-product quality attributes was established and tested in the product. The product remained stable at 2-8°C during 6 months of study. Conclusion: Stable DEX-LPS that could sustain drug release for 7 days was developed.
INTRODUCTION
Arthritis is a progression of dysfunction in joints, with acute or chronic inflammation that has a bearing on one or more joints of the patient. Osteoarthritis and rheumatoid arthritis are most prevalent forms of the disease.1 The manifestations of the disease include pain, swelling, joint stiffness, and damaged cartilage.2 The factors implicated in arthritis include age, weight, hereditary factors, injury of the joints, and atypical reactions of the immune system.3 At present, the treatment is restricted to targeting the symptoms of the disease rather than the disease itself. Patients are prescribed anti-inflammatory drugs and advised regular exercise to slow down the progression of disease.4,5 For RA, glucocorticoids and disease-modifying anti-rheumatic drugs are used to alleviate the signs of the disease.6 Clinical studies demonstrate that Dexmedetomidine (DEX) impedes inflammation and tissue impairment of the joints.7 DEX is receiving lot of attention as drug of choice.8 Al-Metwalli and colleagues evaluated the prospective pain relief influence of DEX when given through intra articular route and concluded that DEX enhanced post-operative analgesia.9
The drugs used in treatment of arthritis are usually given by oral, subcutaneous, and intramuscular routes. Any drug, when administered through these routes, fails to reach sufficient concentration in synovial joints as these joints have a restricted blood supply. Higher dose of the drug therefore has to be administered which in turn leads to severe systemic adverse responses, such as renal and liver damage, bone marrow toxicity and gastrointestinal complications. Intra Articular (IA) injections of drugs offer an advantage of producing required drug concentration at the target site avoiding undue exposure to other organs. However, administration of drugs by IA route provides insufficient assistance. Small molecules cannot be retained for more than 4 hr in the joint as these are susceptible to clearance by the synovial vasculature and rapid draining caused by the lymphatic system.10
Joint clearance can be delayed by increasing the hydrophobicity of drug molecules using chemical modification.11 Formulating drug with polysorbate 80 and the like excipients can delay their joint clearance. Kenalog-40®, a FDA approved IA drug product, contains dispersion of triamcinolone acetate in carboxymethylcellulose sodium and polysorbate 80, and releases drug over several weeks after single IA injection.12 Encapsulating drugs in rationally engineered drug delivery systems like microparticles is by far the most investigated approach for reducing the joint clearance. Joint residence time can be extended by particulate systems that do not cross the ‘leaky’ synovium and can resist the trans-synovial flux.13 Microparticles of size more than 10 microns form depot in the synovial cavity.14 Zilretta®, consists of polylactic-co-glycolic acid particles entraping triamcinolone acetonide, releases drug over a period of 12 weeks after single injection.15
Lipospheres (LPS) dispersion contains water insoluble, spherical lipidic particles of 0.2 to 100 microns. They contain a solid core formed by hydrophobic triglycerides and phospholipid and the drug embedded therein. A single bolus injection of antibiotics and anti-inflammatory agents could sustain the drug release for 3 to 5 days using lipospheres.16 Composition of lipospheres is such that it drug tends to remain stable in the honey comb architecture leading to sustained drug release, the system allows high payload of drugs and the components have minimal toxicity.17 Delivery system based on lipospheres for DEX using intraarticular administration is not reported. Hence current study was undertaken to formulate Dexmedetomidine Lipospheres (DEX-LPS) containing dispersion for delivery through intraarticular route releasing drug over a period of one week.
The design of lipospheres that stay in synovial cavity for 7 days necessitates multi-objective optimization. It is desirable that the lipospheres have a particular particle size and decent amount of entrapped drug, which releases slowly over a period of 1 week. The production of the lipospheres involves several materials and processing steps and associated deviations, which might affect the final product quality attributes. ICH Q9 suggests that the risk involved in product development should be ranked during assessment. The procedure for risk assessment requires the process to be broken down in to smallest units and grasp the factors that might be associated with risk. A relative risk score is derived for these factors and risk associated with each factor is ranked. The factors can then be filtered based on the cut-offs that have been established based on formulation objectives. Statistical tools like Pareto chart can support effective data assessment and decision making with regards to critical material attributes and process parameters.18 Risk assessment was done for factors; critical factors were identified and Box-Behnken design was applied to generate experimental batches for these factors. The data, obtained in the experiments, was treated using response surface methodology. Optimization of predictors to get desirable values for the critical quality attributes was done. The optimum product was thoroughly characterized.
MATERIALS AND METHODS
Dexmedetomidine (DEX) was received from Strem Chemicals (USA), Tricaprylin, dierucoyl phosphatidylcholine (DEPC) and 1,2-Dipalmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt) (DPPG) were acquired from Lipoid GmbH, Germany. Cholesterol was obtained from Avanti Polar Lipids (Alabaster, AL, USA) and, acetonitrile and glucuronate were procured from Merck KGaA, USA. Dichloromethane and high-performance liquid chromatography grade methanol were purchased from Rankem-Avantor, India. All the reagents used in the experiment were of analytical grade. All through the experiments, double distilled water was used.
HPLC Determination of DEX
The concentration of DEX was determined using high-performance liquid chromatography (Waters, USA) coupled to UV/VIS detector operating at 220 nm. Zorbax RX-C8 (150 x 4.6) mm column was used; the mobile phase was a mixture of water and acetonitrile at 70:30 v/v. The column was eluted at a flow rate of 1.5 mL/min at 40°C, and DEX was eluted at 30 min. The sample injection volume was 100 μL.
Preparation of DEX lipospheres
Dispersion of DEX-LPS was prepared using the double emulsion technique. Primary w/o emulsion containing DEX in the aqueous phase and lipids in dichloromethane was prepared using a high shear homogenizer (Polytron, KInematica AG). Double emulsion was then prepared using an inline mixer (Silversion, L5M-A, USA). The ratio of primary emulsion to secondary aqueous phase was kept at 1:10 during preparation of the double emulsion. Solvent was removed from the so formed double emulsion using combination of dilution with secondary aqueous phase and nitrogen flushing. In the final step, the dispersion was centrifuged to obtain a high lipocrit value and clear supernant.
Quality Target Product Profile (QTPP)
Quality target product profile of dispersion containing DEX-LPS was defined taking into consideration the safety and efficacy aspects of the product. (Table 1) QTPP enlisted the quality targets and their respective merits for inclusion in the list. Critical Quality Attributes (CQA) are those characteristics of the product that should fall within a specified range to ensure the quality of the product. CQAs for the final product have also been identified in the QTPP. The level of criticality of the attribute is based on the harm that may be caused to the patient, should the values for these attributes fall outside the set range. The preparation of lipospheres depends on multiple process parameters and attributes of the materials used. The severity and occurrence of deviations associated with these process parameters and material attributes was graded. (Table 2).
Box-Behnken Design (BBD)
In this study, Box-Behnken design was used to examine the effects of three independent variables cholesterol (X1), triglyceride (X2), and speed of homogenization (X3), on ‘critical to quality’ attributes of the product. The predictor variables were studied at three different levels i.e. low (-1), middle (0), and high (+1) levels. Cholesterol was used at 5, 6, and 7 mg/mL for the respective levels, triglyceride was used at 2, 3, and 4 mg/mL, and the speed of homogenization was 12,000, 14000, and 16000 RPM. 15 experimental runs were randomly generated.19 The Particle Size of the lipospheres (PS), Percentage Entrapment (EE), and amount of drug release after 4 hr (DR4h) and after 5 days (DR120h) were studied as the response variables for each experimental run. (Table 3) Response Surface Methodology (RSM) allows the optimization of process and/or composition and helps delineate the main effects, interaction effects or the quadratic effect of the predictors on the response variables.20
The relation between factor (Xi) and response (Yi) is given by a second order model (Eq. 1):
where X1, X2 and X3 are the predictors affecting the response variable Y’s; β0, βi (i = 1, 2, 3), βii (i = 1, 2, 3), and β ij (i = 1, 2, 3; j = 2, 3) are the regression coefficients for the intercept, linear, quadratic and two-way interaction terms, respectively.
The model was reduced to first order model (Eq. 2) for some responses based on the significance value in “anova” function in Rstudio.
| QTPP Element | Target | Justifications |
|---|---|---|
| Dosage form | Lipospheres | Honey-comb like structure and sub-micron size helps in retention and prolonged release. |
| Dosage type | Prolonged release | Prolonged release for one week reduces the frequency of administration making the system more patient-friendly. |
| Route of administration | Intra-articular administration | Localized administration reduces the general toxicity; allows accumulation at the site of action. |
| Therapeutic indication | Analgesic action | Dex has significant analgesic effects in neuropathic pain. |
| Site of activity | Locally in the joint | Local administration leads to dose and side effect reduction compared to systematic administration. |
| Stability | At least 6 months | Stability for at least 6 months is essential for effective shelf life. |
| Container-closure system | Prefilled syringe | Required to achieve the target shelf life and to ensure sterility and integrity. |
| Finished product quality attributes | ||
| Particle Size Distribution (PSD)* | >15 μm | This size is important for retention in the synovial cavity. Size smaller than this might cause the lipospheres to be engulfed and larger would be difficult to inject. |
| % Drug entrapped* | Max possible achieved | Low values of entrapment may lead to increased drug losses. |
| Assay | 100.0% of label claim | Drug variability in assay will affect safety and efficacy. |
| Residual solvents | Dichloromethane not more than 600 PPM | The limit of organic solvents is important for drug product safety of the product. |
| Drug release at the end of 4 hr* | Not more than 20% | It is important that the formulation does not release more than 20% in the first 4 hr else there will be lesser amount of release in the terminal part of week. |
| Drug release at the end of 5 days* | Not less than 70% | Drug release more than 70% indicates that there will be complete release of drug from the formulation and sustained release through the week. |
Particle Size (PS)
The PS of DEX-LPS was measured using a Zetasizer (NanoZS, Malvern, UK) at 25 ± 0.1°C. Angle of scatter was set at 173° to measure the intensity of the scattered light. The DEX-LPS dispersion was suitably diluted and sonicated prior to analysis. The z-average value was recorded.21
Percentage Entrapment (EE)
1 mL Dex-LPS was mixed with 0.5 mL of 0.1% protamine sulphate solution and 4.5 mL of 0.9% NaCl solution and subjected to centrifugation for 1 min at 1000 RPM. 1 mL of supernant was suitably diluted with methanol and subjected to analysis to determine the amount of free drug. In another set up DEX-LPS dispersion was diluted with methanol to rupture the liposphere structure and the total amount of drug present in the formulation was determined by analysing the clear liquid. Entrapped drug was calculated as the difference of total drug and free drug. Percentage Entrapment was calculated as the product of the ratio of entrapped drug to the total drug and 100.
In vitro Drug Release Study
Drug release from DEX-LPS was determined using dialysis bags (Float-A-Lyzer G2, 100 kD, Merck) using a Bottle Rotating dissolution apparatus (Electrolab, I) using 1 mL sample, 80 mL dissolution medium and 12 rotations per min settings. Phosphate buffer saline (pH 7.4) containing 0.02% sodium azide served as the dissolution medium. Temperature was maintained at 37±2°C. Samples were siphoned at predetermined time points and equal amount of fresh dissolution medium was added. Samples were analysed using HPLC, the values obtained are the average of the values obtained after performing the same procedure thrice.
Characterization of the DEX-LPS dispersion
The dispersion was evaluated for pH (Thermo Scientific™ Orion Star™ A121), viscosity (30 rpm, and spindle CP-50-1 using Rheometer (Anton PAAR, M103)), osmolality (Model 3250, Advanced Instruments, Fisher Scientific, USA) and zeta potential (Zeta Seizer, Malvern Instruments, UK). The total drug content, cholesterol content,22 Packed Particle Volume (PPV),23 residual solvent (dichloromethane) were also analysed.
Stability
DEX-LPS were stored at 2-8°C and 25°C ± 2 and 60% ±5% relative humidity in its proposed final package (prefilled syringe). The parameters studied were change in pH, particle size, drug content, % free drug, cholesterol, DR4h and DR120h at initial time point and at the end of 3 and 6 months.
RESULTS
Risk assessment
Primary emulsion was prepared using a high-speed homogenizer. The emulsion was prepared using Tricaprylin, DPPG, DEPC, and cholesterol in dichloromethane as the lipid phase and Dex in the aqueous phase. The pH of the aqueous phase was adjusted to 3.5 using glucuronic acid. The potential material attributes that affected the CQAs of the product were the ratio of drug to lipid and that of aqueous to the oil phase, the concentration of cholesterol, and triglyceride. Process parameters included speed, time, and temperature of homogenization. The determination of risk is related to the severity of harm associated with deviation and the frequency of occurrence for process parameters and material attributes. Severity and occurrence were graded on a three-level scale of high, medium, and low and were assigned values of 9, 3, and 1 respectively. A concise matrix relating the level of severity and Critical Material Attributes (CMAs) and Critical Process Parameters (CPPs) was prepared. (Table 2)24 The severity score for the material attributes and process parameters was calculated. (Figure 1a) The relative occurrence and relative severity of the CMAs and CPPs are depicted in Figure b, and the factors with the highest occurrence and severity rate were subjected to further investigation to identify the optimal value of each examined variable using Box-Behnken design. DPPG and DEPC were used in a concentration of 1mg/mL and 10 mg/mL respectively. The ratio of the aqueous phase to the lipid phase was optimized to 50:50 so as to get an emulsion having low viscosity. Drug concentration (0.5 mg/mL) was kept constant in all formulations. The temperature was controlled between 15 to 20°C and the mixing time was 10 min. The amount of cholesterol and triglyceride and the homogenization speed were found to be high-risk factors and were optimized to identify the region of operation using the design of experiments.
| CQAs | Critical Process Parameters | Critical Material Attributes | |||||
|---|---|---|---|---|---|---|---|
| Temperature | Speed | Time | D/L Ratio | Cholesterol | Triglyceride | Aq/Oil Vol Ratio | |
| PS | Medium | High | Low | Low | Medium | Medium | Medium |
| % E | Medium | High | Low | High | Medium | High | Medium |
| BR | Medium | Medium | Low | Medium | High | High | Low |
| DR120h | Medium | Medium | Low | Medium | High | High | Low |
| Batch | X1 | X2 | X3 | PS | EE | DR4h | DR120h | d1 | d2 | d3 | d4 | D |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | -1 | -1 | 0 | 18.20 | 93.20 | 15.30 | 89.90 | 0.41 | 0.09 | 0.20 | 0.00 | 0.00 |
| 2 | -1 | 1 | 0 | 17.70 | 96.40 | 12.10 | 69.60 | 0.35 | 0.62 | 0.54 | 0.00 | 0.00 |
| 3 | 1 | -1 | 0 | 18.90 | 97.10 | 11.50 | 68.30 | 0.43 | 0.48 | 0.42 | 1.00 | 0.44 |
| 4 | 1 | 1 | 0 | 16.90 | 94.30 | 07.30 | 58.90 | 0.36 | 0.08 | 0.76 | 0.00 | 0.00 |
| 5 | -1 | 0 | -1 | 21.10 | 96.20 | 12.30 | 82.40 | 0.73 | 0.38 | 0.35 | 0.00 | 0.00 |
| 6 | -1 | 0 | 1 | 16.20 | 97.30 | 10.60 | 86.70 | 0.03 | 0.50 | 0.38 | 0.00 | 0.00 |
| 7 | 1 | 0 | 1 | 15.50 | 95.10 | 13.30 | 70.50 | 0.04 | 0.38 | 0.60 | 0.00 | 0.00 |
| 8 | 0 | -1 | -1 | 24.60 | 97.30 | 12.20 | 79.90 | 0.77 | 0.62 | 0.29 | 0.00 | 0.00 |
| 9 | 0 | -1 | 1 | 15.10 | 98.20 | 11.50 | 83.50 | 0.07 | 0.81 | 0.32 | 0.00 | 0.00 |
| 10 | 0 | 1 | -1 | 22.50 | 98.70 | 10.40 | 69.30 | 0.71 | 0.80 | 0.63 | 0.00 | 0.00 |
| 11 | 0 | 1 | 1 | 17.20 | 98.10 | 09.80 | 71.60 | 0.01 | 0.76 | 0.66 | 0.00 | 0.00 |
| 12 | 0 | 0 | 0 | 19.20 | 99.30 | 11.50 | 73.60 | 0.39 | 0.92 | 0.48 | 1.00 | 0.55 |
| 13 | 0 | 0 | 0 | 18.80 | 99.70 | 11.30 | 79.20 | 0.39 | 0.92 | 0.48 | 1.00 | 0.55 |
| 14 | 0 | 0 | 0 | 17.50 | 98.60 | 12.10 | 70.90 | 0.39 | 0.92 | 0.48 | 1.00 | 0.55 |
| 15 | 1 | 0 | -1 | 22.40 | 94.60 | 11.10 | 67.60 | 0.74 | 0.35 | 0.58 | 0.00 | 0.00 |
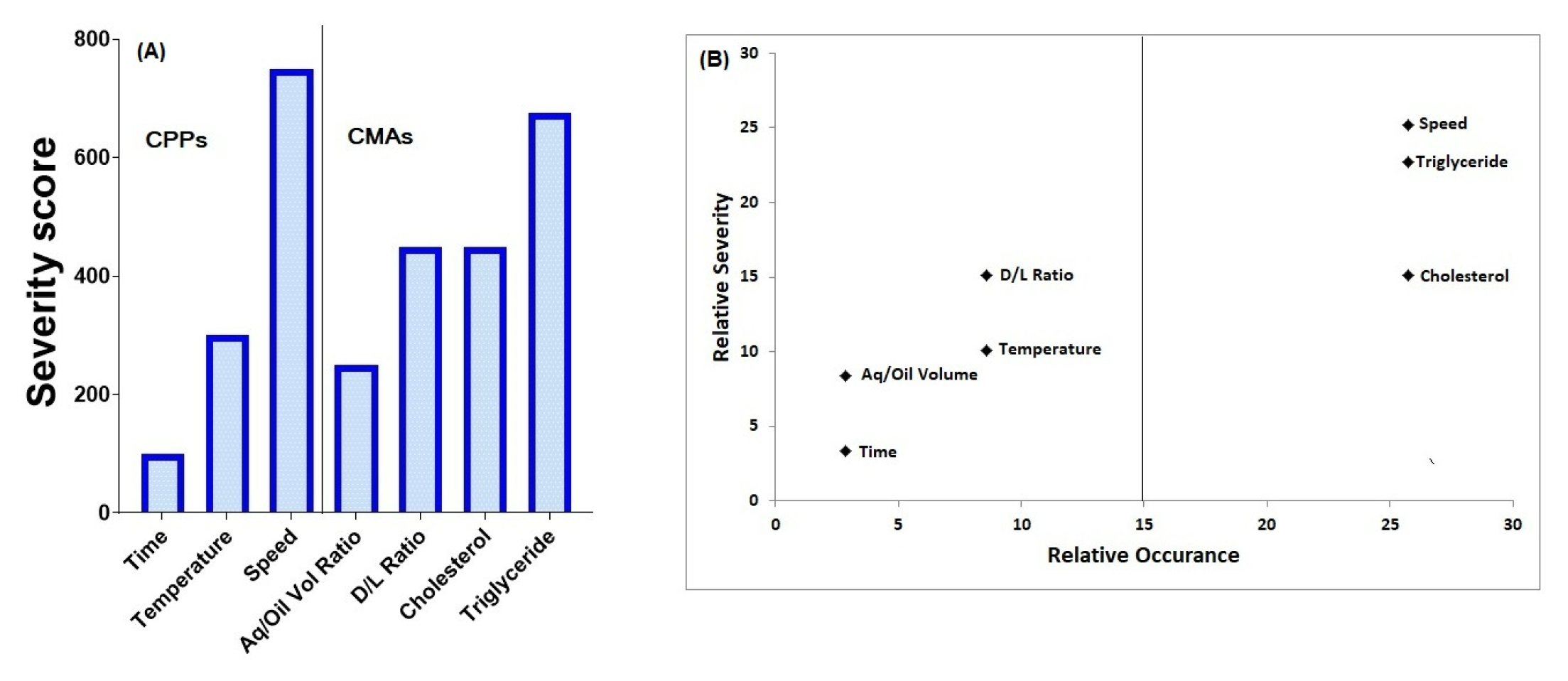
Figure 1.
Relative Severity and Relative Uncertainity of Factors in DEX-LPS formulation.
Formation and separation of lipospheres
The primary emulsion was diluted with a secondary aqueous phase containing glucose (50 mg/mL) and lysine (15 mg/mL) to adjust the osmolality and pH to 9.8 (isoelectric point of lysine) in the Inline mixer. At this pH DPPG lipid becomes fully deprotonated and the pH is above the pKa value of Dexmedtomidine. Thus, during in process steps at pH 9.8, lipospheres remained fully charged and agglomeration phenomenon was least. Moreover, maximum API got entarpped in the aqueous phase of lipospheres. The flow rate of the primary emulsion was optimized at 60 mL/ min and that for the secondary aqueous phase was 700 mL/ min for the Inline mixer. Dichloromethane was removed using dilution with the secondary aqueous phase (1:20 times) and nitrogen flushing for 30 sec at 25 PSI. The obtained dispersion was centrifuged at 2500 rpm for 10 min at 2-8°C. Lipospheres were resuspended in buffer to get a pH of 5.5 to 7.5.
Mathematical Modelling
The data obtained by performing experiments in BBD were subjected to regression analysis to generate mathematical models for all the response variables i.e. particle size, percentage entrapment, drug release at the end of 4 hr and drug release at the end of 120 hr.
Particle Size (PS)
First order model for PS
When comparing the second order and first order model using “anova” function in r the p value was more than 0.11 indicating that first order model is enough for capturing the data. The equation shows that the particle size is predominantly dependent on speed of homogenization. There is a relationship between the predictors and response (PS) as indicated by a p value <0.05. Rsquare value close to 1 indicates that model parameters explain the variation in PS.
The coefficient values for speed are high and have negative sign indicating that particle size decreases with increase in speed.
Percentage Entrapment (EE)
The first order and second order model comparison (p = 0.0185) indicated that retaining all the terms was important to use the model for prediction. The magnitude of the coefficient of interaction between cholesterol and triglyceride, and quadratic term of cholesterol was high indicating the importance of the composition in deciding the drug entrapment. Changes in entrapment with speed might be confounded with effect of speed on the particle size. As the speed of homogenization changes the particle size changes which in turn might affect the amount of drug entrapped.
Drug Release at the end of 4 hr (DR4h)
The drug release was measured at the end of 4 hr to find out the tendency of the lipospheres to give the burst release (DR4h). Since the lipospheres were expected to release the drug over a period of 7 days, the lipospheres should not release unduly high amount of drug in early hours after administration to the site.
The burst release was found to be influenced by speed to a certain degree which can be explained by the fact that speed influences in the particle size and surface of lipospheres.
Drug Release at the end of 120 hr (DR120h)
The equation for DR120h highlighted the importance of composition of lipospheres in determining drug release. Quadratic term for speed also was important to determining the drug release at the end of 5 days.
The first order model was found to be sufficient to capture the data and hence was used for optimization.
Design Space and Numerical Optimization
These equations were used to plot contours where the predictors were put on X and Y axis and the contours were formed for the different values of responses (Figure 2). The contour plot is a two dimensional plot and can represent only two variables at a time. Our experimental design had three variables so three plots were required to visualize how all possible combinations of predictors affected the responses. Figure (A) indicates that the speed is more important predictor as compared to cholesterol. The contours are parallel to the X-axis while there is decrease in particle size as the speed on Y-axis increases. Figure (B) implies that the relationship between the entrapment and predictors is not linear and there is importance of the quadratic effect in deciding the magnitude of entrapment. Figure (C) and 2(D) indicate that drug release reduces as the amount of cholesterol and triglyceride increases but the drug release is less affected by speed as indicate by parallel lines on the vertical axis.
DEX-LPS was expected to be amenable for administration using needle in to the joint, stay there for 1 week and release drug over this period. Specifications were set for individual responses, to satisfy the characteristics desired from the product. Particle size was set to more 15 microns, drug entrapment was set to more than 90% and drug release at 4 hr and 5 days was set to less than 20% and 70 to 75% respectively. Overlay plot were generated using models generated by RSM (Figure 3a) Different combinations of predictors had different levels of desirability in the desirability plot (Figure b), the area shaded in yellow is the area where the combinations of cholesterol and triglyceride will give the product with desired characteristics at medium level of speed (14000 RPM). The region obtained using models generated using RSM included the center point batch and hence it was carried forward for characterization.
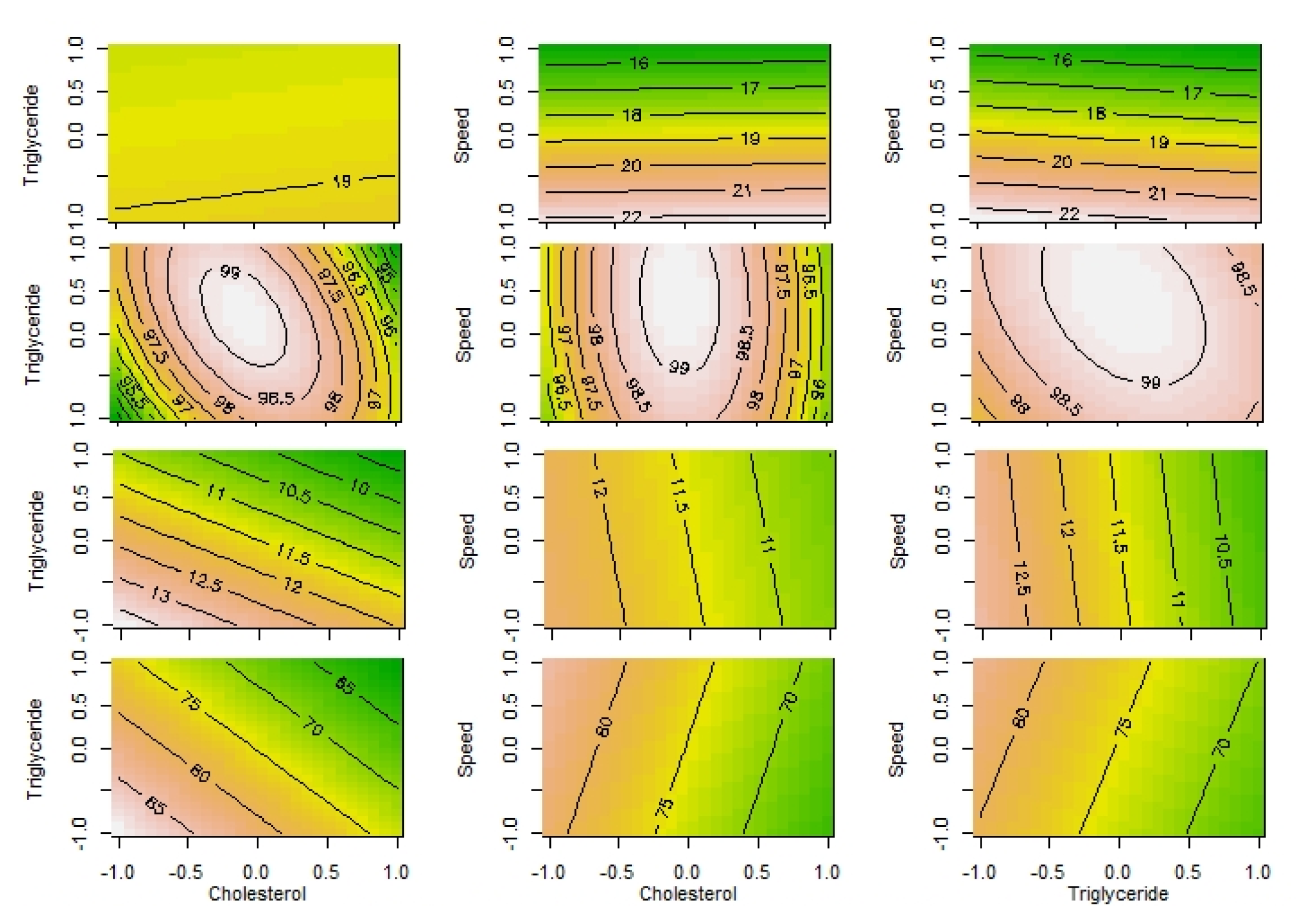
Figure 2.
Contour Plots: A) PS, B) EE, C) DR4h, D) DR120h.
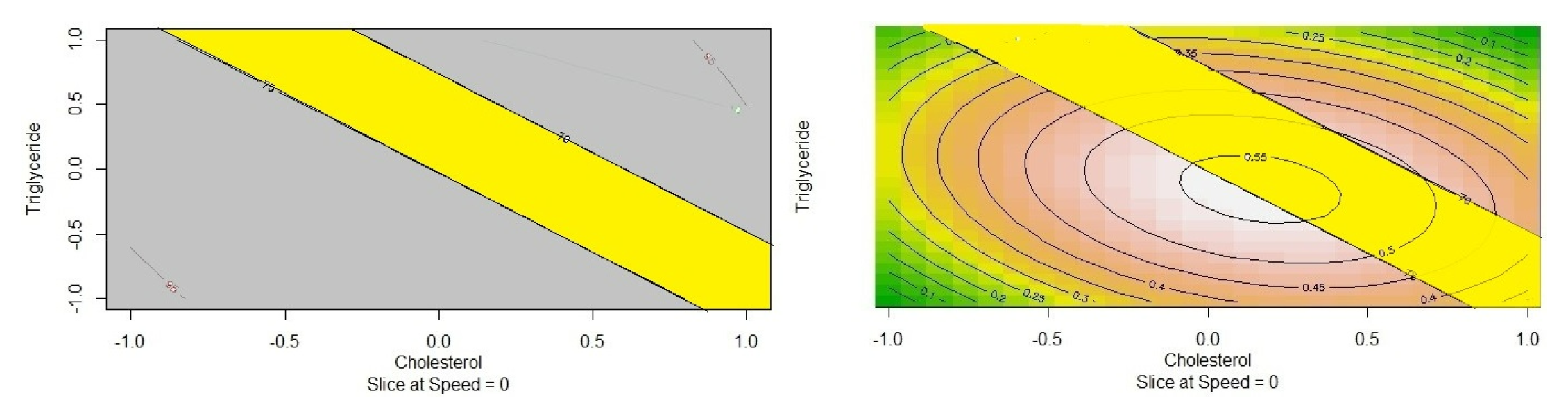
Figure 3.
a) Overlay Contour Plot, b) Desirability Plot.
Characterization of the center point batch
The DEX-LPS formed at the end of process is to be injected intraarticularly and so was expected to have certain characteristics that make it amenable to the route and provide drug release in a controlled fashion for a week. These include pH, osmolality, viscosity, drug loading and release. PPV value indicates the volume occupied by liposphers in the total dispersion and is characteristic to the product. (Table 4).
Stability Study
The product was filled in a prefilled syringe and tested for stability at 25°C and 60% RH and at 2-8°C. The characteristic parameters of the product were evaluated over a period of 6 months. DEX-LPS was found to be stable at 2-8°C during the period of stability testing (Table 4).
| Parameter | Acceptance Criteria | Optimized Batch | 25°C/60% RH 3 Months | 2-8°C 6 Months |
|---|---|---|---|---|
| Description | White Suspension | White Suspension | White Suspension | White Suspension |
| pH | 5.5 to 7.5 | 6.67 | 5.56 | 6.53 |
| Osmolality (mOsm) | 275 to 325 | 283 | 297 | 285 |
| Particle size [D (50)] μ | 15 to 20 | 19.2 | 18.4 | 18.1 |
| Zeta potential mV | -5 to -10 | -9.7 | -3.27 | -8.22 |
| Assay (%) | 90 to 100 | 98.1 | 96.9 | 96.6 |
| Free Drug (%) | NMT 10 | 2.2 | 68.9 | 8.1 |
| Cholesterol (%) | 90 to 100 | 99.7% | 90.6 | 95.6 |
| DR4h (%) | NMT 15% | 11.3 | 74.3 | 14.7 |
| DR120h (%) | NLT 70% | 69.6 | 91/9 | 79.2 |
DISCUSSION
The final product CQAs included the particle size of the lipospheres, the percentage of drug entrapped in the liposphere, and the drug release from the DEX-LPS. The particle size was considered the most critical quality attribute because of the severity of harm it can cause to the product characteristics if it deviates from the chosen range. The particle size influences the injectability of the final product, release rate, and clearance of the drug from the targeted cavity and it may be linked to the amount of drug entrapped in LPS. The percentage of drug entrapped was defined as the second CQA. Any process used in the preparation of particulate systems is expected to give the highest entrapment and less amount of free drug in the dispersion. The amount of drug entrapped may depend on the particle size yet it may be the deciding factor when it comes to drug release. The amount of drug released from the product with respect to time is dependent on the amount of drug entrapped in the matrix structure of the liposphere. Percentage drug entrapped therefore was assigned to a high level of criticality. The drug release over a period of time after administration of the intraarticular injection is an important attribute of the product. The product is expected to release the drug for one week. The lipospheres are monolithic systems, considering the pattern of drug entrapment, and it is inevitable that there will be some amount of burst release. The product should have minimum burst release as well as zero order release over a period of one week. For a product that releases drug in a zero-order fashion, the amount of drug released at the end of 5 days should be close to 70%. The drug release from the product is dependent on the particle size and percentage of drug entrapped; hence the drug release-related attributes were assigned a medium level of criticality. Product assay (Total Amount of Drug) and residual solvent are important aspects but they were not included in the list of critical attributes based on the experience with the system.
Risk assessment for each process parameter and material attribute has to be done after thorough understanding of the whole process. The primary emulsion is formed by mixing of aqueous and oil phases and there are changes in the viscosity of the system as the emulsion is formed this may affect the speed at which the contents are homogenized and so the occurrence level of changes in speed is kept highest. The speed of the homogenization has the highest effect on particle size and drug entrapment so it has been assigned the highest level of severity too. The time for stirring can be better controlled by human intervention and hence it has been assigned the least level of occurrence. The process parameters are more likely to affect the particle size and drug entrapment than the drug release characteristics of the formed lipospheres in the dispersion and have been accordingly graded on the severity scale. The material attributes, though prima facie appear to be directly controlled by formulation scientists, are profoundly dependent on the process parameters. The temperature and speed of homogenization may directly affect the quantity of the material available for emulsion formation; the associated deviation of material attributes, therefore, has been put on the highest level on the occurrence scale. The deviations in the quantities of the material has a direct bearing on the drug release characteristics and so the severity associated with lipid components of the formulation is highest.
Quality by Design (QbD) strategies require that the relative importance of the CMAs and CPPs in determining CQAs should be assessed.25 The mathematical equations generated using regression analyses for a well-designed experimentation provide important insights about the relationship between predictors and responses. The relation between particle size and homogenization speed was more pronounced with the speed at which homogenization was done. Similar findings have been reported in literature and this may be due to the mechanical impact the speed can produce once the lipospheres are formed.26 Entrapment of the drug in the lipid matrix is bound to depend on the composition of the lipospheres. It was found that the concentration of cholesterol was more important for entrapment than the triglyceride followed by speed. Increase in entrapment with increase in cholesterol level might be related to the solubility of drug in cholesterol.27 The burst release depends on the drug present on the surface of the lipospheres. Homogenization might cause abrasion on the surface of lipospheres and therefore its speed might determine the adsorption of drug on the boundary surface of DEX-LPS. The more is the drug on the surface the higher is the burst release. The drug release after 5 days was dependent on composition of the lipospheres. The way the drug is entrapped inside the matrix will be more dependent on the tortuosity and the strength of matrix and therefore dependence of release on the composition seems to be logical extension.
The center point batch has been identified as the batch that satisfied all the constraints that were set in the QTPP. Additionally the product was expected to have certain pH and osmolality to avoid irritation.28 The product is an aqueous dispersion and it is reflected by certain conductivity. Viscosity of the dispersion has to be controlled, for it to be syringable and also because it affects the rate of drug diffusion through the lipospheres.29 The lipospheres in the dispersion should have certain zeta potential to avoid aggregation. Acceptable ranges for % free drug, cholesterol, assay of drug in the product, burst release and release at the end of 120 hr were set and the parameters for product obtained from center point settings fell in this range.
The DEX-LPS dispersion was packed in prefilled syringe and tested for stability. The lipospheres are expected to be sensitive to temperature and it was found that they were more stable at 2-8°C and so this temperature was proposed for storage.
CONCLUSION
Dispersion of DEX containing lipospheres was prepared using a double emulsion technique. Experiments were conducted based on the settings in Box-Behnken Design. The results obtained were subjected to data treatment using response surface methodology. The composition of lipids and the process parameters that satisfied all the constraints on the responses was identified using overlay plot which happened to be the center point batch of the design. The product obtained from the settings of the center point batch was subjected to thorough characterization and stability study and the product was found to be stable at 2-8°C for 6 months.
Cite this article
Gorajiya A, Lalwani A. The Amalgamation of Risk Assessment Using Quality by Design Principles and Prediction of Regression Models in the Development of Dexmedetomidine-loaded Lipospheres for Intraarticular Administration. J Young Pharm. 2023;15(2):298-307.
ACKNOWLEDGEMENT
The authors are grateful to Amneal Pharmaceuticals for facilitating the research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- Hong Y-K, Javaregowda PK, Lee S-K, Lee S-R, Chang K-T, Hong Y-G, et al. Morphological changes of bones and joints with rheumatoid arthritis and osteoarthritis. Reproductive and dDevelopmental bBiology. 2011;35(2):143-9. [Google Scholar]
- Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390-8. PMIDhttps://www.ncbi.nlm.nih.gov/pub med/2264113822641138
[CrossRef] | [Google Scholar] - Chien SY, Huang CY, Tsai CH, Wang SW, Lin YM, Tang CH, et al. Interleukin-1β induces fibroblast growth factor 2 expression and subsequently promotes endothelial progenitor cell angiogenesis in chondrocytes. Clin Sci (Lond). 2016;130(9):667-81. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/2681154026811540
[CrossRef] | [Google Scholar] - Susko AM, Fitzgerald GK. The pain-relieving qualities of exercise in knee osteoarthritis. Open Access Rheumatol. 2013;5:81-91. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/2779002727790027
[CrossRef] | [Google Scholar] - Mobasheri A, van Spil WE, Budd E, Uzieliene I, Bernotiene E, Bay-Jensen AC, et al. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr Opin Rheumatol. 2019;31(1):80-9. PMID//www.ncbi.nlm.nih.gov/pubmed/3046154430461544
[CrossRef] | [Google Scholar] - Kohler BM, Günther J, Kaudewitz D, Lorenz HM. Current Ttherapeutic Ooptions in the Ttreatment of Rrheumatoid Aarthritis. J Clin Med. 2019;8(7) PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/3126178531261785
[CrossRef] | [Google Scholar] - Said Ahmed MA, Saweeres ESB, Abdelkader NA, Abdelmajeed SF, Fares AR. Improved pain and function in knee osteoarthritis with dexamethasone phonophoresis: A randomized controlled trial. Indian J Orthop. 2019;53(6):700-7. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/3167316931673169
[CrossRef] | [Google Scholar] - Akęa B, Ankay Yilbaş A, Üzümcügil F, Büyükakkuş B, Bahador Zırh E, Zeybek D, et al. How does intraarticular dexmedetomidine injection effect articular cartilage and synovium? An animal study. BMC Anesthesiology. 2020;20(1):237 PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/3294300532943005
[CrossRef] | [Google Scholar] - Al-Metwalli RR, Mowafi HA, Ismail SA, Siddiqui AK, Al-Ghamdi AM, Shafi MA, et al. Effect of intra-articular dexmedetomidine on postoperative analgesia after arthroscopic knee surgery. British J of Anaesth. 2008;101(3):395-9. PMID//www.ncbi.nlm.nih.gov/pubmed/1856767518567675
[CrossRef] | [Google Scholar] - Rai MF, Pham CT. Intra-articular drug delivery systems for joint diseases. Current oOpinion in pPharmacology. 2018;40:67-73. PMID//www.ncbi.nlm.nih.gov/pubmed/ 2962533229625332
[CrossRef] | [Google Scholar] - Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGpegylation. Adv Drug Deliv Rev. 2002;54(4):459-76. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/1205270912052709
[CrossRef] | [Google Scholar] - Shah A, Mak D, Davies AM, James SL, Botchu R. Musculoskeletal Corticosteroid Administration:. Musculoskeletal Corticosteroid Administration: Current Cconcepts. Can Assoc Radiol J. 2019;70(1):29-36. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/3069155930691559
[CrossRef] | [Google Scholar] - Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Advanced dDrug dDelivery rReviews. 2006;58(2):226-42. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/1657426716574267
[CrossRef] | [Google Scholar] - Pradal J, Maudens P, Gabay C, Seemayer CA, Jordan O, Alleemann E, et al. Effect of particle size on the biodistribution of nano-and microparticles following intra-articular injection in mice. Int J Pharm. 2016;498(1-2):119-29. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/2668572426685724
[CrossRef] | [Google Scholar] - B Sánchez BA, Calpena AC, Soriano JL, Gálvez P, Clares BB. Anti-inflammatory nanomedicines: what does the future hold?. Nanomedicine. 2020;15(14):1357-60. [CrossRef] | [Google Scholar]
- Swain S, Beg SM, Babu SMS. Liposheres as a novel carrier for lipid based drug delivery: current and future directions. Recent Patents on Drug Delivery & Formulation. 2016;10(1):59-71. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/2642344326423443
[CrossRef] | [Google Scholar] - Patra CN. Liposphere: A versatile controlled release carrier for hydrophobic drugs. J Pharmaceut Drug Develop. 2013;1(2) [CrossRef] | [Google Scholar]
- Quality Risk Management [ICH Q9 Quality risk management]. Available from:https://www.fda.gov/media/71543/download
- Ferreira N, Viana T, Henriques B, Tavares DS, Jacinto J, Colónia J, et al. Application of response surface methodology and box-behnken design for the optimization of mercury removal by Ulva sp. Journal of Hazardous Materials. 2023;445:130405 PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/3643719236437192
[CrossRef] | [Google Scholar] - Pashaei H, Ghaemi A, Nasiri M, Karami B. Experimental modeling and optimization of CO2 absorption into piperazine solutions using RSM-CCD methodology. ACS Omega. 2020;5(15):8432-48. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/3233740532337405
[CrossRef] | [Google Scholar] - Epstein H, Gutman D, Cohen-Sela E, Haber E, Elmalak O, Koroukhov N, et al. Preparation of alendronate liposomes for enhanced stability and bioactivity: in vitro, in vivo characterization. AapsAPS j.J. 2008;10(4):505-15. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/1893707118937071
[CrossRef] | [Google Scholar] - Gorajiya A, Lalwani A. Leveraging the Eexploratory and Ppredictive Ccapabilities of Ddesign of Eexperiments in Ddevelopment of Iintraarticular Iinjection of Iimatinib Mmesylate Ccontaining Llipospheres. AAPS PharmSciTech. 2022;23(7):275 PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/3620760436207604
[CrossRef] | [Google Scholar] - Katre NV, Asherman J, Schaefer H, Hora M. Multivesicular liposome (DepoFoam) technology for the sustained delivery of insulin-like growth factor-I (IGF-I). J Pharm Sci. 1998;87(11):1341-6. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/98114879811487
[CrossRef] | [Google Scholar] - Pallagi E, Bĺró T, Fekete H, Aigner Z, Csóka I. Implementation of Patient Reported Outcome Measures (PROMs) in QbD based formulation development in ophthalmology. Acta Pharmaceutica Hungarica. 2020;90(4):192-204. [CrossRef] | [Google Scholar]
- Yeung E, Ramsey P. Optimization of a Cconventional Gglycosylation Aanalytical Mmethod Uusing Mmachine Llearning and Eexperimental Ddesign. [CrossRef] | [Google Scholar]
- Jenita JJL, Tibrewal R, Rathore SS, Manjula D, Barnabas W, Mahesh AR, et al. Formulation and optimization of albumin nanoparticles loaded ivabursadine hydrochloride using response surface design. Journal of Drug Delivery Science and Technology. 2021;63:102461 [CrossRef] | [Google Scholar]
- Mohammadi-Samani S, Zojaji S, Entezar-Almahdi E. Piroxicam loaded solid lipid nanoparticles for topical delivery: Ppreparation, characterization and in vitro permeation assessment. Journal of Drug Delivery Science and Technology. 2018;47:427-33. [CrossRef] | [Google Scholar]
- Ho MJ, Lee DR, Jung HJ, Song WH, Park JS, Kang MJ, et al. Formulation and analgesic effect of sodium hyaluronate and magnesium sulfate combination in rats following intra-articular injection. Bulletin of the Korean Chemical Society. 2017;38(5):538-43. [CrossRef] | [Google Scholar]
- Magri G, Selmin F, Cilurzo F, Fotaki N. Biorelevant release testing of biodegradable microspheres intended for intra-articular administration. European Journal of Pharmaceutics and Biopharmaceutics. 2019;139:115-22. PMIDhttps://www.ncbi.nlm.nih.gov/pubmed/3090577730905777
[CrossRef] | [Google Scholar]
