ABSTRACT
Background
The development of plant-based alternatives is gaining thrust in contemporary research, with the traditional use as male contraceptive agents. To explore anti-spermatogenic properties of Polyherbal Formulation (PHF) containing hydroalcoholic extracts of seed Neem (Azadirachta indica), seeds of papaya (Carica papaya), fenugreek (Trigonella foenum-graecum), cotton (Gossypium herbaceum) and fruit of Piper (Piper nigrum).
Materials and Methods
Extraction of plant materials was carried out using Soxhlet extraction and maceration. After the extraction process, different polyherbal formulations were prepared using the extract. Experiments were conducted by administering the hydroalcoholic extract of PHF to male rats at different doses of 200, 400, 800 mg/kg body weight/day for 28 days. The evaluation of anti-spermatogenic activity was conducted using parameters such as reproductive organ weights, hormonal analysis, including serum testosterone levels, FSH, and LH, sperm count, and motility were evaluated using semen collected from the cauda epididymis.
Results
After 28 days of treatment, a significant decrease (p<0.05) in testis weight was observed at a dose of 800 mg/kg body weight/day (0.95±0.04 g). A significant reduction in testis weight may result from lower serum testosterone levels and disrupted sperm development, as testosterone is crucial for spermatogenesis. The decreased sperm count observed may be due to lower levels of FSH and LH, which are essential for spermatogenesis. Histological analysis revealed experimental group exhibited sperm head and tail abnormalities compared with the control group.
Conclusion
The study indicates that the potential of plant-based polyherbal formulations exhibits significant anti-spermatogenic activity as a promising, safer alternative to synthetic male contraceptives.
INTRODUCTION
Overpopulation is increasingly recognized as a major global problem that can be addressed through biological control of human fertility. India is a highly populous country with a population of approximately 9.2 billion by 2050 (Vermaet al., 2021P; Manochaet al., 2023). Although advancements in reproductive biomedicine have led to the development of hormonal contraceptives, these often come with side effects (Joshiet al., 2011). Contraception can be achieved through various methods, which can be categorized into the four groups: natural, physical, chemical, and surgical. As most contraceptive methods are primarily designed for women, men have limited participation in family planning (Ghoshet al., 2015; Ghoshet al.,2017). The development of new methods for male fertility management could offer significant social and public health benefits. One of the main reasons for this huge disparity is the lack of safe, reversible, and effective methods (Longet al., 2019).
Phytotherapy has a very long tradition and a scientific explanation of herbal medicine and its extracts (Joshiet al., 2011). It is widely acknowledged today that many traditional medicines are preferred because they are more productive, socially acceptable, and more suitable for the human body, with fewer side effects and proven effectiveness (Longet al., 2019). Although very few contraceptives have been developed from plant extracts, their effectiveness has not been well established, and their mode of action is unknown to us (Goswamiet al., 2020).
Herbal contraceptives are a growing trend in current research, focusing on plants with anti-spermatogenic properties, though their precise mechanisms remain unclear. Global efforts are underway to assess the efficacy of such herbal formulation for male contraception. While some plant-based products have been formulated, their extract modes of action are not fully understood. Several plants, including Azadirachta indica, Albizzia lebbeck, Carica papaya, Piper nigrum, Trigonella foenum-graecum, Gossypium herbaceum, Jatropha curcus, Allium sativum, Momordica charantia, Rubia cordifolia, and Piper nigrum have been proven to have spermicidal effects in various studies (Kamboj and Dhawan, 1982; Riar., 2021; Gupta and Gupta J., 2020).
The reversibility of the anti-fertility effects of herbs and their active components could provide clinical advantages in the development of male contraceptives (Shwetaet al., 2011; Pham et al., 2023). Different herbs contain a variety of bioactive compounds. Combining these herbs in specific ratios may result in synergistic effects, where the overall therapeutic impact is greater than the sum of the effects of individual herbs (Shwetaet al., 2011). The anti-fertility activity of a solid pharmaceutical dosage formulation containing different extracts of seed Neem (Azadirachta indica), seeds of papaya (Carica papaya), fruit of Piper (Piper nigrum), fenugreek (Trigonella foenum-graecum), cotton (Gossypium herbaceum), and several excipients such as starch, microcrystalline cellulose, Lactose, Magnesium stearate, and talc. Different formulations were prepared from the mixture, and a preformulation study was conducted on the raw materials, followed by an evaluation of all selected formulations for final tablet preparation (Drisset al., 2017).
The development of novel herbal medications is becoming increasingly popular in current research. These medications use extracts from various plant components that have potent anti-fertility activity (Ogbuewu, 2011). This formulation would offer an alternative to synthetic contraceptives, giving individuals and couples more choices for family planning that are accessible, convenient, and reversible.
MATERIALS AND METHODS
Procurement and identification of the plant
Plants used in the research were procured from the local market of Bharuch in Gujarat, India. Fresh plant-like seeds of Neem (Azadirachta indica), and seeds of papaya (Carica papaya) were collected from the nearby village of Bharuch, and the dry part of the drug, like the fruit of Piper (Piper nigrum), fenugreek (Trigonella foenum-graecum), cotton (Gossypium herbaceum) was collected from the local Commercial market of Bharuch. Herbarium sheets were prepared and authenticated by Dr. P.S. Nagar, Department of Botany, BARO herbarium of the Maharaja Sayajirao University of Baroda, Gujarat, with voucher specimen no. BARO (AN30725, AN30726, AN30723, AN30724, AN728) and submitted.
Preparation of extract
All plants were cleaned, dried, and powdered with a grinder. After all, the dried powdered plant materials (500 g) were subjected to extraction using different extraction methods such as Soxhlet apparatus, maceration, using various solvents like methanol, water, ethyl acetate, benzene etc. After the extraction process, the solvent is evaporation of the filtrate to obtain a brownish-dark sticky residue. The percentage yield was found to be between 15-20% (w/w), respectively, and the final dry extract was kept in closed vessels at the control temperature and subjected to further study (Kumaret al., 2022; Chainpureet al., 2019).
Preparation of polyherbal formulation
The Wet granulation process is widely used in pharmaceutical herbal preparation for small-scale preparations. The different extracts and excipients are listed in Table 1. Each additional constituent and standardized extract in the formula is individually weighed, crushed, and sieved through a sieve no 80 (Pham et al., 2023; Gupta and Gupta, 2020). The granules were mixed and dried at 105ºC in a hot air oven (Maury and Kumar, 2019). Before punching the tablets, all the granules were mixed with magnesium stearate, the die cavity was adjusted to achieve the desired weight, and then the tablets were punched (Manocha, 2023).
| List of Ingredients | Amount (mg) for One Tablet | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | ||
| 1 | Azadirachta indica seed extract | 100 | 100 | 150 | 150 | 25 |
| 2 | Carica papaya seed extract | 50 | 50 | 100 | 50 | 100 |
| 3 | Gossypium herbaceum seed extract | 10 | 20 | 20 | 40 | 20 |
| 4 | Piper nigrum seed extract | 100 | 25 | 25 | 100 | 100 |
| 5 | Trigonella foenum graecum seed extract | 200 | 200 | 100 | 150 | 100 |
| 6 | Starch | 30 | 30 | 30 | 30 | 30 |
| 7 | Microcrystalline Cellulose | 42 | 30 | 60 | 42 | 60 |
| 8 | Magnesium stearate | 30 | 30 | 12 | 12 | 30 |
| 9 | Lactose | 38 | 115 | 103 | 76 | 85 |
| Total weight | 600 | 600 | 600 | 600 | 600 | |
Experimental Animal
This study utilized 30 healthy male Wistar albino rats, each weighing almost 200±10 g. The rats were kept at controlled environmental conditions, maintaining an ambient temperature of 24±3ºC and an RH of 30-70%, according to a 12-hr light-dark cycle. Animals were acclimatized for the week before the commencement of the experiments (Sharmaet al., 2023; Ghoshet al., 2017). Each rat was marked on the tail, and the cages were label with relevant study details, including study number, title, gender, dose, cage number, and animal number. The rats were provided with a diet and pure drinking water ad libitum (Kambleet al., 2017). The research received approval from the Institutional Animal Ethics Committee (IAEC) under [IAEC PIP/6/23] CPCSEA, and all procedures were conducted under the prescribed guidelines of the Committee for Control and Supervision of Experiments on Animals (CPCSEA), Government of India (Shah and Jhade., 2018).
Acute toxicity study of the extract
The experiments in this study were designed based on OECD’s Fixed Dose Procedure-reproductive Toxicity Guidelines (OECD/OCDE-420, 2001). A minimum lethal dose of the drug extract was determined by giving animals up to 2000 mg/kg of alcoholic extract. After two weeks of treatment, the animals’ clinical symptoms, behavioral patterns, and motility were examined (Jainet al., 2012; Lakshman and Changamma, 2015).
Treatment protocol
Five groups of animals were investigated for the experiments, including the control group. Each group consisted of 06 healthy male rats. Three doses were selected to carry out the experiment these were 200, 400, and 800 mg/kg body wt./day for 28 days to evaluate the anti-spermatogenic effect of the PHF. The treatment plan of each group was as follows (Table 2).
| Experimental Group | Specification |
|---|---|
| Group I (Normal Control) | Normal Saline-Oral. |
| Group II Vehicle Control | 1% Sodium CMC, 10 mL/kg body weight once a day. |
| Group III (200 mg/kg) | Rats were treated with polyherbal extract 200 mg/kg for 28 days. |
| Group IV (400 mg/kg) | Rats were treated with polyherbal extract- 400 mg/kg for 28 days. |
| Group V (800 mg/kg) | Rats were treated with polyherbal extract 800 mg/kg for 28 days. |
At the end of the treatment, the animals were anesthetized, and their body weight was measured on day 28. Before being sacrificed, the rats were weighed again, and their reproductive organs were separated. The testes and epididymis were examined for structural changes and preserved in Bouin’s fluid for further investigation (Ghoshet al., 2015).
Parameters For Antifertility
Body and sex organ weights
Effect on sperm viability and Sperm count
The epididymis was separated from the cauda regions and kept in Petri dishes containing 1 mL of 0.9% NaCl solution at 36ºC to improve sperm viability during the study period. Each rat in each group had its cauda epididymidis removed epididymis and spermatozoa were extracted by flushing with a suspension medium (Bhaktaet al., 2018; Pokharkaret al., 2009; Mishra and Singh, 2009). The existence of spermatozoa in the epididymal suspension sample was confirmed by the eosin-nigrosin staining following the standard protocol. Using a hemocytometer, spermatozoa are counted in specific grid areas under a microscope (Duryatet al., 2025).
Serum Testosterone, FSH and LH Assay
Serum testosterone, FSH and LH levels were determined by ELISA kit. The kit was purchased from Lilac Medicare Pvt. Ltd., Mumbai, India.
Statistical analysis
All data are expressed as Mean±SEM (n=6). Statistical significance (p<0.001) was assessed by comparing the treated group with the control group using ANOVA followed by Bonferroni post hoc analysis.
RESULTS
Toxicity study
The rats did not exhibit any apparent signs of toxicity at a dose of 2000 mg/kg, representing the LD50, which was found to be beyond 2000 mg/kg and was used for antifertility investigation.
Body and relative organ weights
After 28 days of oral administration of PHF, that male rats did not show any significant difference in the body weight but a significant decrease (p<0.01) in the weight of the testis (0.95±0.04 g) at a dose of 800 mg/kg body weight per day as shown in (Table 3) when compared with control.
| Group | Initial Body Weight (g) | Final Body Weight (g) | Testis Actual weight (g) | Testis Relative weight (g) |
|---|---|---|---|---|
| Normal Control | 269±5.64 | 263.83±6.77 | 2.8±0.06 | 1.06±0.03 |
| Vehicle Control | 261±6.43 | 262.17±5.4 | 2.76±0.03 | 1.03±0.03 |
| Group III (200 mg/kg) | 263±7.67 | 258.83±9.28 | 2.72±0.07 | 1.05±0.05 |
| Group IV (400 mg/kg) | 263±7.54 | 261.83±7.54 | 2.6±0.005 | 1±0.04** |
| Group V (800 mg/kg) | 267±7.34 | 263.17±7.51 | 2.5±0.03 | 0.95±0.04** |
Sperm viability and sperm count
Sperm viability of the Vehicle control rats (Group 2) spermatozoa possess normal morphology (Figure 1a, 1b). On the other hand, treated group with a dose of 800 mg/kg Body wt/day showed a significant reduction (p<0.01) compared to control rats (54%). The caudal epididymal sperm count was significantly reduced (p<0.01) that were treated with the extract of PHF (49.27±1.96 with 800 mg/kg b.wt./day in comparison to control rats (Table 4).
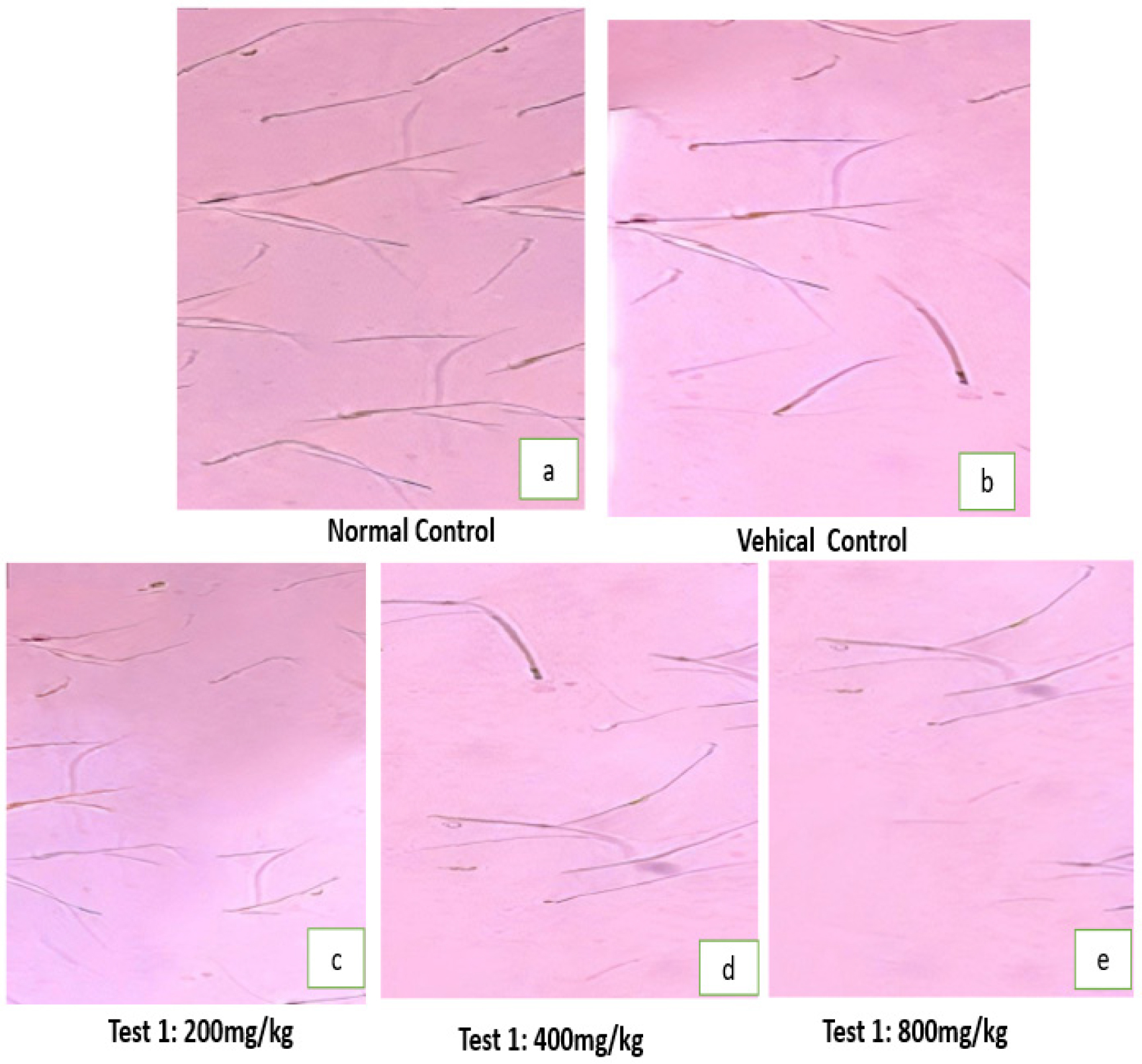
Figure 1:
Photomicrograph of Sperm morphology of treated rats with orally administered doses of PHF.
| Group | % Viability | Total sperm count 106/mL | Testosterone (ng/dL) | FSH (mIU/mL) | LH (mIU/mL) |
|---|---|---|---|---|---|
| Normal Control | 78±5 | 69.78±2.86 | 0.16±0.01 | 3.05±0.19 | 2.03±0.15 |
| Vehicle Control | 77±3.32 | 73.23±2.21 | 0.18±0.01 | 3.12±0.28 | 2.02±0.11 |
| Group III (200 mg/kg | 70.83±2.27 | 65.3±1.72 | 0.14±0.02 | 2.69±0.12 | 1.97±0.21 |
| Group IV (400 mg/kg) | 62.5±2.29 | 57.67±2.12 | 0.11±0.01 | 2.88±0.23 | 1.83±0.11 |
| Group V (800 mg/kg) | 54±4.23 | 49.27±1.96 | 0.1±0.01 | 2.47±0.32 | 1.64±0.13 |
Serum Testosterone, FSH and LH Assay
Serum testosterone levels were lowered significantly (p<0.01) that were treated with a dose of 800 mg/kg b.wt./day in comparison to control rats (0.16±0.01) (Figure 3)(Table 4). Experimental rats were given the drug at a dose of 800 mg/kg body weight per day (p<0.05), which produced a significant reduction in the serum levels of LH and FSH(Figures 4 and 5) (Table 4).
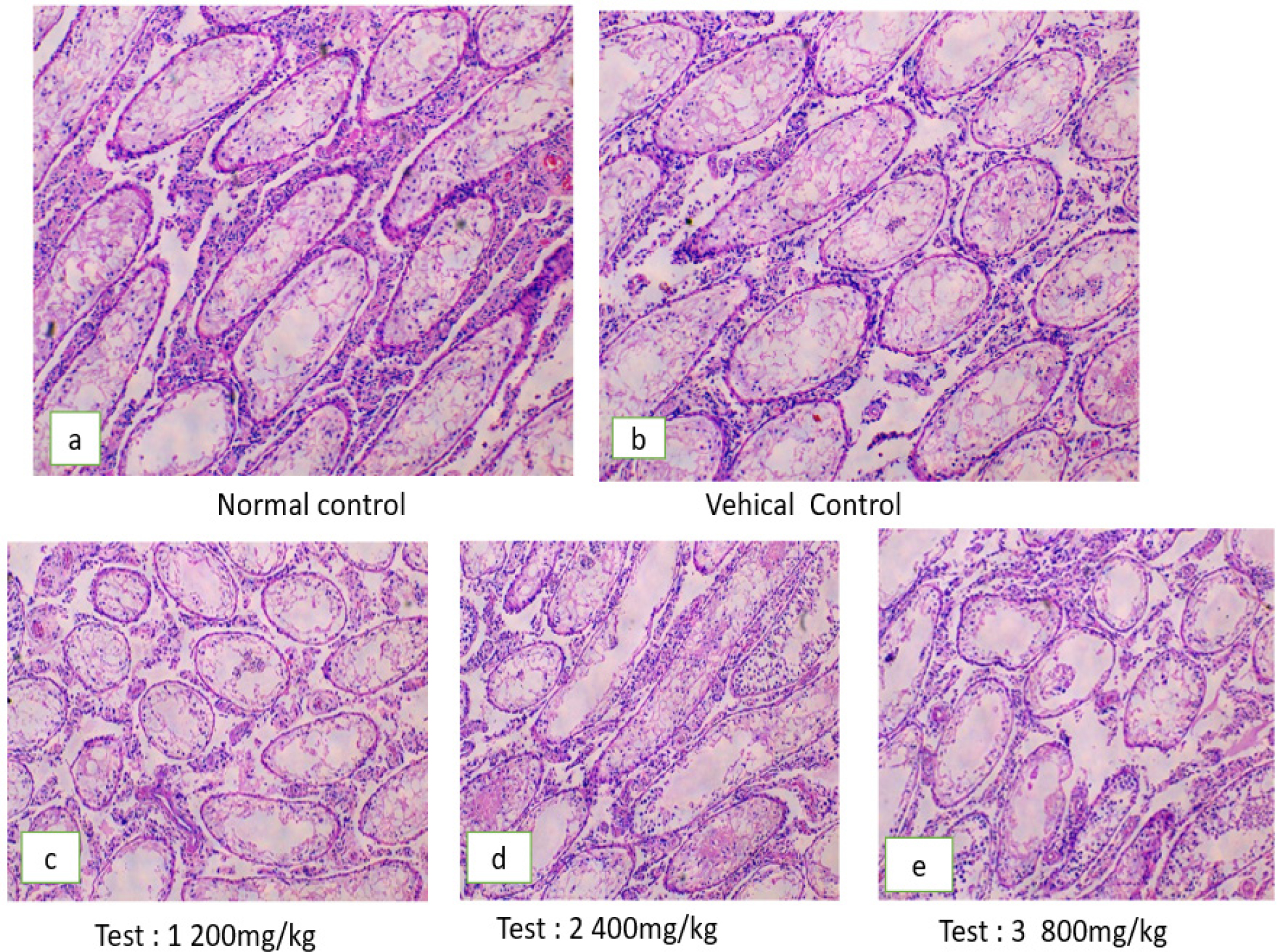
Figure 2:
Microphotograph of rat testis after treatment with different doses of PHF.
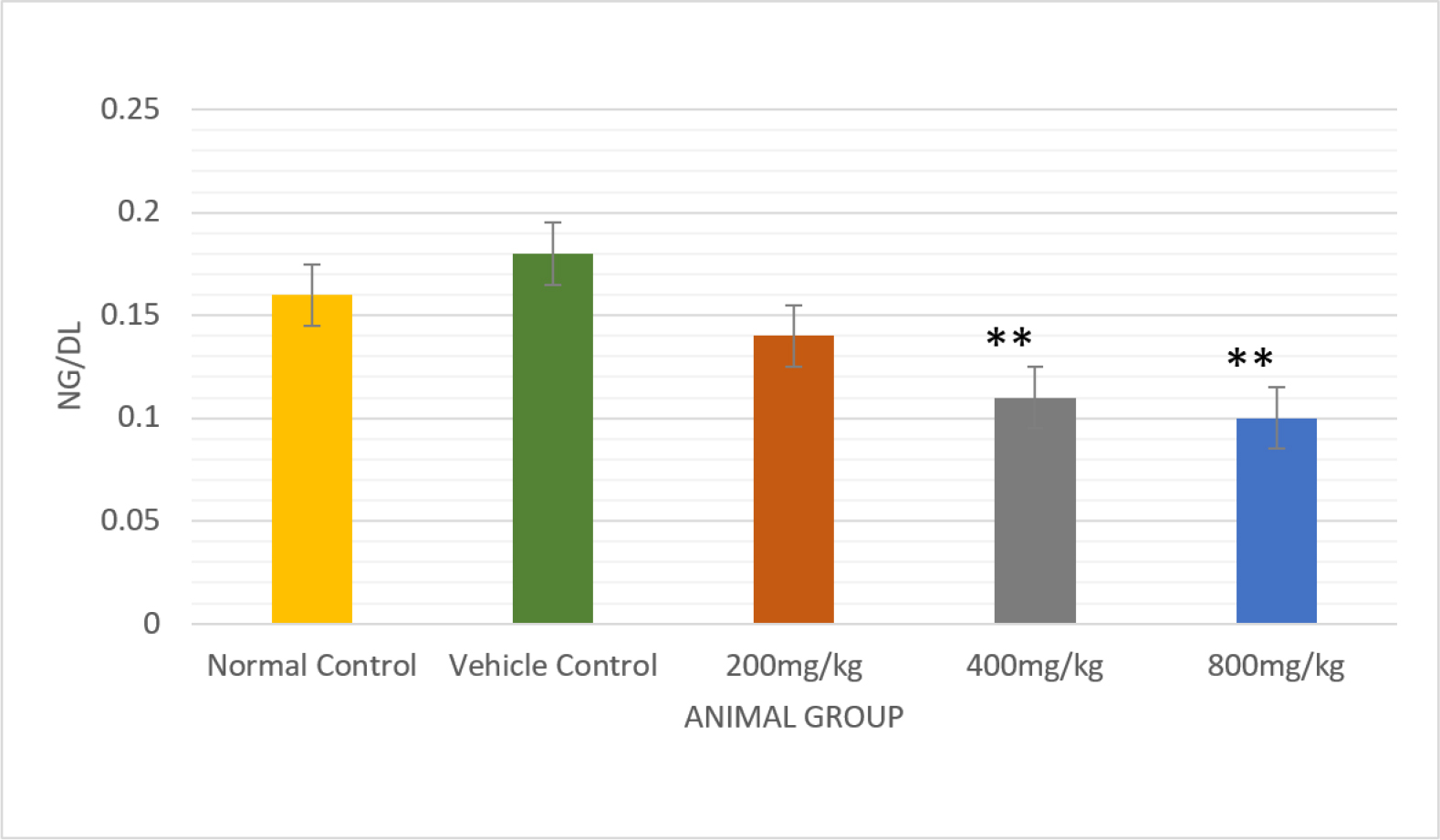
Figure 3:
Comparative analysis of the effect of Testosterone in normal and vehicle control treated rats with different doses of PHF bar graph, with a significant p<0.01.
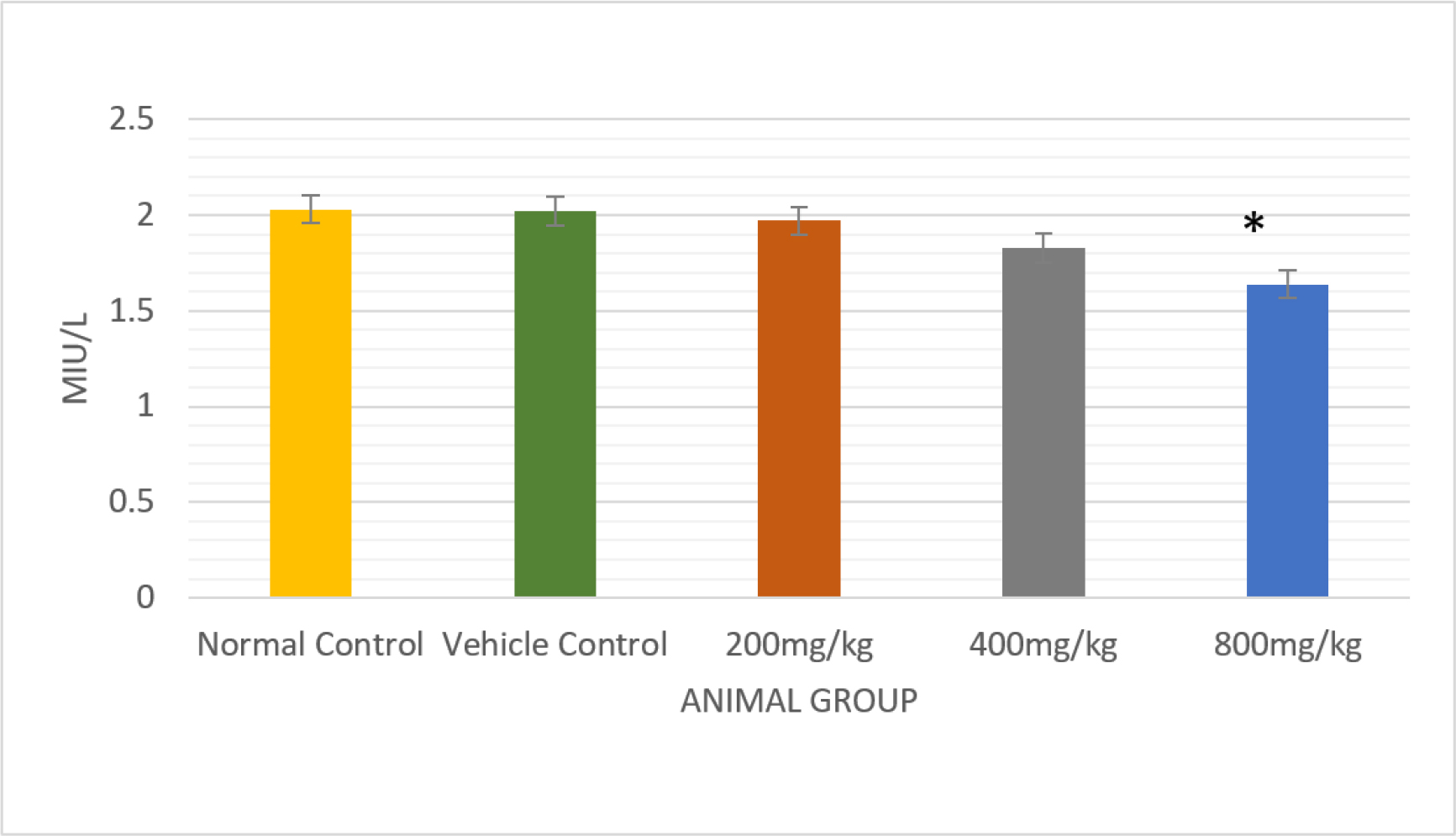
Figure 4:
Comparative analysis of the effect of LH in normal and vehicle control treated rats with different doses of PHF bar graph with significantly p<0.01.
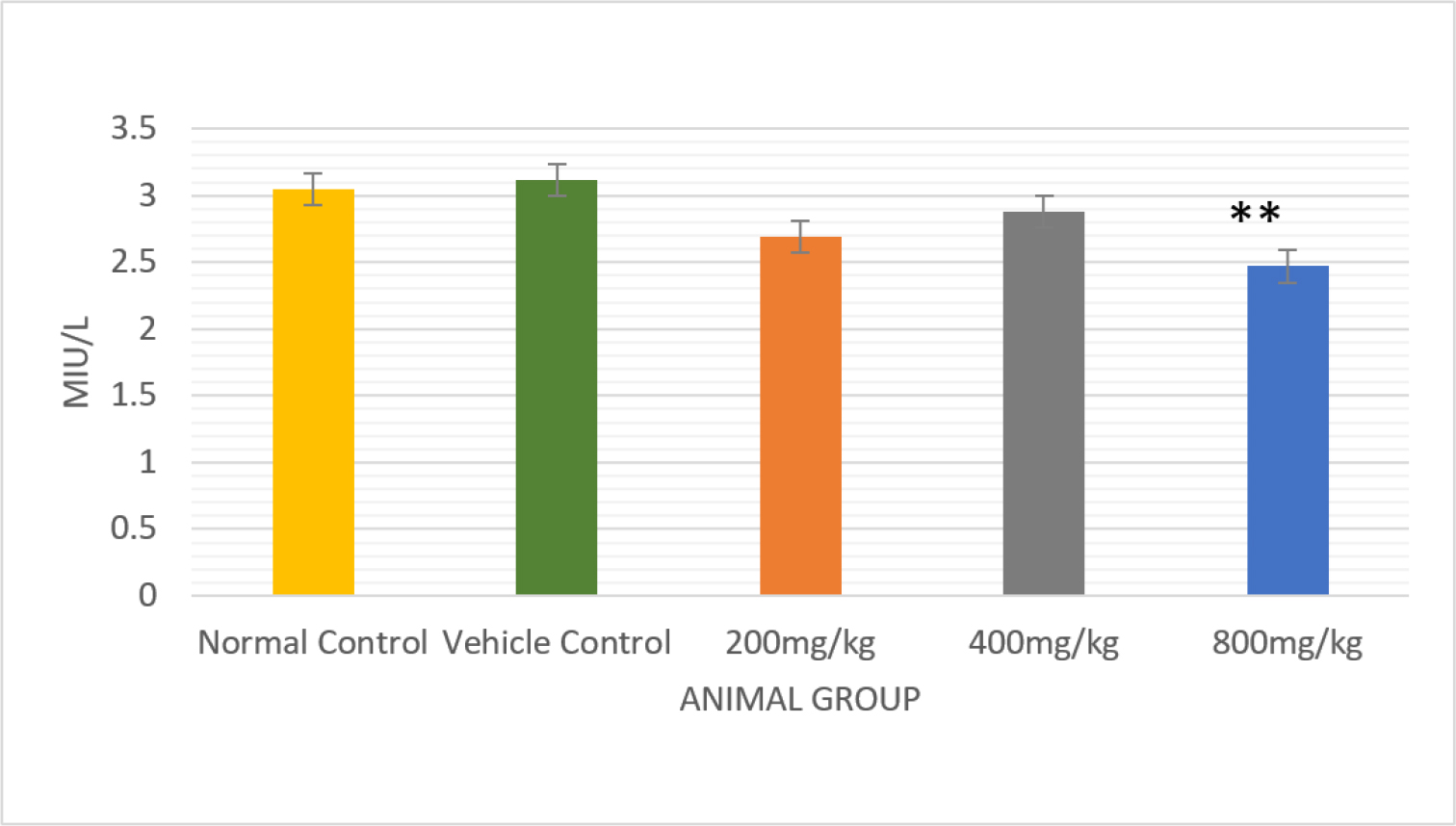
Figure 5:
Comparative analysis of the effect of FSH in normal and vehicle control-treated rats with different doses of PHF bar graph, with a significant p<0.01.
Histopathology of the testis
Histological analysis of the testis of control rats showed normal seminiferous tubules with active spermatogenesis (Figures 2a, 2b). In group II, there was a minor reduction in the quantity of sperm present within the lumen of the seminiferous tubules. (Figure 2c). Groups IV and V showed spermatogonial degeneration lining the seminiferous tubules and decreased sperm count (Figures 2d, 2e). Histological study of sperm dynamics and morphology was normal in the control group, whereas the sperm morphology in the experimental group showed sperm head and tail distortion, suggesting anti-spermatogenic and antifertility activity of PHF.
DISCUSSION
In the present investigation the anti-spermatogenic activity of hydroalcoholic extract of PHF, when administered orally when compared with the control groups rats at different dose levels to male rats did not show any significant difference in the body weight while there was a significant reduction in the weight of testis at 800 mg/kg b.wt./day dose level (p<0.01) (Table 3). Significant reduction in testis weight may be due to decreased levels of serum Testosterone and interference in the formation and maturation of spermatozoa (Manivannanet al., 2009).
The present study found that the extract of PHF, when administered orally to rats at different dose levels, it exhibited a significant reduction (p<0.01) in the levels of LH and FSH after administering the experimental rats with the drug at 800 mg/kg b.wt./day (Table 4). This reduction in hormonal levels might be presence of phytoconstituents to the extract of Azadirachta indica (neem) seed, extract exhibits spermicidal and contraceptive effects in male and female rats by altering hormonal levels (Akihisaet al., 2021).
In this study, from histological analysis of the testes in the control group, sperm structure and shape appeared unchanged, while treated animals exhibited sperm head and tail distortion, these abnormalities suggest that the extract may interfere with spermiogenesis and the structural integrity of developing spermatozoa, likely due to disruption in the maturation processes or altered Sertoli cell function (Gangwaret al., 2023).
A high level of intratesticular testosterone is required for normal spermatogenesis. The serum testosterone levels significantly reduced after administration of the drug at 400 mg/kg b.wt./day and 800 mg/kg b.wt./day when compared to the control group (p<0.01) (Table 4). It is evident that a reduction in testis weight and abnormality of sperm morphology may be attributed to a decline in testosterone production. Papaya seed extract significantly lowers testosterone levels (Kaur, Het al., 2021).
Histological study of sperm dynamics and morphology was normal in the control group (Figure 1a) whereas the sperm morphology in the experimental group showed sperm head and tail distortion (Figure 1e), suggesting anti-spermatogenic and antifertility activity of PHF. In our study, a decrease in the sperm count of the cauda epididymis following treatment with hydroalcoholic extract may be due to inhibition of spermatogenesis.
In conclusion, the purpose of this research is to highlight plant drugs and their bioactive phytoconstituents that work to prevent conception. The development of male contraceptives may benefit clinically from the reversibility of the anti-fertility effects, and future studies should focus on identifying active phytoconstituents, assessing long-term safety, and conducting clinical trials to confirm efficacy in humans.
CONCLUSION
The present study showed that polyherbal extract impaired reproductive activity in treated male rats. The research revealed that the polyherbal extract has a strong potential to decrease spermatogenesis. The study of current research suggests that the administration to groups containing male rats at different dose levels significantly reduced sperm count and viability. This study revealed FSH and LH are critical for normal spermatogenesis, and their suppression is a key strategy in male contraceptive development and anti-spermatogenic therapies. In males, a reduction in testosterone levels can restrict spermatogenesis and lead to male infertility.
This study offers promising insights for the pharmaceutical industry, potentially paving the way for the development of herbal contraception. Such a drug could be an effective herbal drug for reducing spermatogenic activity, thereby promoting male involvement in family planning. Our current research proves that a PHF employs an anti-spermatogenic activity in treated rats
Cite this article:
Vasava P, Chakraborthy GS. Anti-spermatogenic Activity of Polyherbal Formulation on Male Wistar Albino Rats. J Young Pharm. 2025;17(3):661-8.
ACKNOWLEDGEMENT
The author thanks Dr. Snigdha Mandal, head of the Department of Pharmacology, and Mr. Tarun Lal, Assistant Professor, PIPR, Waghodia Vadodara, for providing the necessary resources and support to make this research possible.
ABBREVIATIONS
| μL | Microliter |
|---|---|
| mIU/L | Milliequivalents per liter |
| ng/dL | Nanograms per Deciliter |
| cm | Centimeter |
| B.wt | Body Weight |
| or.wt | Organ Weight |
| RH | Relative Humidity |
| PHF | Polyherbal Formulation |
| LH | Luteinizing Hormone |
| FSH | Follicle Follicle-Stimulating Hormone. |
References
- Akihisa T., Zhang J., Manosroi A., Kikuchi T., Manosroi J., Abe M., et al. (2021) Limonoids and other secondary metabolites of (neem) and var. siamensis (Siamese neem), and their bioactivities. Studies in Natural Products Chemistry 68: 29-65 https://doi.org/10.1016/B978-0-12-819485-0.00013-X | Google Scholar
- Bhakta S., Awal M. A., Das S. K.. (2018) A new polyherbal contraceptive rendering positive effects on hematology in Swiss albino mice. International Journal of Scientific and Engineering Research 9: 1913-1917 https://doi.org/10.1016/B978-0-12-819485-0.00013-X | Google Scholar
- Chainpure P. R., Patwekar S. L., Shivpuje S. S., Sheikh Z. A.. (2019) Formulation and evaluation of and herbal tablets used for anti-obesity. International Journal of Engineering, Science and Mathematics 8: 180-195 https://doi.org/10.1016/B978-0-12-819485-0.00013-X | Google Scholar
- Duryat D., Rodiani R., Maryono T.. (2025) Acute toxicity study of the leaf and fruit extracts of (Forssk.) on Wistar white male mice. Journal of Multidisciplinary Applied Natural Science 5: 288-304 https://doi.org/10.47352/jmans.2774-3047.247 | Google Scholar
- Gangwar C., Kumaresan G., Mishra A. K., Kumar A., Pourouchottamane R., Rai B., et al. (2023) Herbal remedies for male infertility and spermatogenic activity in animals: A review. In The Indian Journal of Animal Sciences Array: 843-852 https://doi.org/10.56093/ijans.v93i9.113326 | Google Scholar
- Ghosh A., Jana K., Pakhira B. P., Tripathy A., Ghosh D.. (2015) Anti-fertility effect of aqueous-ethanolic (1: 1) extract of the fruit of : Rising approach towards herbal contraception. Asian Pacific Journal of Reproduction 4: 201-207 https://doi.org/10.1016/j.apjr.2015.06.002 | Google Scholar
- Ghosh A., Pakhira B. P., Tripathy A., Ghosh D.. (2017) Male contraceptive efficacy of polyherbal formulation, Contracept-TM, composed of aqueous extracts of fruit and seed in rat. Pharmaceutical Biology 55: 2035-2042 https://doi.org/10.1080/13880209.2017.1357734 | Google Scholar
- Goswami P., Laskar M. A., Basak M.. (2020) A review on medicinal plants of the North Eastern Region with potential antifertility activity. Asian Journal of Pharmaceutical Research and Development 8: 162-165 https://doi.org/10.22270/ajprd.v8i3.762 | Google Scholar
- Hind B., Zineb M., Elbachir H., Najat E. A., Siham A., Driss R., et al. (2017) Evaluation of potential effects of the aqueous extract of fenugreek seeds on fertility in male rats. Journal of Ayurvedic and Herbal Medicine 3: 210-215 https://doi.org/10.31254/jahm.2017.3408 | Google Scholar
- Jain S., Jain A., Paliwal P., Solanki S. S.. (2012) Antifertility effect of chronically administered leaf extract on male rats. Asian Pacific Journal of Tropical Medicine 5: 547-551 https://doi.org/10.1016/S1995-7645(12)60096-0 | Google Scholar
- Joshi S. C., Sharma A., Chaturvedi M.. (2011) Antifertility potential of some medicinal plants in males: An overview. International Journal of Pharmacy and Pharmaceutical Sciences 3: 204-217 https://doi.org/10.1016/S1995-7645(12)60096-0 | Google Scholar
- Joshi V. K., Joshi A., Dhiman K. S.. (2017) The Ayurvedic Pharmacopoeia of India: Development and perspectives. Journal of Ethnopharmacology 197: 32-38 https://doi.org/10.1016/j.jep.2016.07.030 | Google Scholar
- Kamble A., Reddy C., Patili S.. (2017) Testicular activity of mice treated with MeOH extract of leaves. Journal of Advanced Medical Sciences and Applied Technologies 3: 93-100 https://doi.org/10.18869/nrip.jamsat.3.2.93 | Google Scholar
- Kassem A., Al-Aghbari A., Al-Habori M., Al-Mamary M.. (2006) Evaluation of the potential antifertility effect of fenugreek () seeds in male and female rats. Contraception 73: 301-306 https://doi.org/10.1016/j.contraception.2005.08.017 | Google Scholar
- Kaur H., Singla N., Mahal A. K.. (2021) Antifertility effect of bait containing L. seed powder in male lesser bandicoot rat, (Gray and Hardwicke). Indian Journal of Experimental Biology (IJEB) 59: 448-457 https://doi.org/10.1016/j.contraception.2005.08.017 | Google Scholar
- Kumar V., Kushwaha V., Charde V., Jagtap C., Gandhi Y., Grewal J., Verma R., Rawat H., Mishra S. K., Thakur A., Babu G., Singh A., Singh R., Srikanth N., Dhiman K. S., et al. (2022) The validated pharmaceutical standard operating procedure and quality control study of the coded polyherbal tablet formulation AYUSH SG-5. South African Journal of Botany 151: 319-327 https://doi.org/10.1016/j.sajb.2022.02.038 | Google Scholar
- Long J. E., Lee M. S., Blithe D. L.. (2019) Male contraceptive development: Update on novel hormonal and nonhormonal methods. Clinical Chemistry 65: 153-160 https://doi.org/10.1373/clinchem.2018.295089 | Google Scholar
- Majumder P., Paridhavi M.. (2016) Physicochemical standardization and formulation development of polyherbal tablet for diabetes. British Journal of Pharmaceutical Research 12: 1-17 https://doi.org/10.9734/BJPR/2016/26599 | Google Scholar
- Manivannan B., Mittal R., Goyal S., Ansari A. S., Lohiya N. K.. (2009) Sperm characteristics and ultrastructure of testes of rats after long-term treatment with the methanol subfraction of seeds. Asian Journal of Andrology 11: 583-599 https://doi.org/10.1038/aja.2009.25 | Google Scholar
- Manocha R.. (2023) Impact of population, trade openness, and foreign investment on India’s environment: An empirical evaluation of a STIRPAT model. Jindal Journal of Business Research 12: 209-232 https://doi.org/10.1177/22786821231177121 | Google Scholar
- Maurya H., Kumar T.. (2019) Formulation, standardization, and evaluation of polyherbal dispersible tablet. International Journal of Applied Pharmaceutics 11: 158-167 https://doi.org/10.22159/ijap.2019v11i1.30113 | Google Scholar
- Mishra R. K., Singh S. K.. (2009) Reversible antifertility effect of aqueous rhizome extract of L. in male laboratory mice. Contraception 79: 479-487 https://doi.org/10.1016/j.contraception.2009.01.001 | Google Scholar
- Mohamed G. A., Ibrahim S. R., Al Haidari R. A.. (2014) A review on natural contraceptive agents. American Journal of PharmacyTech Research 4: 124-158 https://doi.org/10.1016/j.contraception.2009.01.001 | Google Scholar
- Ogbuewu I. P., Unamba-Oparah I. C., Odoemenam V. U., Etuk I. F., Okoli I. C.. (2011) The potential of medicinal plants as a source of new contraceptive principles in males. North American Journal of Medical Sciences 3: 255-263 https://doi.org/10.4297/najms.2011.3250 | Google Scholar
- Omagha R., Idowu E. T., Alimba C. G., Otubanjo A. O., Agbaje E. O., Oyibo W. A., et al. (2023) Alterations in testis histology, reproductive hormones, and abnormal sperm morphology in mice treated with polyherbal antimalarials. Drug Discovery 1: e29dd1947 https://doi.org/10.54905/disssi.v17i40.e29dd1947 | Google Scholar
- Pokharkar R. D., Saraswat R. K., Kanawade M. G.. (2009) Contraceptive evaluations of oil extract of seeds of (L.) in male albino rats. Pharmacologyonline 3: 905-914 https://doi.org/10.54905/disssi.v17i40.e29dd1947 | Google Scholar
- Reena G., Jitendra G.. (2020) Chromatographic fingerprint analysis of piperine in polyherbal and marketed formulation by HPTLC and GC-MS methods. Current Trends in Biotechnology and Pharmacy 14: 164-173 https://doi.org/10.5530/ctbp.2020.2.17 | Google Scholar
- Sharma R., Lakhne R., Gupta R. S.. (2023) Anti-spermatogenic activity of methanolic root extract. Journal of Experimental Biology and Agricultural Sciences 11: 359-370 https://doi.org/10.18006/2023.11(2).359.370 | Google Scholar
- Shweta G., Chetna R., Jinkal S., Nancy S., Hitesh J.. (2011) Herbal plants used as contraceptives. International Journal of Current Pharmaceutical Review and Research 2: 324-328 https://doi.org/10.18006/2023.11(2).359.370 | Google Scholar
- Verma S., Yadav A.. (2021) Rising trends towards the development of oral herbal male contraceptive: An insight review. Future Journal of Pharmaceutical Sciences 7: 1-15 https://doi.org/10.1186/s43094-020-00154-7 | Google Scholar
