ABSTRACT
A fatal disorder named oral submucous fibrosis (OSMF) is marked by the oral submucosa’s progressive fibrosis. OSMF is distinguished by aberrant collagen deposition. In 1.5-15% of patients, it’s a precancerous condition that progresses to a malignant tumour. Although nutrient deficiencies and immunological procedures could perform a role in pathogenesis, epidemiological evidence suggests that betel nut quid (which contains guvacine, arecoline, guvacoline, arecaidine, and chavibetol) contains areca nut, slaked lime, tobacco, is a significant risk factor for OSMF. Submucous fibrosis, xerostomia, ulceration, a burning sensation, and a limited mobility of the mouth are among the symptoms. The patient’s quality of life is seriously affected by each of these components. The present article gives a broad overview of OSMF from a molecular viewpoint and describes what has been learned about its underlying causes, methods of diagnosis, and available treatments. Along with active therapy for OSMF, prophylaxis is essential, and this section gives a quick review of its treatment.
INTRODUCTION
The oral mucous membranes are a separate region of the body that is routinely exposed to a variety of effects, including heat, cold, mechanical irritations, chemicals, and pathogens. In response to these stressors, connective tissue and the epithelium components of the oral mucosal surface show acute and chronic reactive alterations.1 OSMF is one of the areca nut-induced reactions of the oral mucosal collagen.2 It is a chronic disease with a slow start and a prevalent potentially malignant disorder characterised by a juxtaepithelial inflammatory response as well as mucosal fibrosis.3 In 1956, Paymaster found one-third of the OSMF patients had slow- growing basal cell carcinoma, he realised the condition had precancerous characteristics. The deposition of thick collagen in fibrous tissue distinguishes OSMF.4 In ancient Indian texts, Sushruta described the condition as “VEDARI,” mentioning patients suffering from mouth constricting, a burning feeling, and agony.5 It is because of the nutritional, religious, or cultural practises that this disease predominates throughout the Indian subcontinent. OSMF is a long-term condition that affects the mouth. It is a cancerous condition in which 3–19% of OSMF patients develop oral squamous cell carcinoma (OSCC).6 The oral cavity, throat, and digestive system are all affected by OSMF. The consumption of betel nuts is regarded as the major cause of OSMF.7 Areca nuts are the areca palm seeds, which grows throughout most of the tropical pacific, including South Asia and Southeast parts of East Africa.8 It is a chronic, severe illness characterised by oral cavity juxtaepithelial fibrosis. It is considered a precancerous and perhaps cancerous condition.9 Pindborg and Sirsat’s most frequently recognised definition of the illness is that it is an insidious, chronic condition that affects any part of the buccal cavity and occasionally the throat.10 It is always related with inflammation of a juxtaepithelial followed by fibroelastic alterations of the epithelial atrophy and lamina propria. This causes tightness in the oral mucosa, which makes it difficult to eat and causes trismus, even if vesicle formation occurs before or along with it.11 OSMF causes scarring, precancerous lesions and tissue fibrosis in the buccal mucosa, it is fairly prevalent. Pathogenic features comprise persistent inflammation, local inflammation in the deep connective tissues or lamina propria, excessive collagen deposition underneath the oral mucosal epithelium, and muscle wasting.12 Some OSMF sufferers feel severe oral burning after eating spicy foods. Additional signs of OSMF are pain, changes in tone, taste disturbances, dry mouth, trismus, dysphagia, and limited mouth mobility.13 This condition greatly increases fatality and has a high carcinogenic transformation rate of 1.5–15%. OSMF is linked to diet, lifestyle, and culture, and its frequency varies depending on ethnicity and region. OSMF patients are most numerous in India, although Taiwan and other Asian nations are also affected by this disease. Due to the country’s large Indian community, there are a considerable range of OSMF sufferers in South Africa.14 WHO data indicates more than 5 million individuals are suffering from OSMF worldwide. Although it is the contrary in other countries, OSMF affects more women than males in India. The patients’ age range is from 20 to 40 years old.15 The proliferating cell nuclear antigen (PCNA) index is strongly correlated with the possibility of malignant transformation. In comparison to the typical buccal mucosa, the OSMF epithelium had a higher PCNA index. The expression levels of dysplastic OSMF and nondysplastic OSMF were considerably different. p34 (Cdc2), Cyclin B1, and p-survivin all causes impact on mitosis during the G2/M phase, which is critical for carcinogenesis.16 All of these molecules have been found to be more prominent in OSMF than in ordinary mucosa and their expression levels were significantly different between OSMF and OSCC. In the same manner as p53 stimulates cell proliferation and differentiation, P63 does as well. It serves as a proximal sign of cancer progression. Nuclear positivity for p63 continued to rise as OSMF progressed to OSCC. Therefore, p63 is a prognostic marker for the development and malignant potential of OSMF. Further cause of p53 inactivation is HPV infection. Cell cycle regulation is impaired by the inactivation of the tumour suppressor proteins p53 and retinoblastoma carried on by the high-risk HPV E6 and E7 oncoproteins. In precancerous lesions (including OSMF), the prevalence of any HPV type was shown to be higher compared to normal control samples. High-risk HPV strains are more common in OSCC lesions. This implies that whereas high-risk HPV types are common in OSCC and may contribute to its development progression, low-risk HPV types are associated to oral pre-cancerous lesions. OSMF has been staged and classified clinically, functionally, and histologically. Doctors employ specific staging methods to diagnose and treat clinical OSMF.17 Early OSMF is characterised by stomatitis and vesiculation; Severe OSMF is characterised by leukoplakia and erythroplakia, while intermediate OSMF is characterised by a marble-like surfacing with distinct fibroid bands. Maximum interincisal mouth openings in the functional OSMF classifying stages I-V range from 35 mm to 5 mm. Solid biopsies are essential for clinical diagnosis and treatment planning since OSMF can develop into OSCC. The quantity and distribution of collagen fibres, fibroblasts, blood vessels, and inflammatory cells are utilised to evaluate whether OSMF is in an early, moderate, or advanced stage in histological staging and classification. Additionally, mRNAs, non-coding RNAs, and proteins are used to evaluate and categorise OSMF. In recent times, liquid biopsies of serum as well as saliva have now been employed to enhance detecting performance of the equipment. In addition, bioinformatic analysis can be used to decrease the need for invasive or non-invasive diagnostic procedures, as well as to substitute surgical solid biopsies in the diagnosis of OSMF.18 OSMF can be caused by a various factors, such as autoimmune disease, Vitamin B, C, and iron insufficiency, including betel nut chewing, hot food consumption, human papillomavirus (HPV) infection, and also genetic abnormalities. According to epidemiological data, chewing betel nuts is also most significant risk factors for OSMF.19 In China, 62.3% of OSMF patients consume betel nuts. Smoking, tobacco chewing, and alcohol consumption, all have been linked to an increased risk of OSMF. A Taiwanese survey found that the vast majority of betel quid eaters either smoked tobacco (86%) or consume alcohol (74%). When betel nuts and tobacco are chewed together, the severity of OSMF produced is much higher.20 According to other studies, drinking alcohol plus eating betel nuts had a synergistic effect on OSMF induction. Oral cancer has long been assumed to be preceded by OSMF. According to previous research, Oral cancer was more likely to develop in 1.19 % of OSMF patients in China. 7.6% of all OSMF patients in India have oral cancer. Past research has linked the progression of oral cancer to the period of OSMF and the severity of its indications. Oral cancer is common 3–16 years following the first diagnosis of OSMF. Unfortunately, there are no effective therapies available in clinics for OSMF. The goal of this study is to sift through the available research on the aetiology, diagnosis, and therapy of OSMF in order to identify promising molecular preventive, diagnostic, and therapeutic methods. OSMF is a severe public health issue that affects global population who eat areca nuts and their derivatives. It is a complicated illness that causes substantial morbidity in patients and has a complicated pathophysiology that is still unknown after intensive investigation. In certain cases, several mechanisms interact to cause severe fibrosis, trismus, and malignant transformation. The hypothesis of local and systemic copper in OSMF pathogenesis now is believed to be the most feasible, although further research is required to truly comprehend the disease.21
Causes/Aetiology
Factors that cause OSMF are mainly categorised into two different categories: Local and systematic factors, which are as follows:
Local factors
Areca nut
Chillies
Systematic factors
Genetic Predisposition
Autoimmunity
Nutritional deficiency
According to epidemiological and in vitro experimental studies, the main etiological factor is chewing areca nuts. Originally OSMF was assumed to be idiopathic, later found to be multifactorial in nature. The capsaicin in chillies, zinc, iron, and vitamin deficits are suspected etiological agents for the condition. Oral submucous fibrosis has been linked to nutritional inadequacies, immunological processes, the usage of chillies, and betel nuts (Areca catechu) chewing. Malnutrition is a major issue for most people living in places where OSMF is common. Vitamin and iron deficiencies have been related to the development of OSMF. Areca nut has been recognized as the primary etiological factor in OSMF by various epidemiological studies, including cross-sectional surveys, cohorts, case-control studies, and interventional studies. The nut is the endosperm of the fruit of plant Areca catechu palm.
The incidence, frequency, and length of nut chewing correlated with the onset of the disease. The cases-control studies clearly showed a dose-response relationship between disease causation and areca nut chewing. The two areca-nut products that are most frequently used in commerce are betel quid and gutkha in India. It is a popular stress reliever comprised mostly of areca nuts, tobacco, and flavourings. It quickly dissolves in saliva when chewed, giving off central stimulation that is reportedly stronger than tobacco. Commercially prepared products such as Pan masala, Gutkha, and mawa, with high areca nut content per chew, appear to develop OSMF more quickly than handmade traditional betel quid with lower areca nut content. Commercial areca-nut products have largely been replaced by gutkha, which also contains tobacco and significant amounts of the nut.22 Betel quid typically used betel nut, slaked lime, and areca catechu which are wrapped in betel leaf from the Piper betel, a pepper shrub. It is still chewed in India, usually as an appetiser after meals. Pan masala is available in tiny sachets that are easy to carry and can be consumed at any time, unlike pan, which must be freshly prepared before usage. Unlike pan, this requires to be freshly prepared before use. Gutkha is chewed for up to an hour in the oral or labial vestibule and occasionally just beneath the tongue. Alternatively, the excess is swallowed or spit up. Pan is chewed until the nut softens and completely dissolves in saliva. It is usually done multiple times, and for those who are hooked, even more. Many patients keep it in their oral vestibule as they sleep.
Geographical Distribution
OSMF, a highly carcinogenic disorder, affects the buccal mucosa. This disorder can affect persons of any age, although it is most common in those between the ages of 18 and 35. Over the previous two decades, the number of OSMF cases in India has surged from 0.03 – 6.42%, constituting a significant public health risk. OSMF is thought to be strongly linked to betel quid (BQ) chewing, with higher rates recorded in countries and areas where BQ chewing is popular, such as India, Sri Lanka, China, Pakistan, Bangladesh, Kenya, Taiwan, Saudi Arabia, the United Kingdom, and to other regions of the world where Asians have migrated. Over the course of its life, OSMF has a malignant factors regulating that ranges from 7% to 13%.23
Pathogenesis
OSMF is fundamentally a collagen metabolism disorder; still its pathophysiology has remained a mystery after more than three decades of investigation. The areca nut contributes certainly in the genesis of OSMF, but more research is required. Extracellular matrix changes have an important role. Increased collagen synthesis or decreased collagen breakdown have been considered in this research as potential mechanisms in the development of the disease; normal collagen metabolism differs at various stages. The genes COL1A2, COL3A1, COL6A1, COL6A3, and COL7A1 contribute for increased collagen level in OSMF.11,24–26
Areca nut includes alkaloids, flavonoids, and copper all disrupt the equilibrium of extracellular matrix. Arecoline, guvacoline, guvacine, and arecaidine are four alkaloids that are responsible to activate fibroblasts for generation of collagen. Flavonoids (tannins and catechins) stabilise collagen fibrils, block collagenase, and protect them against collagenase breakdown. Mucosal inflammation caused by areca nut or gutkha recruited activated T cells and macrophages, increasing cytokines and tumour growth factor beta (TGF-). Lysyl oxidase dramatically increases collagen synthesis by increasing procollagen proteinase enzymes, procollagen proteinase enzyme activity, and procollagen genes. TGF-collagen suppresses collagen degradation by activating the tissue inhibitor of matrix metalloproteinase (TIMP) genes and plasminogen activator inhibitor (PAI). It has been observed that the high copper content of areca nuts increases the activity of lysyl oxidase, an enzyme necessary for the final cross-linking of collagen fibres. Higher copper levels have been discovered in OSMF-affected mucosa, which supports the role that copper plays in fibrogenesis by elevating lysyl oxidase activity. Areca nuts chewing, regularly causes increased masticator muscle activity, glycogen depletion, and muscular fatigue. Muscle fatigue is increased by the restricted blood supply caused by fibrosis, which leads to severe degeneration and fibrosis.
As previously mentioned, genetic predisposition and autoimmune factors are two additional processes that are probable to coincide. This hypothesis is supported by the discovery of circulating immune complexes, immunoglobulins, and autoantibodies in some OSMF patients, along with altered cellular and humoral responses. HLA-A10, DR3, and B7 levels are higher in OSMF patients than in healthy controls, indicating genetic predisposition. The illness has been observed to run in families in India and South Africa. OSMF appears to be a complex illness involving promoters, activators, and some other modifying cofactors. However, the OSMF postulate loss of extracellular matrix equilibrium and persistent extracellular matrix deposition is now widely acknowledged.27 The pathogenesis of OSMF is as summarized in following Figure 1.
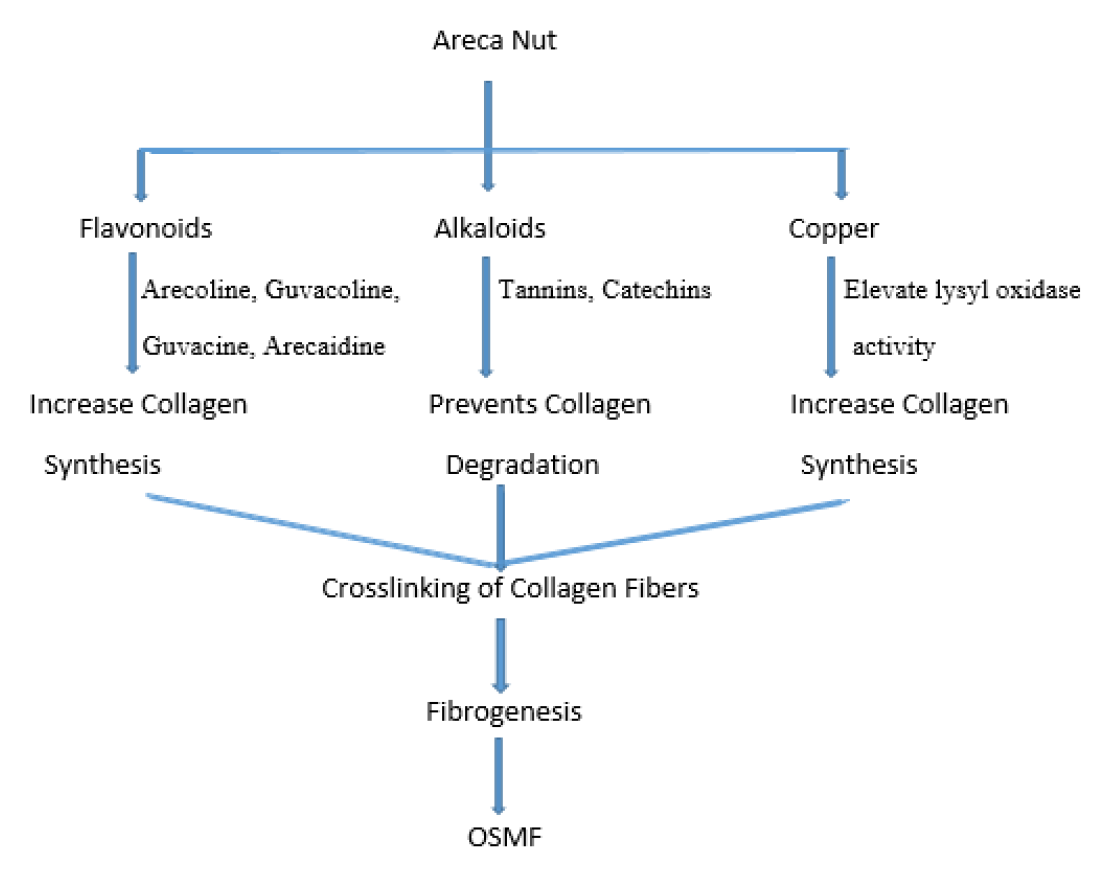
Figure 1.
Mechanism of Pathogenesis of OSMF.
Diagnosis
Differential diagnosis OSMF has been staged and classified clinically, functionally, and histologically. Doctors employ specific staging systems to diagnose and treat clinical OSMF. Stomatitis and vesiculation are symptoms of early OSMF, appearance like a marble with palpable fibrous bands, leucoplakia and erythroplakia are symptoms of severe OSMF.28 Solid biopsies are essential to aid in clinical diagnosis and therapy planning since OSMF may develop into OSCC. The amount and positioning of fibroblasts, inflammatory cells, and collagen fibres including blood vessels, are used in histological staging and classification to assess whether OSMF is in an early, moderate, or advanced stage. OSMF is also staged and classified using biomarkers, including such proteins as mRNAs as well as non-coding RNAs.29 Serum and saliva liquid biopsies have been employed in recent years to increase the precision of diagnostic equipment. In addition, bioinformatic analysis can be used in real time to decrease the need for invasive and/or non-invasive diagnostic procedures, as well as to substitute surgical solid biopsies in the diagnosis of OSMF.30
Solid biopsy
Solid biopsies generate the histological image by the most popular tissue staining method. Methylated PCR, real-time PCR, western blotting, and staining techniques are utilised to identify biomarkers. These are used to determine methylation of promoters, protein expression levels, gene, and marker placements in tissues.31
Immunohistochemical (IHC) staining generally uses H&E staining as a control, since it displays any artefacts and tells if tissue processing was done correctly. Effective fundamental tissue shape elucidates by colouring the nuclei purple and cytoplasm pink, it in addition, H&E staining and other specialised stains and IHC detection in certain situations, are used by pathologists to make a diagnosis.32 According to pathologists, epithelial modifications, rete-peg forms, subepithelial depositions of thick collagen fibres, and inflammatory cells all are indications of OSMF. The comparative efficacies of H&E staining versus Mallory’s, Masson’s, and Van-Gieson staining were studied on 30 OSMF tissues. According to Mallory’s stains, the stratified squamous epithelium’s thick keratin layer, subepithelial edoema, subepithelial hyalinization, pulsatile way, homogeneous collagen, and areas with degenerating skeletal muscle bundles and hyalinization all were affected. It did, however, indicate narrowed blood vessels.33
OSMF tissue Coding Gene and Protein Biomarkers
OSMF condition involves a number of events and compounds associated to hypoxia, the cell cycle, angiogenicity, and epithelial-mesenchymal transition (EMT). Most OSMF patients exhibit PCNA expression in the basal and suprabasal levels. The upper layer of cells from 77% of the patients had positive PCNA expression. CYPA was identified by proteomic two-dimensional electrophoresis (2-DE) as an OSMF biomarker and gene intervention target. Carcinogenesis is aided by CYPA. Caspase inactivation may increase cell growth while inhibiting apoptosis. The latter uses OSMF as a therapeutic target.34
A matrix-assisted laser desorption ionisation imaging mass spectrometry (MALDI-IMS) investigation led to the discovery of nuclear receptor coactivator 7 (NCOA7), which was subsequently validated in 32 pairs of OSCC and noncancerous OSMF tissues, cell lines, and animal models. The cell cycle as well as cell proliferation was controlled by NCOA7-associated proteins. These are promising indicators for detecting OSMF malignant transformation early.35
HIF-1α protein expression is induced by arecoline in a dose-dependent way. The production of HIF-1 was increased greatly in the inflammatory, fibroblastic, and epithelial cells of betel quid consumers. HIF-1 activation promotes the production of PAI-1 and the accumulation of extracellular matrix, leading to OSMF. A TGF signalling receptor named CD105 contributes in both fibrogenesis and angiogenesis. It promotes angiogenesis and endothelial cell development. Compared to CD34, CD105 was a more appropriate biomarker for diagnosing OSMF neo-angiogenesis.36
MALDI-MS, one dimensional sodium dodecyl sulphate polyacrylamide gel electrophoresis (1D SDS-PAGE), and nano liquid chromatography (nano LC) were utilised to discover enolase increased expression in biopsies of dysplastic oral OSMF vs non-dysplastic OSMF and normal oral mucosa.37 By modulating PI3K/AKT signalling, the α-enolase stimulates cell proliferation, induces cancer by stimulating plasminogen, and increases the Warburg effect. Western blotting, IHC, and RT-qPCR are all used to detect α-enolase activity in OSMF tissues.
Cell proliferation is assessed by Ki67 and cyclin D1, whereas tumour suppressor genes p16 and p53 are upregulated. The transcriptional activity of β-catenin and c-Jun is correlated. Tumor invasion is promoted by insulin-like growth factor II mRNA-binding protein 3 (IMP3) and hepatocyte growth factor receptor c-Met. While catenin is decreased in OSMF while cyclin D1, Ki67, IMP3, and c-Met are all raised. Between transforming and non- transforming OSMF, Ki67 over expression paired with p16 suppression differs dramatically.38 Wnt inhibitory factor-1 (WIF1) an antagonist, blocks Wnt/β-catenin transmission by binding specifically to Wnt proteins. The methylation of the WIF1 regulator may be accountable for the β-catenin stimulation seen in OSMF carcinogenesis. However, frizzled-related proteins 1 (FRP1) and FRP5, which are connected to cytosolic accumulation in OSMF carcinogenesis, are produced less when regulator methylation is present. It’s worth looking at the levels of catenin transcription in the regular, metaplastic, dysplastic, and OSCC phases of OSMF.39
OSMF tissue non-coding gene biomarkers
Reverse-transcription quantitative PCR can still be employed to explore low copy numbers even though some micro RNAs are stable in paraffin-embedded tissues or frozen tissues. The miR-200b and miR-200c were shown to be highly suppressed in OSMF tissues. Arecoline treatment decreased the transcription of miR-200c in buccal mucosal fibroblasts.40 MiR-200c and miR-200b, which target ZEB1 and ZEB2 respectively, upregulate E-cadherin. ZEB1 interacts with the regulator of α-smooth muscle actin (α-SMA) and causes the upregulation of α-SMA in myofibroblasts during fibrogenesis. In OSMF tissues, the lncRNA GAS5-AS1 was significantly inhibited. It inhibited phosphorylated Smad2, decreased TGF/Smad transmission and α-SMA transcription in myofibroblasts. LINC00974, on the other hand, produced the reverse effect. In OSMF tissues and myofibroblasts, LINC00974 was abnormally elevated.41
Liquid Biopsy
The modern biochemical and bimolecular processes compared to earlier techniques are more precise and stable. In bodily fluids, even minute amounts of free ions, enzymes, nucleic acids, proteins, and circulating cells can be identified. In comparison to normal tissues, globulin and serum protein levels were considerably lower in OSMF.42 As OSMF transformed into OSCC and the betel quid habit lasted longer, serum copper levels significantly rose. For the last decade, saliva samples have been employed as diagnostic tests since they are easy to acquire from patients. In serum and saliva, OSMF biomarkers have been discovered in the past few years, and the possibility of using them in OSMF diagnosis has advanced as confirmation and sample numbers have grown.43
Biomarkers in OSMF Serum
Sister chromatid exchange per cell was substantially greater in individuals with OSMF and pan chewers than in control subjects. Genome instability is caused by ROS-induced DNA damage. The provitamin A carotenoid β-carotene levels dropped as OSMF progressed. Glutathione peroxidase (GPx) and erythrocyte superoxide dismutase (E-SOD) levels were significantly lower in the OSMF, oral leukoplakia, and oral cancer groups than in the control group. Several potentially malignant lesions and diseases, including oral cancer, have dramatically elevated levels of the enzyme lactate dehydrogenase (LDH), which catalyses the conversion of lactate to pyruvate.44
Serum LDH levels were found to be directly linked to frequency of betel chewing and mouth openness in individuals suffering with OSMF. Salivary LDH, and did not show any such links. Serum LDH, rather than salivary LDH, may be a superior biological indication of OSMF. OSMF patients expressed higher levels of DNA damage and lipid peroxidation when compared to healthy controls. Using a comet test to measure the levels of malondialdehyde (MDA), a lipid peroxidation marker, may aid in identifying OSMF patients at a high risk of acquiring cancer.45
Biomarkers in OSMF Saliva
8-hydroxy-2-deoxyguanosine (8-OHdG) and MDA can be detectable in saliva, serum, and urine. MDA and Salivary 8-OHdG were greater in OSMF patients than in healthy normal subject, where as salivary Vitamin C and Vitamin E was low.46 Multiple biomarkers, such as 8-OHdG, MDA, Vitamins C, and E, may improve OSMF diagnostic specificity and sensitivity. In other research, lipid peroxides and salivary protein were greater in OSMF patients compared to controls, although salivary SOD, GPx, Vitamin A, E, and C were low. As a result, oxidative stress is linked to OSMF progression. Salivary LDH in the OSMF group was significantly higher than in control group. S1007 was observed in squamous epithelial cells of psoriatic skin. OSMF patients had higher salivary S100A7 levels than the healthy control group. Higher levels of S100A7 has been linked to the likelihood of malignant transformation inside buccal dysplasia in patients with potentially malignant oral diseases.47
Instrumentation for OSMF diagnosis
Biopsies are commonly used to verify a clinical diagnosis. Because of OSMF’s moderate transformation rate, several individuals with the disease declined incisional biopsy. To aid incision biopsy in OSMF diagnosis, Fourier transform infrared spectroscopy (FTIR), autofluorescence spectroscopy, and optical coherence tomography (OCT), were utilised.48
As different diseased tissues exhibit a variety of distinctive histomorphological characteristics, autofluorescence spectroscopy utilizes this fact. Intrinsic fluorophores emit diverse fluorescence emission spectra when tissues are activated to an appropriate wavelength. The maximum emissions of tryptophan, collagen, and nicotinamide adenine dinucleotide (NADH) are detected at 340 nm, 400 nm, and 460 nm, respectively. When excited at 330 nm, OSMF displayed a significantly different emission peak at 380 nm and 460 nm in normal oral mucosa. The emission peaks differed significantly when compared between betel quid chewers and OSMF patients. Following treatment with the OSMF, the mucosa showed a decrease in intensity at 385 nm and an increase in intensity at 440 nm.49
The time domain OCT technology was initially utilised on human teeth and oral mucosa in 1988. In an optical scattering medium, OCT captures two and three-dimensional images with micrometre precision using low-coherence light. Effective diagnostic biomarkers for OSMF include epithelial thickness and the standard deviation (SD) of the A-mode scan intensity in the laminar propria layer.50
Diagnosis of OSMF using instrumentation and sera
FTIR is used to develop the infrared emission spectra of solid and liquid. As previously indicated, there were substantial differences in globulin, enzymes, blood protein, specific protein, vitamin, nucleic acid and copper levels between OSMF patients and normal subjects. In the diagnosis of OSMF, combining the two procedures equipment and sera may be more effective and result in a time and reagent cost savings. Preoperative screening and diagnosis could benefit from using FTIR spectroscopy on the serum of OSMF patients.
Ki67 might identify significant OSMF transition rates to malignant cells when used in conjunction with p16 and NCOA7. Liquid biopsies are less intrusive and more effective than traditional biopsies. In the serum of OSMF patients, globulin and protein levels are much lower. Betel chewing may raise copper levels in OSMF patients’ blood sister chromatid exchange in OSMF patients’ lymphocytes is one of the markers for genomic instability used to diagnose the disease. To detect DNA damage, a comet test is used. Between healthy individuals and OSMF patients, there were differences in the serum levels of ROS products (MDA), anti-ROS enzymes (GPx, E-SOD), and cellular metabolic enzymes (LDH).51
SOD, GPx, MDA, LDH, and 8-OHdG, among other enzymes and ROS products, were found in both saliva and sera. The sera of OSMF patients were devoid of 8-OHdG. When compared to normal controls, OSMF saliva had lower amounts of Vitamin C and E. Instruments such as autofluorescence spectroscopy, FTIR, and OCT was developed for OSMF diagnosis, and molecular biological techniques were employed to screen for OSMF biomarkers.52
Symptoms
In an early stage, the juxta-epithelial region demonstrates early hyalinization. Collagen is still visible as individual, thicker bundles. In moderate concentrations, plump immature fibroblasts are found. Blood vessels are commonly dilated and clogged. eosinophils, Lymphocytes and plasma cells are the most common inflammatory cells.53 Collagen has significantly undergone hyalinization at a moderately advanced stage. The amorphous transformation starts from a juxta-epithelial basement membrane. Slight residual oedema can sometimes make thicker collagen bundles visible separately. The nuclei of mature fibroblastic cells are elongated and spindle-shaped, and the cytoplasm is scanty. Inflammatory lymphocytes, plasma cells, and eosinophils are found in the inflammatory exudates. The blood vessels are either compressed or normal due to the expanded surrounding tissue. At this stage, with no oedema or apparent bundles, collagen is fully hyalinized and appears as a smooth sheet. With thin elongated cells, hyalinised connective tissue becomes hypocellular. Inflammatory lymphocytes, plasma cells, and eosinophils are found in the inflammatory exudates. Blood vessels are eliminated or constricted totally. The dense collagen that surrounds the melanin-containing cells in the lamina propria accounts for the clinically observed pigmentation fall. The photographs showing symptoms of OSMF are given in Figure 2.
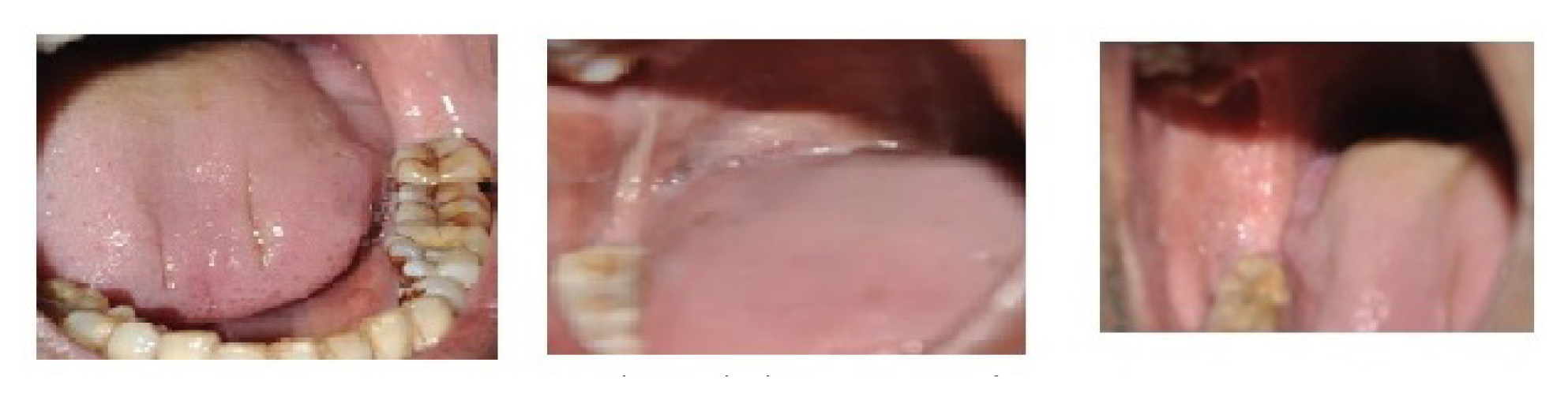
Figure 2.
Photographs showing symptoms of OSMF
TREATMENT
OSMF is treated by surgery and conservative approaches, such as molecular methods. This section will go over how to treat OSMF naturally using physical, chemical, and natural compound therapies.
Corticosteroids
Their anti-inflammatory properties suppress the inflammatory response. Reduced fibroblast proliferation and collagen synthesis prevent fibrosis from developing. Topical treatments, intralesional injections, and mouth washes are all alternatives.
Hyaluronidase
This causes the reduction of the intracellular cement material and collagen synthesis by breaking down hyaluronic acid. Intralesional injections of 1500 IU hyalase, 5000 IU chymotrypsin, and fibrinolytic agent (Hyalase) dissolved in 2% lignocaine were utilised.
Placental Extracts
Dexamethasone, hyaluronidase, and placental extract were found to work better together than they did separately.
Nutritional Support
High protein, vitamin B complex, calories, other vitamins and minerals.
Physiotherapy
Forcefull mouth openings, heat therapy.
Surgical treatment
More fibrosis and impairment resulted after cutting the fibrotic bands.
Discontinuation of the habbits of betal quid, tobacco, gutakha chewing is the main advice given to patients to get rid of OSMF.54
Prevention and management
One of the greatest ways to avoid areca nut chewing is to reduce or eliminate the habit. However, smoking and areca nut (commonly known as betel nuts) tobacco chewing should be avoided. Consumption of spicy foods, such as chilies, should be limited. Maintaining appropriate oral hygiene can help to prevent OSMF. Supplementing the diet with foods high in Vitamin A, Vitamin B complex, Vitamin C, and iron has a significant impact on OSMF prevention. OSMF treatment in ayurveda includes the use of Triphala Kashayam or Yashtimadhu Kashayam to rinse twice a day. Drinking a glass of aloe vera juice once a day can help to alleviate the burning sensation. In the case of constipation, take Triphala churna or Avipattikar churna with warm water before going to bed. Also, by including a combination of micronutrients and minerals in the diet, this has a great impact on eliminating bad habits. It also includes mental stability in order to avoid risky behaviours such as chewing betel nuts and tobacco, as well as tobacco smoking. Health education is likely to have an impact on this behaviour.55
CASE STUDY
The research involved 185 individuals with OSMF who were diagnosed in the outpatient department of Ragas Dental College and Hospital, Chennai (Tamil Nadu). The research study was conducted over a period of three years, starting from April 2000 to February 2003. The hospital on an average treat about 100 patients a day. Of those, 60 are new patients, and the other 40 are all returning for treatment. The gender and age distribution of patients and controls in this case was, OSMF was found in 90.8% of males and 9.2% of females, resulting in a male to female ratio of 9.9:1. The median age of the patients and controls was 29 years, and the mean age was 32.4 ± 10.4 years. OSMF affects men’s between 16 to 76 years of age and women’s between 24 to 57 years of age.50
This study found a significant rate of OSMF in males, which is similar to what Shah and Sharma found in Delhi, India. In this study, there were five times as many men as women. According to case-control research from Pakistan, females have a higher prevalence.51
A 14-year-old Indian girl complained of restricted mouth opening and a burning sensation while eating. She was referred for a routine dental examination while at a camp sponsored by the hospital to the paedodontics and preventive dentistry department of the hospital. For a year, she chewed two packets of flavour-infused areca nut and perfumed tobacco every day. The three principal therapeutic approaches available to treat OSMF patients are medical therapy, surgical treatment, and oral physiotherapy. The initial step was to encourage and advise the patient and her parents to quit smoking. She has given one daily tablet of Vitamin B complex 500 mg. She was forced to eat five meals a day, each one should have included grains, fruits, and vegetables. Fresh green vegetables like spinach, beans, egg yolk, milk, wheat bread, peanuts, and fruits, provide the body with the micronutrients and vitamins it needs on a daily basis. The patient was taught oral physiotherapy, which included inflating exercises. For one month, the balloon was to be blown 15–20 times per day. After a month, the patient returned for a follow-up appointment, and the mouth opening had increased by 1 mm. The patient was arranged for a 3-month follow-up visit and told to follow the same course of treatment until changes were advised during the follow-up.52
Between January 2000 and December 2004, approximately 3 lakh patients consulted the outpatient department of the Government Dental College and Hospital in Nagpur, India.53 The study sample consisted of 1000 patients who had been diagnosed with OSMF. In case record forms, the entire clinical history was collected, including demographic data, eating habits like pan, gutakha, (frequency, duration, and type), and tobacco use.
The overall prevalence of OSMF ranged from 2.42 per 1000 woman (17%) in 2000 to 6.42 per 1000 woman (17%) in 2010, with a male to female proportion of 4.9:1. In this study, the majority of OSMF patients (48.3%) were of grade 20-29 mm severity, with 24.62 mm of average mouth opening, whereas the average mouth opening in Nepalese OSMF cases was 34 mm according to Cox’s study.54
An Indian man at the age of 38 years was referred to the oral and maxillofacial surgery department at the Goldman School of Dental Medicine, Boston University for an assessment and treatment of his trismus. The sufferer complains of being unable of chewing food and having restricted mouth opening. For the past 15 years, the patient has been chewing a betel quid of Indian tobacco. The severity of the condition governs the course of treatment. Nowadays, this disorder is categorised as mild to moderate. Physical therapy along with medicinal treatment is performed as part of the treatment at this stage. Weekly submucosal steroid injections are the only kind of medical care available in the US to decrease the progression of OSMF.55
Steroids and glucocorticoids having anti-inflammatory function were the first drugs used to treat OSMF. Several glucocorticoids used are hydrocortisone, triamcinolone, betamethasone and dexamethasone. Steroids were less effective at reversing inappropriate fibrotic tissue deposition, and so considerable rate of relapse was attributed to this course of the treatment.56–59
CONCLUSION
OSMF is very common among Asians that chew betel nuts. OSMF disturbs collagen homeostasis by causing structural and compositional anomalies by increasing collagen synthesis and decreasing collagen clearance. Inflammation, ROS generation, and mutations can all trigger abnormal oral submucous collagen deposition. Functional and molecular pathology methods are used to establish a clinical diagnosis of OSMF. Xerostomia, dysphagia, trismus, restricted mouth opening, and loss of oral function are all indications of OSMF. Molecular pathology techniques for OSMF detection include both sensitive and non-tests and concentrate on biomarkers that lead to abnormal collagen deposition. Biomarkers in tissue and sera are identified using an intrusive detection approach. The non-invasive procedure looks for biomarkers in saliva and uses optical tools to analyse the mucosa. The biomarkers that affect collagen homeostasis are suppressed by molecular OSMF therapy. Drug therapies for OSMF are often effective. People, who are susceptible to OSMF and possibly towards development of OSCC should avoid unhealthy habits like smoking and chewing betel nuts, instead ingest raw foods which have anti-inflammatory and antioxidant properties.
Cite this article
Lote S, Kotkar K, Nikam M, Dhadiwal Y, Zade S, Patil V. Oral Submucosal Fibrosis: A Potentially Malignant Condition to be Considered. J Young Pharm. 2023;15(2):224-32.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- Groeger S, Meyle J. Oral Mucosal Epithelial Cells. Front Immunol. 2019:1-22. [CrossRef] | [Google Scholar]
- Prabhu R, Prabhu V, Chatra L, Shenai P, Suvarna N, Dandekeri S, et al. Areca nut and its role in oral submucous fibrosis. J Clin Exp Dent. 2014;6(5):e569-75. [CrossRef] | [Google Scholar]
- Rao NR, Villa A, More CB, Jayasinghe RD, Kerr AR, Johnson NW, et al. Oral submucous fibrosis: A contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. J Otolaryngol – Head Neck Surg. 2020;49(1):3 [CrossRef] | [Google Scholar]
- Uchiyama Y, Sumi T, Marutani K. Neurofibromatosis Type 1 in the Mandible. Ann Maxillofac Surg. 2018;8(1):121-123. [CrossRef] | [Google Scholar]
- Pillai R, Balaram P, Reddiar KS. Pathogenesis of oral submucous fibrosis. Relationship to risk factors associated with oral cancer. Cancer. 1992;69(8):2011-2020. [CrossRef] | [Google Scholar]
- Chowdhury S, pratim Chakraborty P. Universal health coverage – There is more to it than meets the eye. J Fam Med Prim Care. 2017;6(2):169-170. [CrossRef] | [Google Scholar]
- Ali F, Aher V, Prasant M, Bhushan P, Mudhol A, Suryavanshi H, et al. Oral submucous fibrosis: Comparing clinical grading with duration and frequency of habit among areca nut and its products chewers. J Cancer Res Ther. 2013;9(3):471 [CrossRef] | [Google Scholar]
- Wetzel SL, Wollenberg J. Oral Potentially Malignant Disorders. Dent Clin North Am. 2020;64(1):25-37. [CrossRef] | [Google Scholar]
- Shih YH, Wang TH, Shieh TM, Tseng YH. Oral Submucous Fibrosis: A Review on Etiopathogenesis, Diagnosis, and Therapy. Int J Mol Sci. 2019;20(12):2940 [CrossRef] | [Google Scholar]
- Rajalalitha P, Vali S. Molecular pathogenesis of oral submucous fibrosis – a collagen metabolic disorder. Published online. 2005 [CrossRef] | [Google Scholar]
- Arakeri G, Brennan PA. Oral submucous fibrosis: An overview of the aetiology, pathogenesis, classification, and principles of management. Br J Oral Maxillofac Surg. 2013;51(7):587-93. [CrossRef] | [Google Scholar]
- Gupta S, Jawanda MK. Oral submucous fibrosis: An overview of a challenging entity. Indian J Dermatol Venereol Leprol. 2021;87(6):768 [CrossRef] | [Google Scholar]
- Agarwal S. Surgical Management of Trismus Due to Oral Submucous Fibrosis Using Diode Laser. Otolaryngol Head Neck Surg. 2015;1(1):1-4. [CrossRef] | [Google Scholar]
- Chattopadhyay A, Gopal J. Molecular Pathology of Malignant Transformation of Oral Submucous Fibrosis. 2016;35(3):193-205. [CrossRef] | [Google Scholar]
- Shen YW, Shih YH, Fuh LJ, Shieh TM. Oral Submucous Fibrosis: A Review on Biomarkers, Pathogenic Mechanisms, and Treatments. Int J Mol Sci. 2020;21(19):7231 [CrossRef] | [Google Scholar]
- Narayanappa U. Expression of Proliferating Cell Nuclear Antigen (PCNA) in Oral Submucous Fibrosis: An Immunohistochemical Study. J Clin Diagnostic Res. 2015;9(5):ZC20-3. [CrossRef] | [Google Scholar]
- Mello FW, Miguel AFP, Dutra KL. Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis. J Oral Pathol Med. 2018;47(7):633-40. [CrossRef] | [Google Scholar]
- Aziz SR. Oral Submucous Fibrosis: Case Report and Review of Diagnosis and Treatment. J Oral Maxillofac Surg. 2008;66(11):2386-9. [CrossRef] | [Google Scholar]
- Kamath VV, Satelur K, Komali Y. Biochemical markers in oral submucous fibrosis: A review and update. Dent Res J (Isfahan). 2013;10(5):576-84. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3858729 [CrossRef] | [Google Scholar]
- Anand R, Dhingra C, Prasad S, Menon I. Betel nut chewing and its deleterious effects on oral cavity. J Cancer Res Ther. 2014;10(3):499-505. [CrossRef] | [Google Scholar]
- More CB, Das S, Patel H, Adalja C, Kamatchi V, Venkatesh R, et al. Proposed clinical classification for oral submucous fibrosis. Oral Oncol. 2012;48(3):200-2. [CrossRef] | [Google Scholar]
- Warnakulasuriya S, Johnson NW, Van Der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575-80. [CrossRef] | [Google Scholar]
- Bhowate RR, Lanjekar AB, Bakhle S, Narayane A, Pawar V, Gandagule R, et al. Comparison of Efficacy of Topical Curcumin Gel with Triamcinolone-hyaluronidase Gel Individually and in Combination in the Treatment of Oral Submucous Fibrosis. J Contemp Dent Pract. 2020;21(1):83-90. [CrossRef] | [Google Scholar]
- Cheng RH, Wang YP, Chang JY, Pan YH, Chang MC, Jeng JH, et al. Genetic Susceptibility and Protein Expression of Extracellular Matrix Turnover-Related Genes in Oral Submucous Fibrosis. Int J Mol Sci. 2020;21(21):8104 [CrossRef] | [Google Scholar]
- Liao P, Yang H, Huang Y. Genetic expression signatures of oral submucous fibrosis and oral cancer—A preliminary microarray report. Journal of Dental Sciences. 2016;11(4):457-62. [CrossRef] | [Google Scholar]
- Rajalalitha P, Vali S. Molecular pathogenesis of oral submucous fibrosis–a collagen metabolic disorder. Journal of Oral Pathology and Medicine. 2005;34(6):321-8. [CrossRef] | [Google Scholar]
- Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006;42(6):561-8. [CrossRef] | [Google Scholar]
- . Role of Areca Nut Induced TGF-β and Epithelial-Mesenchymal Interaction in the Pathogenesis of Oral Submucous Fibrosis. PLoS One. 2015;10(6):e0129252 [CrossRef] | [Google Scholar]
- Passi Deepak, Prateek Bhanot DK, Chahal D, Mansi Atri YP. Oral submucous fibrosis: Newer proposed classification with critical updates in pathogenesis and management strategies ABSTRACT. Natl J Maxillofac Surg. 2017;8(1):89-94. [CrossRef] | [Google Scholar]
- Reshma V, Varsha B, Rakesh P, Radhika M, Soumya M, D′Mello S, et al. Aggrandizing oral submucous fibrosis grading using an adjunct special stain: A pilot study. J Oral Maxillofac Pathol. 2016;20(1):36 [CrossRef] | [Google Scholar]
- Mauri G, Vitiello PP, Sogari A. Liquid biopsies to monitor and direct cancer treatment in colorectal cancer. Br J Cancer. 2022;127(3):394-407. [CrossRef] | [Google Scholar]
- Mathai R, Vidya R, Reddy B. Potential Utility of Liquid Biopsy as a Diagnostic and Prognostic Tool for the Assessment of Solid Tumors: Implications in the Precision Oncology. J Clin Med. 2019;8(3):373 [CrossRef] | [Google Scholar]
- Onul A, Colvard MD, Paradise WA. Application of Immunohistochemical Staining to Detect Antigen Destruction as a Measure of Tissue Damage. J Histochem Cytochem. 2012;60(9):683-93. [CrossRef] | [Google Scholar]
- Suwasini S, Shrikaar Manisha, Kumari Nishu, Singh Abhishek, Khusboo Kumari MK. Expression of p63 and Proliferating Cell Nuclear Antigen in Oral Submucous Fibrosis. J Int Soc Prev Community Dent. 2005;8(831):34-7. [CrossRef] | [Google Scholar]
- Xie X, Jiang Y, Yuan Y. MALDI imaging reveals NCOA7 as a potential biomarker in oral squamous cell carcinoma arising from oral submucous fibrosis. Oncotarget. 2016;7(37):59987-60004. [CrossRef] | [Google Scholar]
- Zhang P, Chua NQE, Dang S. Molecular Mechanisms of Malignant Transformation of Oral Submucous Fibrosis by Different Betel Quid Constituents—Does Fibroblast Senescence Play a Role?. Int J Mol Sci. 2022;23(3):1637 [CrossRef] | [Google Scholar]
- Bag S, Dutta D, Chaudhary A. Identification of α-enolase as a prognostic and diagnostic precancer biomarker in oral submucous fibrosis. J Clin Pathol. 2018;71(3):228-38. [CrossRef] | [Google Scholar]
- Bazarsad S, Zhang X, Kim KY. Identification of a combined biomarker for malignant transformation in oral submucous fibrosis. J Oral Pathol Med. 2017;46(6):431-8. [CrossRef] | [Google Scholar]
- Ejaz I, Ghafoor S. Wnt Signaling Pathway in Oral Lesions. J Pak Med Assoc. 2019;69(11):1 [CrossRef] | [Google Scholar]
- He Y, Wang W, Jiang P. Long Non-Coding RNAs in Oral Submucous Fibrosis: Their Functional Mechanisms and Recent Research Progress. J Inflamm Res. 2021;14:5787-800. [CrossRef] | [Google Scholar]
- Nourmohammadi B, Tafsiri E, Rahimi A. Expression of miR-9 and miR-200c, ZEB1, ZEB2 and E-cadherin in Non-Small Cell Lung Cancers in Iran. Asian Pacific J Cancer Prev. 2019;20(6):1633-9. [CrossRef] | [Google Scholar]
- Ankolekar Kode M, Rashmiraj Karjodkar F. Estimation of the serum and the salivary trace elements in OSMF patients. J Clin Diagnostic Res. 2013;7(6):1215-8. [CrossRef] | [Google Scholar]
- Aishwarya KM, Reddy MP, Kulkarni S, Doshi D, Reddy BS, Satyanarayana D, et al. Effect of Frequency and Duration of Tobacco use on Oral Mucosal Lesions – A Cross-Sectional Study among Tobacco Users in Hyderabad, India. Asian Pac J Cancer Prev. 2017;18(8):2233-8. [CrossRef] | [Google Scholar]
- Sivaramakrishnan M, Sivapathasundharam B, Jananni M. Evaluation of lactate dehydrogenase enzyme activity in saliva and serum of oral submucous fibrosis patients. J Oral Pathol Med. 2015;44(6):449-52. [CrossRef] | [Google Scholar]
- Narayan Biswal B, Narayan Das S, Kumar Das B, Rath R. Alteration of cellular metabolism in cancer cells and its therapeutic. J oral Maxillofac Pathol. 2017;21(3):244-251. [CrossRef] | [Google Scholar]
- Divyambika CV, Sathasivasubramanian S, Vani G, Vanishree AJ, Malathi N. Correlation of Clinical and Histopathological Grades in Oral Submucous Fibrosis Patients with Oxidative Stress Markers in Saliva. Indian J Clin Biochem. 2018;33(3):348-55. [CrossRef] | [Google Scholar]
- Chen HM, Wang CY, Chen CT. Auto-fluorescence spectra of oral submucous fibrosis. J Oral Pathol Med. 2003;32(6):337-43. [CrossRef] | [Google Scholar]
- Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical Coherence Tomography: An Emerging Technology for Biomedical Imaging and Optical Biopsy. Neoplasia. 2000;2(1-2):9-25. [CrossRef] | [Google Scholar]
- Ho PS, Chen PL, Warnakulasuriya S, Shieh TY, Chen YK, Huang IY, et al. Malignant transformation of oral potentially malignant disorders in males: A retrospective cohort study. BMC Cancer. 2009;9(1):260 [CrossRef] | [Google Scholar]
- Kaur J, Politis C, Jacobs R. Salivary 8-hydroxy-2-deoxyguanosine, malondialdehyde, Vitamin C, and Vitamin E in oral pre-cancer and cancer: Diagnostic value and free radical mechanism of action. Published online. 2015 [CrossRef] | [Google Scholar]
- Radhika T. Qualitative Analysis of Collagen Fibers in Oral Submucous Fibrosis using Picrosirius Red Stain and Polarising Microscope. J Clin DIAGNOSTIC Res. 2016;10(2):4-7. [CrossRef] | [Google Scholar]
- an Der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45(4-5):317-23. [CrossRef] | [Google Scholar]
- Ranganathan K, Devi MU, Joshua E, Kirankumar K, Saraswathi TR. Oral submucous fibrosis: A case-control study in Chennai, South India. J Oral Pathol Med. 2004;33(5):274-7. [CrossRef] | [Google Scholar]
- Maher R, Lee AJ, Warnakulasuriya KAAS, Lewis JA, Johnson NW. Role of areca nut in the causation of oral submucous fibrosis: A case-control study in Pakistan. J Oral Pathol Med. 1994;23(2):65-9. [CrossRef] | [Google Scholar]
- Deshpande A, Kiran S, Dhillon S, Mallikarjuna R. Oral submucous fibrosis: A premalignant condition in a 14-year-old Indian girl. Case Reports. 2013;2013(dec12 1):bcr2013200786-bcr2013200786. [CrossRef] | [Google Scholar]
- Hazarey VK, Erlewad DM, Mundhe KA, Ughade SN. PDFlib PLOP: PDF Linearization, Optimization, Protection Page inserted by evaluation version Oral submucous fibrosis: study of 1000 cases from central. [CrossRef] | [Google Scholar]
- Cox SC, Murray Walker D. Establishing a normal range for mouth opening: its use in screening for oral submucous fibrosis. Br J Oral Maxillofac Surg. 1997;35(1):40-2. [CrossRef] | [Google Scholar]
- Alshadwi A, Bhatia I. Excision of Oral Submucous Fibrosis and Reconstruction with Full Thickness Skin Graft: A Case Study and Review of the Literature. Case Rep Dent. 2012;2012:1-5. [CrossRef] | [Google Scholar]
- Kakar PK, Puri RK, Venkatachalam VP. Oral Submucous Fibrosis—treatment with hyalase. J Laryngol Otol. 1985;99(1):57-60. [CrossRef] | [Google Scholar]
