ABSTRACT
Objectives: The present study describes about the Antibiotic-Food Interactions (AFI) and development of Antimicrobial resistance (AMR). Materials and Methods: The Micromedex medication database was used to find and identify potential antibiotic interactions. Micromedex is a trustworthy drug database that used to research medications, interactions, diseases, and dose estimations. Results: As a result, a total of 68 AFIs were obtained, including major, moderate, and Minor AFIs among them. AMR-causing interactions were found in 34 of the 68 AFIs (50%). Only 3 (4.41%) of these 34 (50%) interactions were minor, whereas the remaining 31 (45.58%) were moderate. Only moderate and minor interactions were expected to induce AMR evolution; no major interactions were found to be unrelated to AMR development. Conclusion: Antimicrobial resistance is an important public health concern throughout the world. A pharmacist is a healthcare expert who analyzes a prescription for possible medication interactions and eliminates them in order to improve the patient’s prognosis.
INTRODUCTION
Drugs taken at the same time can interact and affect the pharmacokinetics and pharmacodynamics of the medicine. Drug-Food Interactions (DFIs) have the same clinical significance as Drug-Drug Interactions (DDIs). When a drug is taken with food, the absorption, distribution, metabolism, excretion, and pharmacological impact of the medication are all affected. DFIs can result in therapeutic failure, major life-threatening side effects, or ADRs, and a patient’s hospital stay being extended. The DFI may influence the drug’s effectiveness and concentration available to be reduced or increased in some cases. It can result in treatment failure and drug toxicity.1–3
The antibiotics are the focus of this database study. Antibiotics, also known as antimicrobial medications, are secondary metabolites produced by bacteria, and synthetic or semi-synthesized compounds can suppress bacterial growth and survival, allowing the immune system the upper hand. As a reason, these medications can be used to treat infectious diseases.4,5 As a consequence, when antibiotics are used concurrently, there is a risk of developing Antimicrobial Resistance (AMR). It’s possible that the reason for prescription numerous antibiotics. Actually, these medications areto improve therapeutic efficacy and patient outcomes. Antibiotic interactions, on the other side, can be both synergistic and antagonistic, leading to the development of AMR.6,7 In the same way, AMR might arise as a result of Antibiotic-Food Interactions (AFIs). The AFIs can cause changes in the antibiotic’s pharmacokinetics and pharmacodynamics, which can lead to the development of AMR. The objective of this study is to predict the AMR through the AFIs by using the Micromedex database, the results are analyzed and documented for the future use to council the patients about the possibilities of AMR with food.
MATERIALS AND METHODS
IBM Micromedex 20.0 database was used to identify the AFIs. Micromedex is an evidence-based drug information database that contains information on drugs, drug interactions, patient care comments, IV compatibility, dose calculations, drug comparisons, and disease information.
Antibiotics Search Strategy
A Micromedex database keyword search revealed the entire number of antibiotics available in databases. When you type in the name of an antibiotic in a keyword search, we willget about 200 results. Some antibiotics in the above list were not found in the Micromedex database which includessulfadoxine, sulfamethopyrazine, sulfasalazine, mafenide, co-trimoxazole, pefloxacin, prulifloxacin, ceftazolin, ceftamet pivoxil, cefpirome, ceftobiprole, faropenem, sisomicin, framycetin, tedizolid, fusidic acid, colistin, methenamine, phenazopyridine, and isoniazid.
Procedure for Drug Interactions
The technique for getting medication interactions for the available antibiotics in the database, as well as the findings of interactions, is depicted in Figure 1. Select the drug interaction option after logging into the database, and then enter the 200 antibiotic drugs one by one using the database’s choices. By giving the name of the antibiotic and transferring it into the drug to check, you can find the necessary antibiotic in the matching antibiotic medications. Once entered all of the antibiotics, click the submit button. It lists all of the antibiotic interactions and allows the user to switch to alternative interactions options as needed (drug, food, ethanol, lab, pregnancy, and lactation).
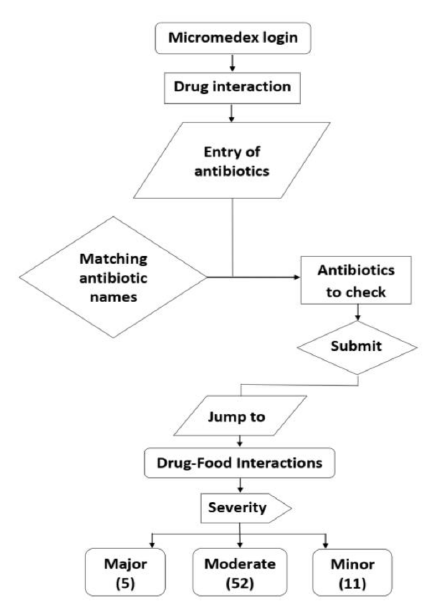
Figure 1.
The procedure for determining DFIs and their outcomes.
Drug Interaction Analysis
After receiving all the antibiotic medication interactions, the information was double-checked to see if there were any antibiotics that had been missed. During the drug’s entry into the system, no antibiotics were missed, according to the analysis.
RESULTS
A total of 68 AFIs were obtained as a result, in those AFIs some was major, moderate and minor. Among the 68 AFIs 34 (50%) were identified as AMR causing interactions. In these 34 (50%) interactions, only 3 (4.41%) were minor interactions and the remaining 31 (45.58%) were as moderate interactions. There were no major interactions, which were not relating to AMR development; only moderate and minor interactions were predicted as to cause the AMR evolution. The all predicted AFIs were displayed in the Table 1. As a result of the concomitant use of antibiotics with food or dairy products, the concentration of antibiotics was decreased/ altered, and the antibiotic’s efficiency was reduced. Penicillins, macrolides, cephalosporins, fluoroquinolones, and tetracyclins were the most involvedantibiotics in AFIs. The prediction of AMR was done based on the spectrum of activity antibiotics. The AMR prediction information was given the Table 2.
| S. No | Drug involved in drug-food interaction | Severity | Summary | Documentation | |
|---|---|---|---|---|---|
| Drug | Food | ||||
| 1 | Ampicillin | Food | Moderate | Concurrent use of ampicillin and food may results in decreased ampicillin concentration | Good |
| 2 | Ampicillin sodium | Decreased concentration | Good | ||
| 3 | Ampicillin sodium + Sulbactam sodium | Decreased concentration | Good | ||
| 4 | Benzoyl peroxide + erythromycin | Altered concentrations. | Good | ||
| 5 | Cefaclor | Decreased concentration | Good | ||
| 6 | Enoxacin | Decreased effectiveness | Fair | ||
| 7 | Erythromycin | Altered concentrations | Good | ||
| 8 | Erythromycin estolate | Altered concentrations | Good | ||
| 9 | Erythromycin ethylsuccinate | Altered concentrations | Good | ||
| 10 | Erythromycin ethylsuccinate + sulfisoxazole acetyl | Altered erythromycin concentrations | Good | ||
| 11 | Erythromycin gluceptate | Altered concentrations | Good | ||
| 12 | Erythromycin lactobionate | Altered concentrations | Good | ||
| 13 | Erythromycin stearate | Altered concentrations | Good | ||
| 14 | Lincomycin hydrochloride | Decreased exposure | Good | ||
| 15 | Demeclocycline hydrochloride | Minor | Decreased levels | Good | |
| 16 | Norfloxacin | Reduced effectiveness | Fair | ||
| 17 | Nafcillin sodium | Decreased concentrations | Fair | ||
| 18 | Penicillin G benzathine | Moderate | Decreased peak concentrations | Good | |
| 19 | Penicillin G procaine | Decreased peak concentrations | Good | ||
| 20 | Penicillin G potassium | Decreased peak concentrations | Good | ||
| 21 | Penicillin G potassium/sodium chloride | Decreased peak concentrations | Good | ||
| 22 | Penicillin G sodium | Decreased peak concentrations | Good | ||
| 23 | Oxacillin sodium | Decreased concentration | Fair | ||
| 24 | Loracarbef | Prolonged time to peak concentration | Good | ||
| 25 | Ciprofloxacin | Dairy Foods | Decreased concentrations | Good | |
| 26 | Ciprofloxacin hydrochloride | Decreased concentrations | Good | ||
| 27 | Ciprofloxacin lactate | Decreased concentrations | Good | ||
| 28 | Democlocycline hydrochloride | Decreased absorption and efficacy | Fair | ||
| 29 | Gemifloxacin mesylate | Decreased concentrations | Fair | ||
| 30 | Minocycline hydrochloride | Decreased concentrations | Good | ||
| 31 | Norfloxacin | Reduced mean peak plasma concentration. | Good | ||
| 32 | Oxytetracycline hydrochloride | Decreased effectiveness | Good | ||
| 33 | Oxytetracycline hydrochloride+polymyxin B sulfate | Decreased effectiveness | Good | ||
| 34 | Tetracycline hydrochloride | Decreased concentrations. | Good | ||
| Family/Antibiotic | Risk of resistant microorganism | ||
|---|---|---|---|
| Gram Positive | Gram Negative | ||
| Ampicillin | Listeria monocytogenes | Escherichia coliProteus species Salmonella typhiShigellaHaemophilus influenzaeHelicobacter pyloriPseudomonas aeruginosaKlebsiella | |
| Cefaclor | Streptococcus pneumonia Streptococcus pyogenes | Haemophilus influenzaeEscherichia coliM catarrhalisProteus speciesKlebsiella | |
| Loracarbef | Marketing end on 2006 | ||
| Enoxacin | Staphylococcus epidermis | Neisseria gonorrhoeaeEscherichia coliKlebsiellaProteus speciesPseudomonas aeruginosa | |
| Erythromycin | Streptococcus pyogenesStreptococcus pneumoniaClostridium perfringensCorynebacterium -diphtheriaeListeria monocytogenes | Neisseria gonorrhoeaeMycoplasmaLegionella pneumophilaChlamydia trachomatisBordetella pertusis | |
| Lincomycin | Penicillin resistant staphylococci | Bacteroides fragilis | |
| Tetracyclines:e.g: DemeclocyclineMinocyclineOxytetracyclineTetracycline | Bacillus anthracis | Rickettsiae speciesChlamydiae speciesBrucella abortusMycoplasmaLeptospira | |
| Semisynthetic penicillins:e.g: Nafcillin Oxacillin | Staphylococci species | – | |
| Penicillin G | Streptococcus species, Bacillus anthracis Corynebacterium diphtheriae, | Neisseria gonorrhoeaeNeisseria meningitidisTreponema speciesLeptospira | |
| Fluoroquinolones:e.g: CiprofloxacinGemifloxacinNorfloxacin | Bacillus anthracis Staphylococcus aureus Mycobacterium Tuberculosis Streptococcus species Enterococcus species Mycobacterium avium | Escherichia coliKlebsiellaProteus speciesSalmonella typhiShigellaHaemophilus influenzaePseudomonas aeruginosaLegionella pneumophilaH. ducreyiVibrio choleraNeisseria gonorrhoeaeNeisseria meningitidisMycoplasma | |
DISCUSSION
Antibiotics proved effective in treating bacterial infections. Unfortunately, antibiotics have been misused for the past 50 years, and experts have recommended combination therapy to improve patient outcomes.8
Oral administration of medications is the most commonly prescribed dosage form by clinicians, and these dosage forms may be contributing to the emergence of AMR. When an antibiotic is taken with food, it causes an interaction that affects the patient’s Absorption, Distribution, Metabolism and Excretion (ADME) and therapeutic action.1–3
Finally, the efficiency of antibiotics will be reduced, and their concentration will be reduced. This was the rationale for predicting the AMR. That information was given in Table 1. Penicillins, macrolides, fluoroquinolones, cephalosporins, and tetracyclines were the most commonly involved classes in AFIs. The antibiotic efficacy and peak concentration of antibiotics were both reduced in all AFIs. In similar,and the repeated use of these drugs with foods surely can cause AMR development. AFIs can contribute to the emergence of AMR in patients and treatment failure as a result of these processes predicting that. The spectrum of activity of antibiotics was used to predict resistant microbes. The spectrum, which were illustrates how antibiotics affect microorganisms. The antibiotic either may beNarrow-spectrum, broad-spectrum, or extended-spectrum if involved in AFIs chances to get AMR. Even while antibiotics are effective at killing or inhibiting the growth of bacteria, misuse or improper use can lead to AMR.9-11
In Indian marketing, enoxacin was banned drug. Thus enoxacin was not available in the market. Although the risk of AMR was estimated, antibiotic and food interaction was available in the database. Loracarbef was a carbacephem antibiotic sometimes grouped together with the second-generation cephalosporin antibiotics and that was banned in the year 2006. As a reason, there was no information provided about loracarbef.12-13
To combat AMR, the consumer or patient required education and awareness. Health professionals will play a vital role in solving this important global health issue. The pharmacist must educate and counsel patients on the use of antibiotics, and the patient will be free of such outcomes and other consequences as a result. Because the pharmacist is a healthcare practitioner, they must aware of possible and actual DDIs as well as drug-food interactions. Before discharging a patient, pharmacists analyze the prescription and examine the drug chart for any drug-related issues, DDIs, and AFIs. Not only theassessment ofthe prescription, but it’s also important to educate and counsel the patient about medications, diseases, and lifestyle changes. Theclinical pharmacy is a professional service that is widely available in all nations and will be beneficial in this regard. To ensure the rational use of drugs, clinical pharmacists will attend to ward rounds and review every prescription for drug, dose, duration, frequency, and dosage forms. Thus, the clinical pharmacist will act as a link between the patient and the physician, leading to improved pharmaceutical care for the patient.3,14-15
One of the advantages of this database research is the ability to predict AMR using antibiotics and interactions. The databases offered documentation for interactions, but the risk must be further assessed using AMR databases, which validates AMR by microorganisms against antibiotics; this is the study limitation. This study suggests that if the AMR database is employed in this study, it will be able to confirm antimicrobial resistance predictions.
CONCLUSION
Antimicrobial resistance is a major public health concern around the world. The pharmacist is a healthcare expert who examines a prescription for possible medication interactions and eliminates them in order to improve the patient’s prognosis.
Cite this article
Battula P, Kumar BP. Potential Food Interactions of Antibiotics and Risk of Antimicrobial Resistance: A Database Research Study. J Young Pharm. 2022;14(4):420-4.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- Koziolek M, Alcaro S, Augustijns P, Basit AW, Grimm M, Hens B, et al. The mechanisms of pharmacokinetic food-drug interactions – A perspective from the UNGAP group. Eur J Pharm Sci. 2019;134:31-59. [PubMed] | [CrossRef] | [Google Scholar]
- Petric Z, Žuntar I, Putnik P, Bursac Kovacevic D. Food–drug interactions with fruit juices. Foods. 2020;10(1):33 [PubMed] | [CrossRef] | [Google Scholar]
- Zawiah M, Yousef AM, Khan AH, Al-Ashwal FY, Matar A, ALKhawaldeh B, Abduljabbar R, Abdo Ahmed AA, et al. Food-drug interactions: knowledge among pharmacists in Jordan. PLOS ONE. 2020;15(6):e0234779 [PubMed] | [CrossRef] | [Google Scholar]
- Ben Y, Fu C, Hu M, Liu L, Wong MH, Zheng C, et al. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: a review. Environ Res. 2019;169:483-93. [PubMed] | [CrossRef] | [Google Scholar]
- Michel JB, Yeh PJ, Chait R, Moellering RC, Kishony R. Drug interactions modulate the potential for evolution of resistance. Proc Natl Acad Sci U S A. [PubMed] | [CrossRef] | [Google Scholar]
