ABSTRACT
Background: The herbal antidiabetic drugs are safe but effective alternate to conventional oral antidiabetic therapy. The single phytoconstituents of single plants are inadequate to produce the required efficacy therefore polyherbal can be tested for the desired results. The objective of the present study was the assessment of total phenolic, flavonoids, tannins content, in vitro antioxidant and antidiabetic activity of Azadirachta indica leaves, Picrorhiza kurroa rhizomes, Pterocarpus marsupium heartwood, and Withania coagulans berries and fruit coat. Materials and Methods: The ethanolic extract of all four drugs were prepared and mixed in a ratio of 1:1:1:1 to make the polyherbal extract. It was checked for the presence of phenols, flavonoids, and tannins. The High-Performance Liquid Chromatographic (HPLC) analysis for phenolic compounds as well as the beta carotenoids was done. The in vitro antioxidant activity as well as in vitro antidiabetic activity were calculated. Results: Quantitative estimations showed that maximum phenolic content was in Azadirachta indica and Pterocarpus marsupium, the flavonoid in Withania coagulans, and tannins content in Pterocarpus marsupium. The HPLC analysis showed the marked presence of beta carotenoids along with other phenolic compounds. The radical scavenging activity of Pterocarpus marsupium and the polyherbal extract was comparable to the standard Gallic acid. The total antioxidant capacity of polyherbal extract was found to be 15.22±.051 mg Quercetin equivalent (QE)/g dry plant extract (DPE). The polyherbal extract showed an IC50 value of 58.81 µg/ml in case of α- amylase inhibition and 64.81 µg/ml in case of α- glucosidase inhibition. Conclusion: The antioxidant activity, as well as antidiabetic activity, may be attributed to the phenolic, tannins, flavonoid content as well as beta carotene found in the polyherbal extract.
INTRODUCTION
Diabetes is a serious public health concern; its global incidence has risen quickly during the last four decades. However, the World Health Organization (WHO) has programmed to inhibit the rise in diabetes incidence and premature mortality from 1/3rd by the year 2030 but the results are not promising. It has been estimated recently that the cases of diabetes from 20-79 years age will rise from 41 million in 2010 to 51 million in 2030. (1 in 10 adults).1,2
The oral antidiabetic have the undesirable side effects and toxicity along with nausea, vomiting, obstructive jaundice, hyponatremia, haematological- dermatological reactions and intolerance of alcohol and weight gain.3 The oral antidiabetic drugs have certain types of adverse effects on the body like Sulphonylureas leads to hypoglycemia and weight gain. Biguanides cause lactic acidosis, abdominal discomfort, bloating. The drug is also contraindicated in patients with liver, kidney, heart or lungs diseases. Alpha-glucosidase inhibitors cause gastrointestinal intolerance due to fermentation of undigested carbohydrates. It is also contraindicated in a patient with Irritable Bowel Syndrome (IBS) liver or kidney disorders. Thiazolidinedione use may lead to anemia, congestive heart failure, edema, pulmonary edema.4–6
The herbal medicines are considered safe as they have fewer side effects than an allopathic system of medicines. Compared to single herbal products polyherbal formulations show high performance, a broad therapeutic window, cost efficiency, easy availability and minimal side effects.7,8 Therefore,in the present study, we are going to work on polyherbal extract consisting of an equal ratio of Azadirachta indica leaves, Pterocarpus marsupium heartwood, Picrorhiza kurroa rhizomes, and Withania coagulans berries and fruit coat that could work on different targets and at the same time show improved patient adherence and therapeutic behavior.
Azadirachta indica (Family: Meliaceae) contains various phytoconstituents like Nimbin, nimbidin, azadirachtin, epicatechin, catechin, gallic acid, limonoid, flavonoid and margolone.9,10 The drug works as an antinociceptive, immunomodulatory, anti-inflammatory, antiviral, neuroprotective, antimicrobial, hepatoprotective, antidiabetic, and antioxidant.11,12 It delays diabetes onset as well as increases the glucose uptake and glycogen deposition.13
Pterocarpus marsupium Roxb. (Red Kino Tree) is used as an anti-inflammatory, anthelminthic, aphrodisiac, and antipyretic, in the management of mental disorders and ulcers.14,15 The phenolic components like marsupsin, pterosupin, and pterostilbene which reduce blood glucose levels and diabetic wounds.14,16 The drug contains alkaloids, flavonoids, saponins, phenols which may be responsible for the α- amylase and α- glucosidase inhibition activity.17,18
Picrorhiza kurroa Royle ex Benth. (Scrophulariaceae), has antiallergic, anticancer, cardiovascular, cholerectic, hypoglycemic, hypolipidemic, molluscicidal, and leishmanicidal properties.19 It contains Kutkin , kutkoside as well as picrosides I, II, III and V.20,21 It shows antioxidant activity along with α- amylase and α- glucosidase inhibition activity.22 Withania coagulans also known as ‘tukhm-e-hayat’ berry has hypoglycemic, hypolipidemic, immunosuppressive, hepatoprotective, anti-flatulent, anti-inflammatory, wound healer, anticancer, and cardiovascular effects. It contain Withanolide A, Withaferin A, Withaferin and Withanone.23 Withacoagin, withasomninne, might be the agents responsible for the free radical scavenging activity.24
MATERIALS AND METHODS
The Collection as well as Authentication of the Plant Material
Herbal drugs were gathered from the local areas of Ropar and validated at NIPER’s Natural Product Field Laboratory and Nursery, SAS Nagar, Pb. Azadirachta indica (Neem) leaves, Withania coagulans (paneer dodi) fruit, Picrorhiza kurroa (Kutki) rhizomes, and Pterocarpus marsupium (Vijay Sar) wood have all been verified. The authenticated plants’ voucher specimens were subsequently sent to the A.S.B.A.S.J.S. College of Pharmacy in Bela, Ropar, and Pb. for future reference.
Preparation of Extracts
The authenticated drugs Azadirachta indica (Neem) leaf, Picrorrhiza kurroa (Kutki) rhizomes, Pterocarpus marsupium (Vijay Sar) wood, and Withania coagulans (paneer dodi) fruit, were dried, and powdered properly in a grinder and were extracted using ethanol by using cold maceration method for 18 hr individually. The extracts so obtained were concentrated to dryness on a water bath below 60oC. The dried extracts were then stored in a cool place for further usage. A polyherbal extract was prepared using the above-obtained extracts by mixing them in the ratio of 1:1:1:1.
Qualitative Phytochemical Identification Test
Quantitative Estimation of Phytoconstituents Total phenolic content
This method was taken from Stoilova et al. The UV spectrophotometer was used to check the absorbance at 750 nm using Gallic acid as standard. The result was expressed in mg of Gallic acid Equivalent (GAE)/1g of DPE (dry plant extract).28 (y = 2.51x + 0.0921R² = 0.9958).
Total Flavonoids contents
The procedure given by Ajay et al. was used. The UV spectrophotometer was used to check the absorbance at 510 nm using rutin trihydrate as standard. The results were expressed in rutin trihydrate equivalents (RE)/1 g of DPT as standard was used to check the UV spectrophotometer.28 (y = 0.6733x + 0.0214, R² = 0.9926).
Total Tannins content
The Folin—Ciocalteu technique was used for the estimation of Total tannins content. The UV spectrophotometer was used to check the absorbance at 725nm using gallic acid as standard. The results were expressed in mg of Gallic acid Equivalent to mg/g GAE.29 (y = 6.0367x +0.0555, R² = 0.995).
High-Performance Liquid Chromatographic analysis
The method used by Piyush Kashyap et al. was used. The phenolic compounds as well as beta carotene were analysed using High Performance Liquid Chromatography technique. The qualitative as well quantitative evaluation was done. The ethanolic extracts of Azadirachta indica (Neem) leaf, Withania coagulans (paneer dodi) fruit, Picrorhiza kurroa (Kutki) rhizomes, Pterocarpus marsupium (Vija Sar) wood as well as polyherbal extract samples were investigated using Water’s HPLC 2489 equipped with C18 column (Phenomix; 100 mm × 4.6 mm, 5 μm). The system was operated at a flow rate of 1 mL/min consisting of (A) Acetonitrile and formic acid (99.8:0.2 v/v) and (B) water, acetonitrile, and formic acid (96:3.8:0.2 v/v). The gradient of elution comprised of A:B (95:5) at 5min, 85:15 at 25 min, 80:20 at 30 min, 75:25 at 39 min, 55:45 at 43 min, 5:95 at 48 min to 55 min, 80:20 at 55 min and 100:0 at 60 min. The injection volume of 10 μL was used. The mobile phase was run for total of 60 min. and phenolic as well as beta carotenoids were noticed at 280 nm. The column was washed using the mobile phase concentration increased to 100% of B for 1 min and upheld for 5 min prior to coming back to the earlier situation. The peak of each phenolic compound as well beta carotene was recognized with the standards by comparing the retention time and their quantification was done using standard curves.30
Antioxidant activity α- α-Diphenyl-β-picryl-hydrazyl radical scavenging (DPPH) Assay.
The procedure mentioned by Ajay et al. where gallic acid was used as a standard was employed. The results were evaluated in mg GAE/g dried extract. The percentage inhibition (I%) was calculated using the equation given below:

Total Antioxidant capacity
In vitro Antidiabetic Activity Determinations of α-Amylase inhibition activity
The method given by Demelash Z et al. was used to estimate the in vitro antidiabetic activity using acarbose as a positive control.
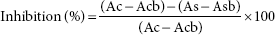
Where AC- control aborbance-samples %[DPPH free radical = Acb standard solutions absorbance. The IC31 valuew values were calculated by plotting mylase inhibition against extract concentration.32
α- Glucosidase Assay
The method given by Rege A. A. et al. was used to estimate the in vitro antidiabetic activity by α- Glucosidase assay method. Standards contained acarbose at different concentrations (10-100 g/ml). Parallel to this, negative control without extracts was put up. It was estimated as percentage inhibition.32

Where A is absorbance.
RESULTS
Qualitative Phytochemical Identification Test
The primary screening of polyherbal extract showed the presence of tannins, phenolics, flavonoids, saponins, terpenes and sterols along with glycosides in the polyherbal extract.
Total Phenolic, Flavonoid and Tannin Content
The total phenolic, flavonoid, and tannin content is mentioned there in Figure 1. The phenolic content of neem leaves (387.76±.23) (mg GAE/g DPE) was found to be maximum as well as comparable to Pterocarpus marsupium (386.50±.718) followed by Withania coagulans fruit (273.15±1.82) and in last Picrorhiza kurroa rhizomes (219.96±.87).
The flavonoid content was again found to be maximum in Pterocarpus marsupium i.e. 173.17±10.39 ((RE)/1 g) followed by Withania coagulans (211.79±5.35), Azadirachta indica (32.08±6.81) and in last Picrorhiza kurroa (9.31±4.53) mg RE/g DPE.
The pattern is the same as Pterocarpus marsupium wood showed a maximum amount of tannin (10.04±.028) followed by Withania coagulans (5.02±.075), Picrorhiza kurroa (2.64±.050) and in last Azadirachta indica (0.16±.047) mg GAE/g DPE.
High-performance Liquid Chromatographic Analysis
The phytochemical investigation report of ethanolic polyherbal extract for phenolic as well beta carotene has been shown in Table 1 and Figure 2. are 13 compounds in order as Beta carotene> rutoside> Hesperidin > tannic acid> ellagic acid> caffeic acid> chlorogenic acid> gallic acid> ferulic acid> benzoic acid > quercetin From above results it has been observed that beta carotene followed by hesperidin is present in maximum amount and quercetin the follow the routine of minimum. The presence of phenolic and beta carotene compounds may be found responsible for its various activities.
| Standards | Polyherbal Extract | |
|---|---|---|
| RT | Conc. ug/g of DPE | |
| Gallic acid | 2.2 | 7.14 |
| Chlorogenic acid | 14.52 | 7.58 |
| Caffeic acid | 17.91 | 7.77 |
| Catechin HYD | —– | —- |
| Ellagic acid | 26.87 | 12.73 |
| Rutoside | 28.82 | 17.20 |
| Ferulic acid | 29.95 | 5.81 |
| Rutin | —- | —- |
| Benzoic acid | 32.06 | 2.18 |
| Tannic acid | 35.22 | 14.69 |
| Hesperidin | 34.11 | 16.83 |
| Quercetin | 44.43 | 0.35 |
| β-carotene | 50.46 | 251.50 |
In vitro Antioxidant Activity
The range of radical scavenging activity of various extracts is from 88.29 to 22.69. The radical scavenging activity of Pterocarpus marsupium was found to be maximum 88.29±0.265 followed by polyherbal extract 71.14±0.321, Picrorhiza kurroa 65.94±0.655, Withania coagulans 51.29±0.769 and in last Azadirachta indica 22.69±0.89. The scavenging activity of Pterocarpus marsupium was found to be comparable to Gallic acid (90.940 ± 0.684).
The results of TAC of the different extracts varied between 21.53 to 12.88 mg RE/g DPE. The order was Pterocarpus marsupium (21.53±0.050)>
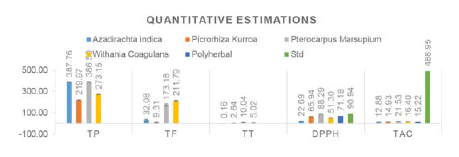
Figure 1.
Quantitative estimation of TP, TF, TT, DPPH, TAC.
TP: Total Phenolic content (mg GAE/g DPE), TF: Total Flavonoid content (mg RE/g DPE), TT: Total Tannin content (mg GAE/g DPE), DPPH: α- α-Diphenyl-β-picrylhydrazyl radical scavenging (DPPH) Assay (mg GAE/g DPE); TAC: Total antioxidant capacity (mg QE/g DPE); Poyherbal: Polyherbal extract, Std: Standard. Gallic acid is standard in DPPH assay, and Quercetin is standard in TAC.
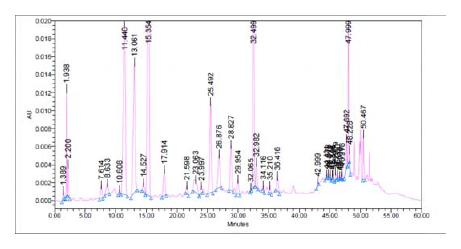
Figure 1.
HPLC analysis of the polyherbal extract.
Withania coagulans (16.40±0.11)> Polyherbal Extract (15.22±.051)> Picrorhiza kurroa (14.93±.151)> Azadirachta indica (12.88±0.06). Here it is to be mentioned that the results were compared to the standard Quercetin which showed total antioxidant capacity (488.95 ±0.79) which is very much high than those found in herbal drug extracts.
In vitroAntidiabetic Activity
Hyperglycaemia is controlled by inhibiting α-amylase and α-glucosidase.33 As a positive control, acarbose was used to test the polyherbal extract’s putative inhibitory effects on α-amylase. The in vitro experiments were used to determine the effect of polyherbal ethanolic extracts on α-glucosidase enzymes.32,34 IC values of acarbose, crude extract, and fractions for inhibition of α-amylase and α-glucosidase are 50 given in Table 2.
| Conc. (µg/ml) | α- amylase | α- glucosidase | ||
|---|---|---|---|---|
| Acarbose | PHE | Acarbose | PHE | |
| 10 | 25.39±0.25 | 22.43±0.21 | 18.21±3.2 | 15.67±2.98 |
| 20 | 35.64±0.37 | 32.65±0.63 | 26.73±1.4 | 19.73±4.2 |
| 50 | 49.27±0.28 | 43.28±0.82 | 45.81±2.7 | 39.27±3.9 |
| 75 | 68.28±0.38 | 59.27±0.27 | 69.12±3.4 | 59.78±7.3 |
| 100 | 75.24±0.51 | 72.35±0.38 | 75.89±8.1 | 72.61±6.3 |
| IC50 | 49.64 | 58.81 | 55.96 | 64.81 |
DISCUSSION
The combination of various herbal drugs in a specific proportion will improve the therapeutic efficacy and decrease toxic effects by simultaneously acting on multiple targets, which meanderingly leads to enhanced patient compliance and therapeutic outcome. They show high performance, a broad therapeutic window, cost efficiency, easy availability, and minimal side effects.7,8 Therefore, in the present study, we are taking 4 different herbal medicines which act in the treatment of diabetes mellitus through various mechanisms of actions.35 The quantitative estimations showed the TPC > flavonoid content > tannins content in all four drugs. The TPC was found maximum in Azadirachta indica leaves and Pterocarpus marsupium, TFC was maximum in Withania coagulans and tannin content was maximum in Pterocarpus marsupium. If we see the overall results the Pterocarpus marsupium sample showed good performance in all fields. While doing HPLC analysis, it has been found that the polyherbal extract contains much amount of beta carotene followed by hesperidin, catechin, tannic acid, ferulic acid, caffeic acid, chlorogenic acid, etc. As is found in the literature that all the above-mentioned phytoconstituents are very good antioxidants. Therefore, the antioxidant activity may be found responsible for the antidiabetic effect of all the above-mentioned samples and polyherbal formulations. Hesperidin help by defending against hepatotoxicity caused by STZ-induced diabetes. β-carotene significantly chelates the reactive singlet oxygen and free radicals the conjugation is responsible for its activity, more the conjugation in structure, so is the ability of carotene to capture ROS. It may eliminate up to 1000 ROS. Hyperglycaemia leads to the production of ROS, where β-carotene controls the redox balance, inhibiting the production of new ROS (reactive oxygen species) as well as lipid peroxidation.36 It has been seen in literature that Ferulic acid treatment leads to a reduction in plasma sugar levels and an increase in insulin levels in the blood. It helped in increasing glucokinase activity and decreasing glucose-6-phosphatase and phosphor-enol-pyruvate carboxykinase activities in the liver. Caffeic acid has been found to show hypoglycaemic activity, improvement in insulin levels, and glucose tolerance in diabetic animals. Chlorogenic acid protects against retinopathy via inhibiting retinal neo-angiogenesis. It lowers the fasting plasma glucose and HbA1c levels. Tannic acid stimulates insulin-like glucose transport in adipocytes.37
Oxidative stress, an imbalance of production as well as the destruction of very reactive species, may cause impairment of lipid and protein oxidations, insulin sensitivity, impaired glucose tolerance, and β-cells’ dysfunction leading to NIDDM. All those conditions can be counteracted by the use of antioxidants.38
The antioxidant activity was calculated using DPPH and TAC method. It is here to be noted that the results of both methods followed the same pattern as that is seen in total tannin contents. The total tannins content was found to be maximum in Pterocarpus marsupium >Withania coagulans > Picrorhiza kurroa> Azadirachta indica. So from the above results, the antioxidant activity may be attributed to the tannins content of the drug. It is here found that the antioxidant activity of Polyherbal formulation is found to be lower than that of the individual extract of Pterocarpus marsupium. The reason behind this may be found in further research.
As carbohydrates are broken down into glucose, they may be broken down by α- amylase and α- glucosidase, two enzymes. α- amylase hydrolyses starch, which is broken down into glucose before absorption. A decrease in postprandial hyperglycemia can be achieved by inhibiting α-amylase, an enzyme that breaks down disaccharides in the small intestine to form glucose (α- glucosidase). In addition to boosting insulin secretion and glucose uptake by cells, plant metabolites can also decrease glucose synthesis and absorption. Inhibition of glucose absorption is one of the most significant methods used in the treatment of diabetes. Polysaccharides are hydrolyzed into little absorbable pieces by digestive enzymes that are inhibited. This prevents postprandial high blood glucose. α-amylase is regarded as one of the most essential digestive enzymes due to its function in the breakdown of polysaccharides. It is present in saliva and pancreatic juice. One of the promising treatments to reduce excessive postprandial blood glucose is to block this enzyme. A significant enzyme in digestion, α -glucosidase is found in the mucosal brush border of the small intestine. Processing and breakdown of complicated carbs into simple, absorbable sugars is its main function. An effective way to reduce excessive postprandial blood glucose levels is to block this enzyme.39 The drug behaved in a concentration-dependent manner and the IC values of the drug was found to be 58.81 in case of α- amylase inhibition and 64.81 in case of α- glucosidase inhibition for 50 the polyherbal extract. Table 2: In vitro antidiabetic activity of the polyherbal extract. The values are denoted in mean ± SEM. (n=3) . The units are denoted are in mg/ml Conc. Concentration, PHE: Polyherbal Extract
CONCLUSION
The ethanolic extracts of all four drugs showed the presence of phenolic, tannins, and flavonoid content. The HPLC analysis confirmed the above phytoconstituents along with beta carotene. An in vitro study of a poly-herbal combination of plants showed strong antioxidant effects. The polyherbal extract significantly reduced the alpha amylase as well as alpha glucosidase levels. The above results showed that the selected drug extracts and polyherbal extracts have polyphenols, tannins and flavonoids, which might be responsible for their antioxidant as well as antidiabetic effect. For further validation of above findings, it is necessary to perform the in vivo study for antidiabetic activity. To determine the mechanism by which this activity occurs, more investigations utilizing more specific approaches are necessary.
Cite this article
Kaur N, Sharma S. Quantitative Estimation of Phytoconstituents, Antioxidants and in vitro Antidiabetic Activity of Polyherbal Extract. J Young Pharm. 2022;14(4):382-6.
ACKNOWLEDGEMENT
The author is thankful to IKGPTU, Jalandhar, Director of ASBASJSM College of Pharmacy, Bela, Ropar, Punjab, for providing the facilities for the successful conduction of the research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care. 2018;41(5):963-70. [PubMed] | [CrossRef] | [Google Scholar]
- Nagja T, Kumar V, Sanjeev A. Anti-diabetic activity of a polyherbal formulation in streptozotocin induced Type 2 diabetic rats. J Nat Rem. 2017;16(4):148-52. [CrossRef] | [Google Scholar]
- Geberemeskel GA, Debebe YG, Nguse NA. Antidiabetic effect of fenugreek seed powder solution ( L.) on Hyperlipidemia in Diabetic Patients [ L]. J Diabetes Res. 2019;2019:8507453 [PubMed] | [CrossRef] | [Google Scholar]
- Krentz AJ, Bailey CJ. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs. 2005;65(3):385-411. [PubMed] | [CrossRef] | [Google Scholar]
- Cheng AYY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005;172(2):213-26. [PubMed] | [CrossRef] | [Google Scholar]
- Scheen AJ, Lefèbvre PJ. Oral antidiabetic agents. A guide to selection. Drugs. 1998;55(2):225-36. [PubMed] | [CrossRef] | [Google Scholar]
- Petchi RR, Vijaya C, Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin – nicotinamide induced diabetic Wistar rats. J Tradit Complement Med. 2014;4(2):108-17. [PubMed] | [CrossRef] | [Google Scholar]
- Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of Ayurveda. Pharmacogn Rev. 2014;8(16):73-80. [PubMed] | [CrossRef] | [Google Scholar]
- Lakshmi T, Krishnan V, Rajendran R, Madhusudhanan N. Azadirachta indica: A herbal panacea in dentistry – an update. Pharmacogn Rev. 2015;9(17):41-4. [PubMed] | [CrossRef] | [Google Scholar]
- Moaty AAA, El-Kholie EA, Adarous RA. The anti-diabetic effect of neem leaves (), in alloxan-induced diabetic rats. 2022;32(2):19-31. [PubMed] | [CrossRef] | [Google Scholar]
- Saleem S, Muhammad G, Hussain MA, Bukhari SNA. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother Res. 2018;32(7):1241-72. [PubMed] | [CrossRef] | [Google Scholar]
- Agrawal S, Bablani Popli D, Sircar K, Chowdhry A. A review of the anticancer activity of (Neem) in oral cancer. J Oral Biol Craniofac Res. 2020;10(2):206-9. [PubMed] | [CrossRef] | [Google Scholar]
- Rekha UV. Known data on the therapeutic use of (neem) for type 2 diabetes mellitus. Bioinformation. 2022;18(2):82-7. [CrossRef] | [Google Scholar]
- Singh P, Bajpai V, Gupta A, Gaikwad AN, Maurya R, Kumar B, et al. Identification and quantification of secondary metabolites of Pterocarpus marsupium by LC–MS techniques and its lipid lowering activity. Ind Crops Prod. 2019;127:26-35. [CrossRef] | [Google Scholar]
- Mishra A, Srivastava R, Srivastava SP, Gautam S, Tamrakar AK, Maurya R, et al. Antidiabetic activity of heart wood of Roxb. and analysis of phytoconstituents. Indian J Exp Biol. 2013;51(5):363-74. [PubMed] | [Google Scholar]
- Chawla R, Thakur P, Chowdhry A, Jaiswal S, Sharma A, Goel R, et al. Evidence based herbal drug standardization approach in coping with challenges of holistic management of diabetes: A dreadful lifestyle disorder of 21st century. J Diabetes Metab Disord. 2013;12(1):35 [PubMed] | [CrossRef] | [Google Scholar]
- Rashida V, Nisha A. Phytochemical and chromatographic analysis of flavonoid fraction isolated from methanolic extract of . J Phytopharmacol. 2022;11(2):79-88. [PubMed] | [CrossRef] | [Google Scholar]
- Bagyalakshmi J, Haritha H. Green Synthesis and Characterization of Silver Nanoparticles using and Assessment of its Antidiabetic Activity. Am J Drug Deliv. 2017;5(3) [CrossRef] | [Google Scholar]
- Thani PR. A comprehensive review on Royle ex Benth. J Pharmacogn Phytochem. 2021;10(3):307-13. [CrossRef] | [Google Scholar]
- Misar Wajpeyi S. Hepatoprotective and hypolipidemic effect of kutaki ( Royle ex benth.) – a review. Int J Res Anal Rev. 2020;6(1):782-8. [CrossRef] | [Google Scholar]
- Raut A, Dhami-Shah H, Phadke A, Shindikar A, Udipi S, Joshi J, et al. Array. J Ayurveda Integr Med. 2022:100558 [PubMed] | [CrossRef] | [Google Scholar]
- Nisar J, Shah SMA, Akram M, Ayaz S, Rashid A. Phytochemical Screening, Antioxidant, and Inhibition Activity of against α-amylase and α-glucosidase. Dose-Response. 2022;20(2):15593258221095960 [PubMed] | [CrossRef] | [Google Scholar]
- Ahmad R, Fatima A, Srivastava AN, Khan MA. Evaluation of apoptotic activity of methanolic extract against human breast cancer and Vero cell lines. J Ayurveda Integr Med. 2017;8(3):177-83. [PubMed] | [CrossRef] | [Google Scholar]
- Kumar P, Ram H, Kala C, Kashyap P, Singh G, Agnihotri C, et al. DPP-4 inhibition mediated antidiabetic potential of phytoconstituents of an aqueous fruit extract of (Stocks) Dunal: , and assessments. J Biomol Struct Dyn. 2022:1-23. [CrossRef] | [Google Scholar]
- Rani BS. Phytochemical Analysis and Evaluation of Latex of three plants L., R. Br. ex Roem and Schult, Wrightia tinctoria R. Br., Mem. Wern. Soc. Ann Rom Soc Cell Biol. 2020;24(2):1013-7. [CrossRef] | [Google Scholar]
- Makwana S, Mehere N, Bedarkar P, Biswajyoti P, Harisha CR. Comparative pharmacognosy and phytochemical evaluation of leaf, root and stem of Linn. (Bakuchi). Ayu. 2020;41(4):235-41. [PubMed] | [CrossRef] | [Google Scholar]
- Harborne JB. (Jeffrey B. Phytochemical methods: A guide to modern techniques of plant analysis. Chapman and Hall. 2007:p302 [PubMed] | [CrossRef] | [Google Scholar]
- Sharma A, Cannoo DS. Comparative evaluation of extraction solvents/techniques for antioxidant potential and phytochemical composition from roots of and quantification of polyphenolic constituents by RPHPLC-DAD. Food Measure. 2016;10(3):658-69. [CrossRef] | [Google Scholar]
- Izuegbuna O, Otunola G, Bradley G. Chemical composition, antioxidant, anti-inflammatory, and cytotoxic activities of cladodes. Plos One. 2019;14(1):e0209682 [PubMed] | [CrossRef] | [Google Scholar]
- Kashyap P, Riar CS, Jindal N. Effect of extraction methods and simulated gastrointestinal digestion on phenolic compound profile, bio-accessibility, and antioxidant activity of Meghalayan cherry () pomace extracts. LWT. 2022;153:112570 [CrossRef] | [Google Scholar]
- Sharma A, Cannoo DS. Effect of extraction solvents/techniques on polyphenolic contents and antioxidant potential of the aerial parts of and the analysis of their phytoconstituents using RP-HPLC-DAD and GC-MS. RSC Adv. 2016;6(81):78151-60. [CrossRef] | [Google Scholar]
- Demelash Z, Seid KJ, Seyfe Y, Atnafie A. Evaluation of and anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of (Rosaceae). J Exp Pharmacol. 2021;195(15) [CrossRef] | [Google Scholar]
- Telagari M, Hullatti K. Array. Indian J Pharmacol. 2015;47(4):425-9. [PubMed] | [CrossRef] | [Google Scholar]
- Rege AA, Chowdhary AS. Evaluation of alpha – amylase and alpha – glucosidase inhibitory activities of . Int J Pharm Sci Res. 2014;5(6):2261-5. [PubMed] | [CrossRef] | [Google Scholar]
- Jayakumar R. Herbal medicines for type-2 diabetes. Int J Diab Dev Ctries. 2010;30(3):111 [CrossRef] | [Google Scholar]
- Marcelino G, Machate DJ, Freitas KC, Hiane PA, Maldonade IR, Pott A, et al. β-carotene: Preventive Role for type 2 diabetes mellitus and Obesity: A review. Molecules. 2020;25(24) [PubMed] | [CrossRef] | [Google Scholar]
- Sun C, Zhao C, Guven EC, Paoli P, Simal-Gandara J, Ramkumar KM, et al. Dietary polyphenols as antidiabetic agents: Advances and opportunities. Food Front. 2020;1(1):18-44. [CrossRef] | [Google Scholar]
- Ali AM, Gabbar MA, Abdel-Twab SM, Fahmy EM, Ebaid H, Alhazza IM, et al. Antidiabetic potency, antioxidant effects, and mode of actions of Fruit Peel Hydroethanolic extract, hesperidin, and quercetin in nicotinamide/streptozotocin-induced Wistar diabetic rats. Oxid Med Cell Longev. 2020;2020:1730492 [PubMed] | [CrossRef] | [Google Scholar]
- Mechchate H, Es-Safi I, Louba A, Alqahtani AS, Nasr FA, Noman OM, et al. Array. Molecules. 2021;26(2) [PubMed] | [CrossRef] | [Google Scholar]
