ABSTRACT
Background
Onychomycosisis is a fungal nail infection mainly caused by funguses like Trichophyton rubrum, Aspergillus, and various species of Candida that impact the nail. Symptoms can manifest as a color change of the nails, appearing white or yellow, along with becoming thicker of the nails and separation of the nail plate from the nail bed. Ketoconazole-loaded fungicidal nail lacquer has been manufactured in the present study. The objective of the formulation’s development was to achieve enhanced adherence with the nail plate and sustained release of ketoconazole over a prolonged time frame, thereby reducing the frequency of administration, minimizing the systemic toxicity, improving the drug permeation and clinical effectiveness, and enhancing patient acceptance.
Materials and Methods
Nail lacquer formulations were created through simple mixing methods. Among all formulations, nail lacquers coded as F2, F3, F5, F6, and F8 were optimized based on drying time and subsequently examined for their physicochemical, molecular, and in vitro antimicrobial investigation.
Results
The drug permeation investigation indicated that the optimized formulations F2 and F3 showed the most prominent drug permeation. FTIR analysis indicated compatibility between the drug and excipients. The optimized formulations F2 and F3 exhibited a substantial inhibitory zone against Candida albicans.
Conclusion
The optimized compositions of nail lacquers may be regarded as a viable topical therapy method for onychomycosis.
INTRODUCTION
Onychomycosis, also known as tinea unguium, is a fungal condition that impacts the nail. Symptoms can manifest as a color change of the nails, appearing white or yellow, along with becoming thicker of the nails and separation of the nail plate from the nail bed (Dhingraet al., 2022). This is mainly linked to funguses like Trichophyton rubrum, Aspergillus, Epidermophyton floccosum, Trichophyton violaceum, Trichophyton soudanense, Interdigitale, Microsporum gypseum and various species of Candida (Bormanet al., 2015). Available treatment options are oral and topical therapy. The most used treatment method is oral therapy. Still, it has some unavoidable drawbacks like hepatotoxicity, and chances of drug-drug interaction, and as the disease is chronic it requires a longer treatment period which enhances the treatment cost also (Guptaet al., 2020). The topical administration of active pharmaceutical substances is advantageous because of its non-invasiveness, targeted distribution to the site of action, minimized systemic side effects and medication interactions, and enhanced patient compliance (Zhanget al., 2022). But the main issue of transungual drug delivery is the adherence of the formulation to the nail plate and the permeation of the drug through the nail plate (Elkeebet al., 2010). The nail plate of humans has a far more intricate construction than the situation seems at first appearance. It protects the nail bed, the area just underneath the nail plate having blood vessels, and the nail matrix, located at the proximal ventral surface of the nail, which is responsible for cellular proliferation and nail development (Berker et al., 2019). To enhance nail permeability, Transungual drug delivery mostly focuses on modifying the structure of the nail plate (Baranet al., 1996 and Vejnovicet al., 2010).
Physical and chemical methods are there but among both chemical permeation enhancers are most widely used due to their easy availability and they provide self-application options to the patients (Yuanet al., 2022 and Leeet al., 2024). Chemical penetration-enhancing substances such as mercaptans, urea, sulfites, hydrogen peroxides, keratolytic agents, and keratinolytic enzymes are used in topical formulations to improve the nail permeability of antifungal drugs (Kauret al., 2024). Optimal antifungal effectiveness may be attained with the use of medicated nail lacquers (Puriet al., 2022). Ketoconazole is a synthetic antifungal drug of the imidazole family with broad-spectrum efficacy. It serves as a model medication, functioning as an antifungal agent with both topical and systemic effects, suitable for incorporation into other pharmaceutical formulations (Maksimovet al., 2021). After application, medicated nail lacquer forms an occlusive film over the nail plate, serving as a drug reservoir and enhancing the formulation’s adherence to the nail plate. This facilitates sustained release of the antifungal, extending the duration of therapy, while the inclusion of chemical permeation enhancers improves nail permeation (Rahal et al., 2024). This study examined the efficacy of ketoconazole loaded nail lacquers in the topical treatment of onychomycosis.
MATERIALS AND METHODS
Chemicals Required
Ketoconazole was procured from Yarrow Chemicals. Ethyl cellulose and Nitrocellulose were obtained from SRL Laboratories. Ethanol, Ethyl acetate, and Propylene glycol were procured from CDH Laboratories. Sodium lauryl sulfate, PEG 300, and Lactic acid were of SRL Laboratories.
Preparation of nail lacquer
In one beaker required amount of ethyl cellulose and the drug was taken and ethanol was added to it. In another beaker, nitrocellulose was taken and ethyl acetate was incorporated to it. Both the beakers were covered with aluminum foil and were stirred properly with the help of a magnetic stirrer till a clear solution was obtained. The beaker containing nitrocellulose was incorporated into the beaker containing ethyl cellulose solution (Aggarwalet al., 2020 and Dehariet al., 2022). After that other ingredients were incorporated (Table 1).
| Ingredients (%) | F2 | F3 | F5 | F6 | F8 |
|---|---|---|---|---|---|
| Ketoconazole | 2 | 2 | 2 | 2 | 2 |
| Nitrocellulose | 4 | 6 | 4 | 6 | 4 |
| Ethyl cellulose | 3 | 1 | 3 | 1 | 3 |
| Lactic acid | 3 | 3 | 3 | 3 | 3 |
| Salicylic acid | 0.3 | 0.5 | 0.6 | 0.7 | 0.8 |
| PEG 300 | – | – | 5 | 5 | 5 |
| Ethanol: Ethyl acetate | 7 :3 | 7:3 | 7:3 | 7:3 | 7:3 |
Evaluation of Nail Lacquer
Optimization through drying time
A small amount of the prepared composition was poured and applied onto a glass slide using a brush. The required time to establish a dry-to-touch film was recorded by a stopwatch (Kushwahaet al., 2017 and Puriet al., 2022). According to the drying time, formulations were optimized and selected for further study.
Physical Properties
pH
Non-volatile content
Smoothness to flow
Water resistance
All the optimized nail lacquers were mixed with red color and poured into Petri dishes separately and allowed to dry and form the film. 20 mL water was poured into each petri dish and kept aside for 24 hr in ambient conditions (Murdanet al., 2017). After this period the leaching was visually analyzed.
Viscosity
The viscosity of the developed formulations was checked with the help of a rotational viscometer at 2 RPM (LABMAN), using the spindle number 4. A beaker having 30 mL of the developed formulation was placed in the platform of the viscometer and the spindle was dipped in the beaker containing the formulation and the viscosity was recorded (Laubéet al., 2019).
Adhesion
Equipment created at the laboratory was utilized to evaluate the adhesive properties of nail lacquer. The equipment was modified from a laboratory balance. Material holding vessel of the weighing balance was substituted with two plates and 1 mL of the formulation was affixed between the plates and evaluated the weight required to detach the plates (Brysonet al., 2022).
Thickness
The film’s thickness was measured at several locations using a digital vernier caliper. The thickness was recorded in three distinct locations on the prepared lacquer film and the mean was calculated (Shivakumaret al., 2010).
Drug content
1 mL of developed nail lacquer was measured and mixed with 100 mL of methanol. The was filtered using a 0.45 μm syringe filter, and after appropriate dilution, the sample was analyzed using a UV-visible spectrophotometer (UV-3200 LABINDIA, Mumbai, India) at 210 nm, from which the % drug concentration was estimated (Giri et al., 2024).
FTIR
Using an ATR-FTIR spectrometer, the formulations and the pure drug’s FTIR spectra were captured. A comparison was made between the drug’s obtained spectrum and the final formulation. The analysis was done to confirm the interaction between the drug and excipients (Aliet al., 2023 and Giriet al., 2024).
DSC
Differential scanning calorimetry was done to check the transformation of the drug from crystalline to amorphous state. Each sample was divided into 6 g and added to a separate aluminum pan. The temperature was recorded between 25 to 350ºC, and the heating scan rate was adjusted by 20ºC/min. With STARe 15.00 software, data were evaluated (Giriet al., 2024).
In vitro drug permeation
The permeation study was performed using a modified USP-II dissolution apparatus (DS-8000, LABINDIA, India). A semi-permeable membrane was attached to one end of a diffusion tube, which is open at both ends and has an inner diameter of 3.4 cm. 1 mL of the formulation was administered into the diffusion tube, which is often referred to as the donor chamber was tied with the paddle of the apparatus and dipped in the receptor chamber containing the 400 mL dissolution medium (normal saline with 1% tween-80). The receptor chamber was kept at 37±2ºC and of RPM 50. A volume of 5 mL of the sample was extracted at fixed intervals and substituted with an equivalent amount of new dissolving media. The samples were examined at a wavelength of 210 nm using a UV spectrophotometer (Giri et al., 2024).
Rheology
The rheological behavior of optimized nail lacquers was assessed using a parallel plate rheometer (Mars III, Thermo Fisher Scientific, Germany). For the studies, parallel plate geometry was used, with a diameter of 40 mm. Storage modulus (G’), Loss modulus (G”), and intrinsic viscosity of modified nail lacquers were investigated.
Anti-fungal study
Sabourad Dextrose Agar (SDA) was used as a medium for the study. Sterile SDA was poured into the Petri dish and allowed to solidify. After solidification of SDA, the fungal strain was inoculated and a sterile disc was dipped into the nail lacquer and placed in the center of the inoculated petri dish. Incubated for 36 hr and the zone of inhibitions were measured and noted down (Giriet al., 2024).
Stability
The temperature and relative humidity of the stability chamber were maintained at 40ºC and 75% RH throughout the experiment. At fixed intervals, samples were taken out and tested for color, odor, pH, texture, and drug content and followed as per ICH recommended guidelines of stability testing’s (Aliet al., 2023, Jena BRet al., 2021 and Giriet al., 2024).
RESULTS
Optimization through drying time
It was found that the F2, F3, F5, F6, and F8 showed the desired drying time and were optimized for further study (Table 2).
| Formulations | Drying time (sec) | Nonvolatile content | Smoothness to flow | Water resistance | Glossiness | Viscosity (cPs) | Adhesion | Thickness (mm) |
|---|---|---|---|---|---|---|---|---|
| F2 | 140± 1.2 | 33±0.38 | Excellent | No Leaching | Excellent | 122±2.24 | Yes | 0.1 |
| F3 | 123±2.6 | 33±0.38 | Excellent | No Leaching | Excellent | 136±3.41 | Yes | 0.2 |
| F5 | 80±1.3 | 41±0.81 | Good | No Leaching | Excellent | 139±1.16 | Yes | 0.1 |
| F6 | 92±2.42 | 39±0.40 | Excellent | No Leaching | Good | 168±2.46 | Yes | 0.1 |
| F8 | 96±1.83 | 35±0.71 | Good | No Leaching | Good | 156±1.62 | Yes | 0.1 |
Physical properties
Optimized nail lacquers were evaluated for color, order, and foreign particles against the clarity test apparatus. All the formulations were transparent and no particulate matter was found.
Non-volatile content
The increase in polymer concentration directly increases the non-volatile content of nail lacquer. The results are displayed in Table 2.
Smoothness to flow
Following the pouring of the nail lacquer over the glass plate and the subsequent raising of the plate to spread it, a uniform and smooth film developed. The study findings are presented in Table 2.
Water resistance
The mixtures exhibited no notable turbidity, blistering, or alterations in weight when in contact with water (Table 2).
Viscosity
The viscosity of all the optimized compositions was found in the range of 122 to 168 centipoise and findings are showcased in Table 2.
Adhesion
Formulation F3 exhibited the least film peel-off while demonstrating the highest adhesion, whereas formulation F6 displayed the greatest film peel-off accompanied by the lowest adhesion, results are illustrated in Table 2.
Thickness
The thickness of the films was measured with the help of a digital vernier caliper and was found in the range of 0.1 to 0.2 mm (Table 2).
Drug content
The drug content present in preparations was found to be satisfactory, with results of 97.12±0.16, 96.21±0.22, 98.19±0.23, 94.68±0.19, and 95.86±0.26%, for the optimized F2, F3, F5, F6, and F8, respectively.
FTIR
FTIR spectra of pure ketoconazole showed the characteristic peak in the region of 1646 cm-1 (C=O stretch), 1512 cm-1 (C=C aromatic stretch), 1444 cm-1 (3º amines), 1092 cm-1, and 814 cm-1 (C-Cl stretch). All the characteristic peaks were also present in the FTIR spectra of optimized formulations (Figure 1).
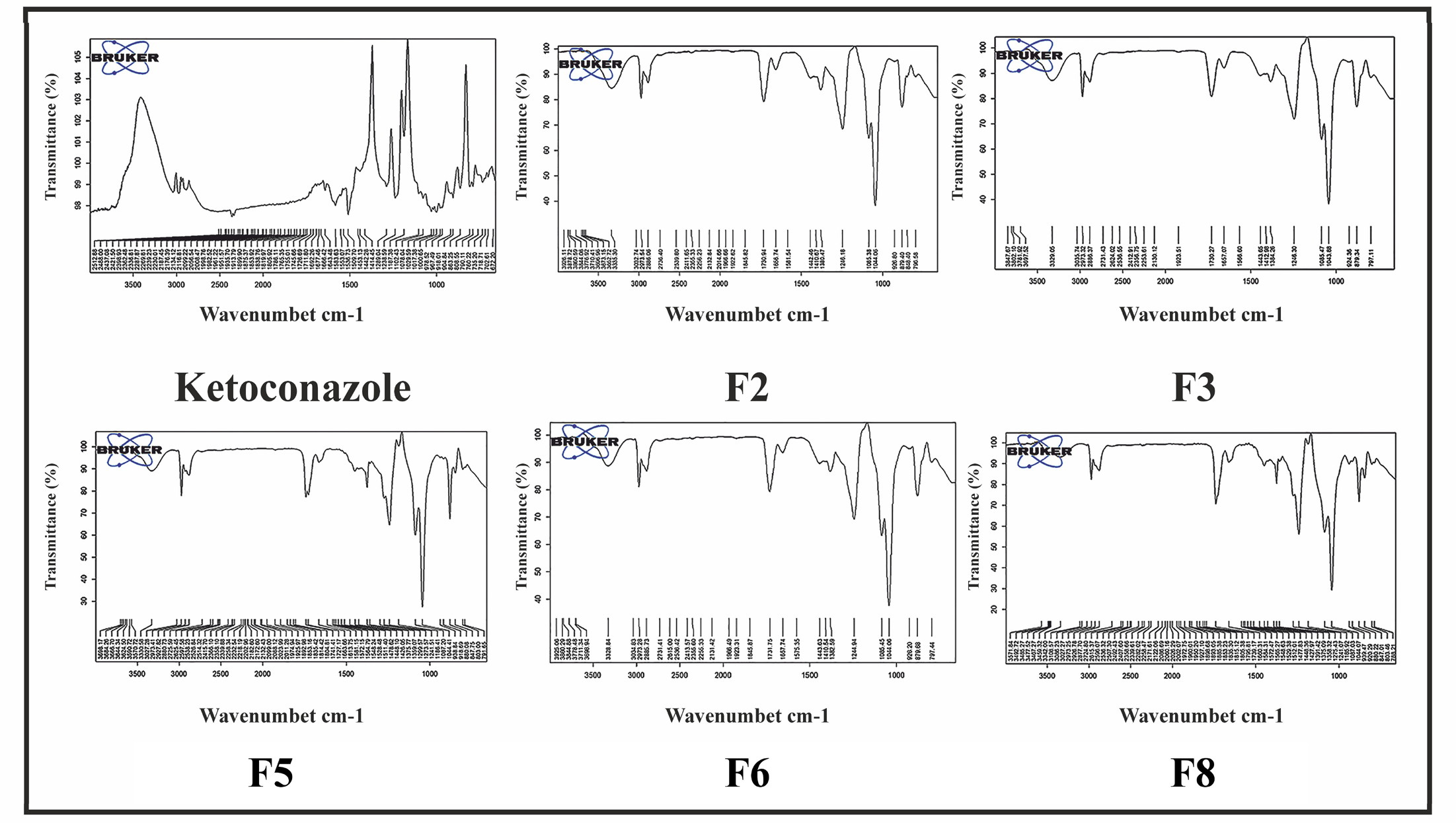
Figure 1:
FTIR analysis of pure drug and optimized formulations.
DSC
DSC spectra of pure ketoconazole showed a sharp peak in the melting point region of ketoconazole. This was confirming that the drug was in crystalline form. While the DSC spectra of ketoconazole-loaded formulations revealed no sharp melting point in the melting region of ketoconazole. That confirmed the conversion of the crystalline form to the amorphous form of the drug after incorporation into the formulations (Figure 2).
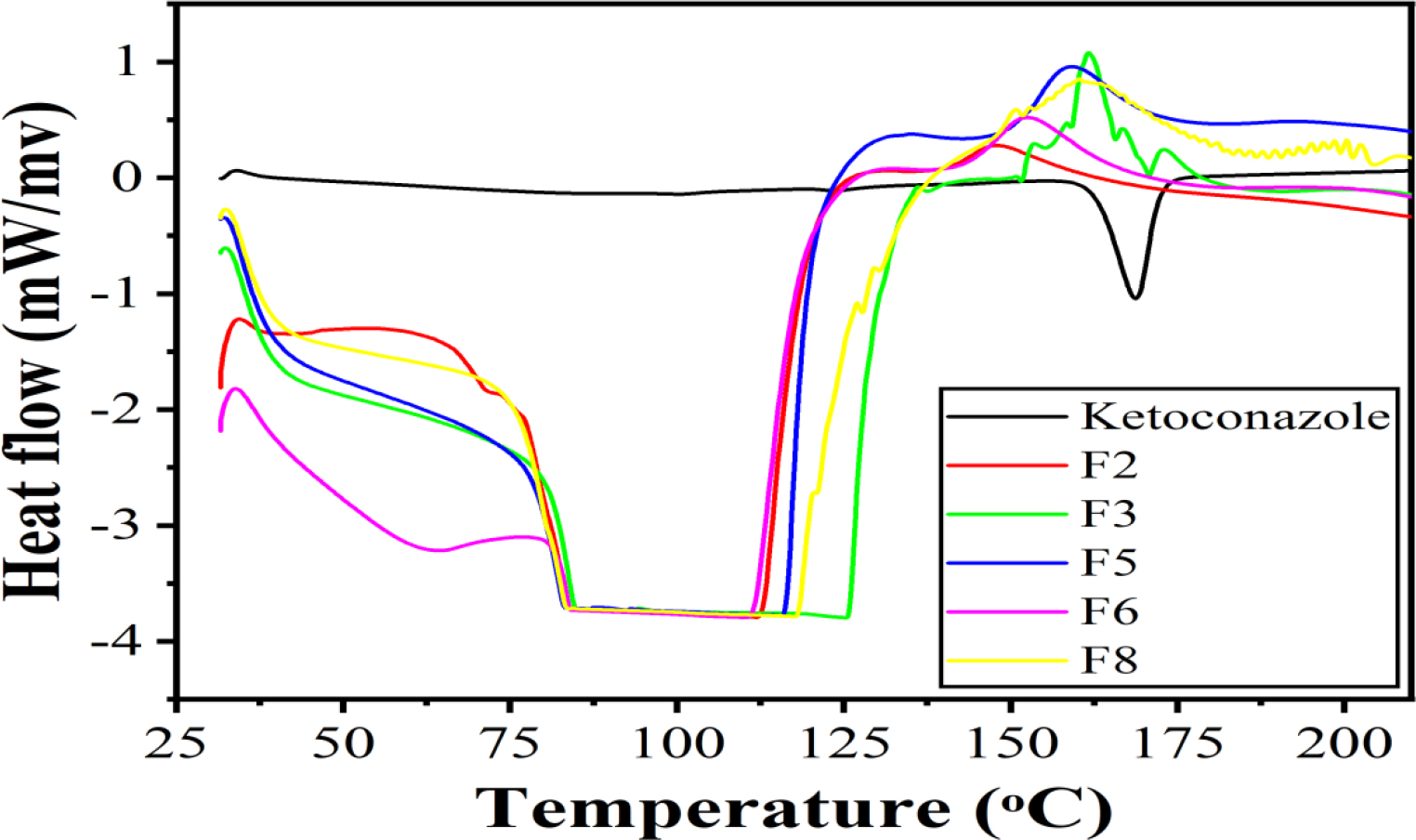
Figure 2:
DSC analysis of pure drug and optimized formulations.
Rheology
The observed results of optimized F2 and F3 showed that the storage and loss modulus both increase significantly with the increase in frequency after some time the G” gets disturbed and near 10 Hz its get falls and increases till 100 Hz in the F2, while in F3 the same phenomena happened after 50 Hz (Figure 3).
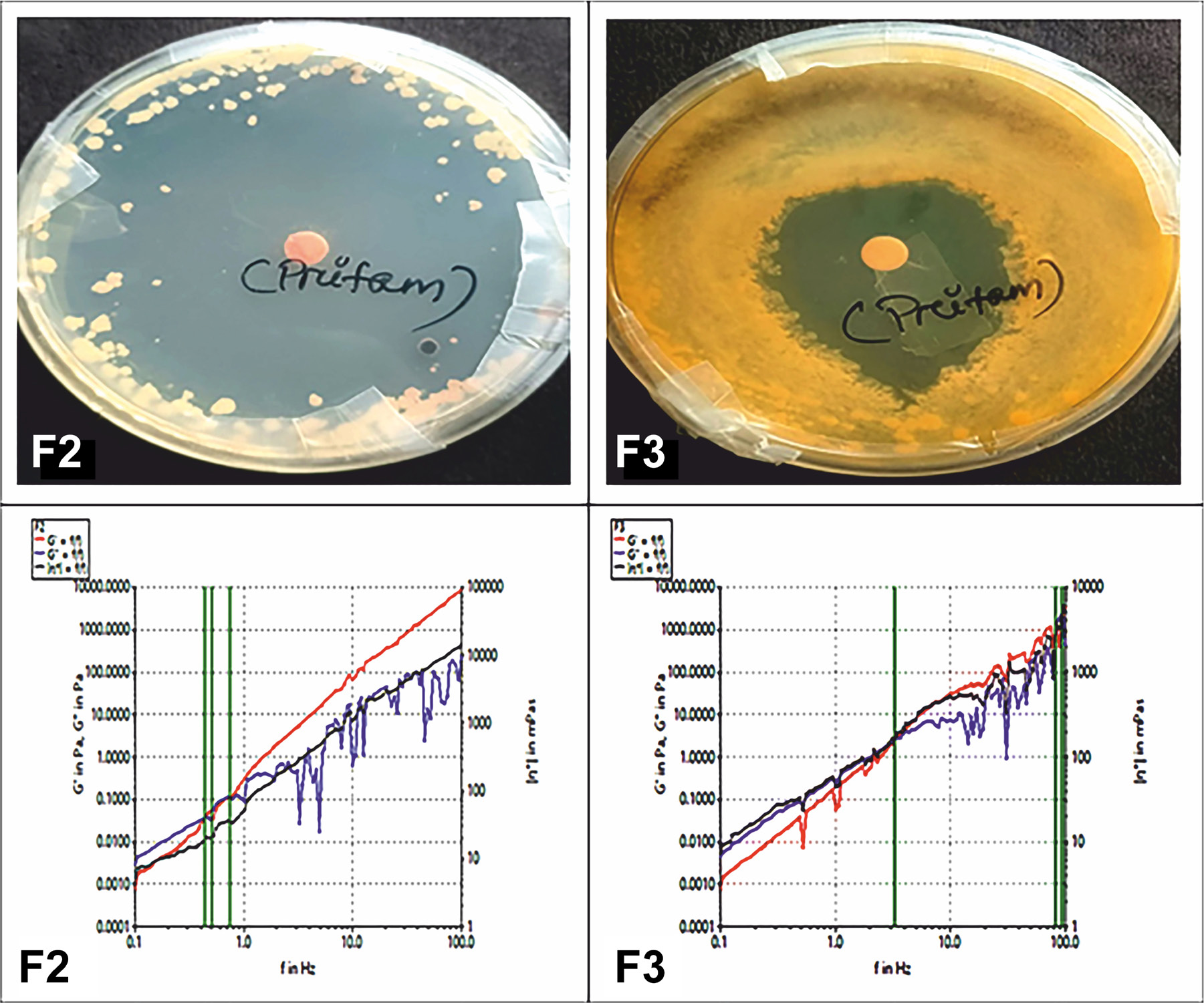
Figure 3:
Antifungal study and rheological analysis of optimized formulations.
In vitro drug permeation
The permeation rate was found highest in F3 and F8 showed the minimum permeation among all the tested nail lacquers. However, the sequence of permeation was F3> F2> F6> F5> F8. All the formulations showed good permeation and negligible difference in the rate of permeation. The results are displayed in Figure 4.
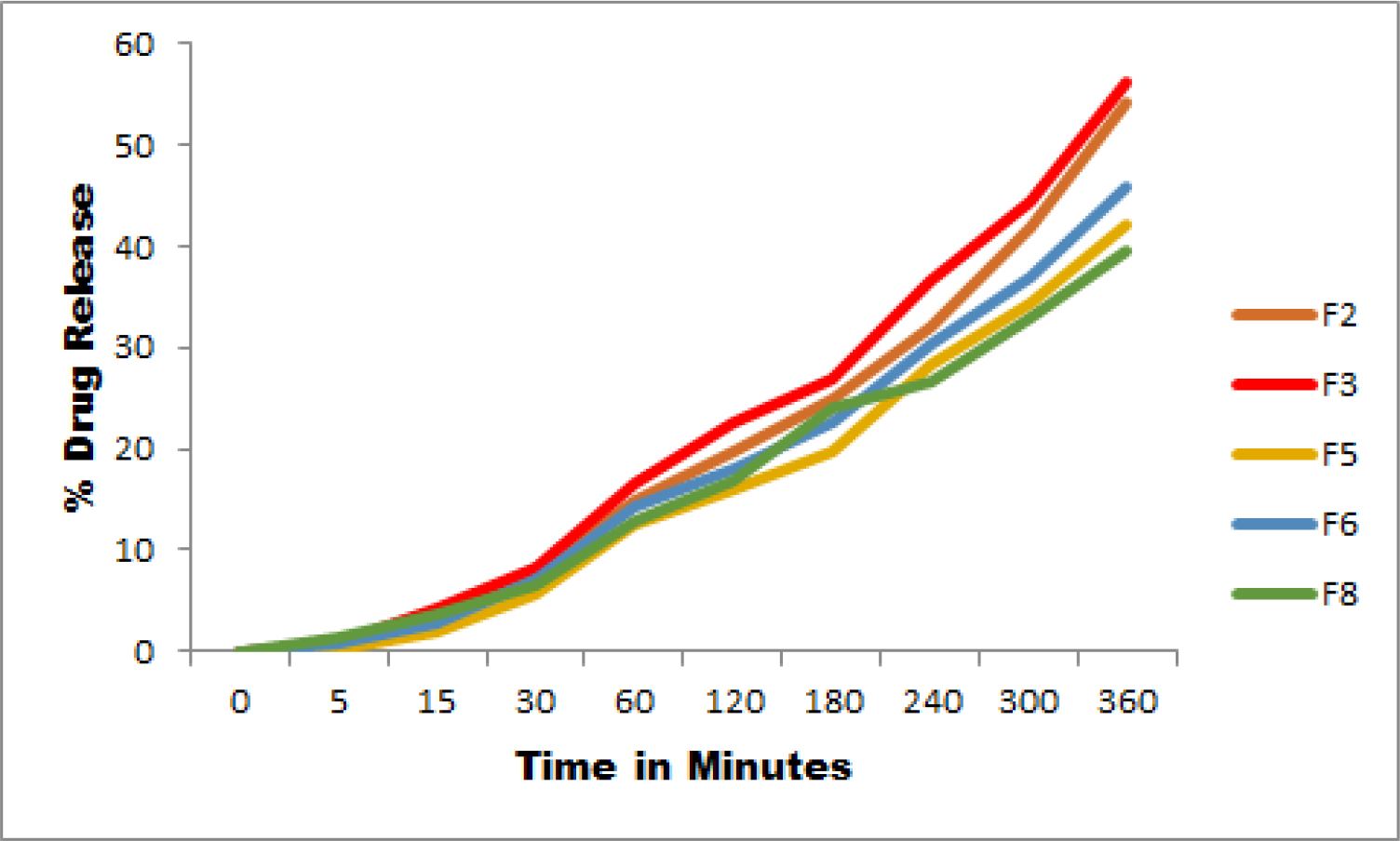
Figure 4:
Drug permeation of optimized formulations.
Anti-fungal activity
The formulations F2 and F3 exhibited excellent results in anti-fungal examination tested fungi compared to the marketed formulation. The findings are displayed in Figure 3.
Stability
At specific time intervals, the samples’ physical characteristics, pH, and drug content were assessed. It can be deduced from the findings that no such alterations have been identified in the parameters mentioned above.
DISCUSSION
The ideal nail lacquer should not be quick drying nor delayed drying after the application. The drying time was used to optimize the formulations. Optimized nail lacquers were evaluated for color, order, and presence of any foreign particles, as the formulation was transparent and no particulate matter was found, it was thought that it will not give any stain after application into the nail plate. There is a correlation between the level of cover that the nail lacquer film provides after the application and the non-volatile content of the formulation. Also the non-volatile content can interfere the drying time of the formulation (Aggarwalet al., 2020). Optimum concentration of non-volatile content is required. Upon the completion of the solvent evaporation process, the solid, non-volatile component that takes the form of a film that covers the entire nail surface is retained. All the tested formulations showed satisfactory results in non-volatile content test (Dehariet al., 2022). Optimized formulations showed a smooth flow over the glass slide suggested that the formulations will be readily spreadable upon application on the nail plate. All the optimized nail lacquers were found to be water resistant as the formulations were of nitrocellulose and ethyl cellulose; both the polymers are non-hydrophilic in nature. Viscosity is an important parameter for topical nail lacquers as viscosity influences the spreadability and can interfere with the drying time also. Increased viscosity may produce clouding, less spreadability and decrease in gloss which will not be cosmetically acceptable (Aggarwalet al., 2020, and Dehariet al., 2022). However, the observed viscosity range of optimized KCZ-nail lacquers was satisfactory and provided good adherence and flow property. The thickness of the nail lacquer is an important parameter that influences the drug-holding capacity of the preparation. The observed thickness of films formed by KCZ nail lacquers were in under desired range and exhibited good drug holding capacity. All the optimized formulation exhibited % drug content of more than 94%, shows the high amount of drug present in the formulations, making sure that the methods of formulation and the ingredients selected were capable to prepare a stable formulation and are not impacting on the stability of the drug after incorporation into the preparation. High drug content of the formulations also provides an assurance that the therapeutic effect of the formulations will be desired. In FTIR analysis all the characteristic peaks were available in the spectra of pure KCZ were also available in all the drug-loaded nail lacquers. No additional peak presence and vanishing were observed in formulations when compared with the spectra of pure KCZ. Suggesting that, there was no interaction between drug and excipient (Thataiet al., 2018). DSC thermogram of pure KCZ exhibited a sharp peak in the reported melting point area of the drug, confirmed that the drug was present in crystalline form. While the DSC thermogram of KCZ-loaded nail lacquers have not showed any sharp peak in the melting point region of KCZ. This conversion of the drug from crystalline to the amorphous state suggested the enhancement in the solubility of KCZ after incorporation into the polymer matrix of the formulation (Thataiet al., 2018). The observed results of rheology suggested the complex shear thinning and thickening properties of the nail lacquers. However, tested nail lacquers exhibited non-Newtonian flow; they showed a decrease in viscosity against increase in frequency, which will help in the easy spreading of the lacquer after application on to the nail plate. The result of in vitro drug release suggested that the F2 and F3 was precursor among tested formulations. Moreover, addition of permeation enhancer improves the permeation of drug was also observed. After incorporation of penetration enhancers like PEG 300 and salicylic acid the drug permeation of the formulations are significantly increased (Giriet al., 2024).The results of antifungal activity of optimized KCZ-loaded nail lacquers suggested that the modified formulations F2 and F3 showed a significant zone of inhibition against the tested fungal strains responsible for onychomycosis (Giriet al., 2024). It can be thought that the formulation F2 and F3 will be effective in terms of antifungal activity when applied on the infected nail. All the formulations showed satisfactory stability during the stability test and no such significant changes were observed in the tested parameters, suggesting the formulations were stable and could be store for longer period of time. However, the F2 and F3 exhibited the most favorable results in tested parameters making them precursor among all the optimized formulations. The F2 and F3 were able to overcome the limitations faced by transungual drug delivery, so they can be used in the treatment of onychomycosis effectively.
CONCLUSION
Onychomycosis, as a long-lasting and recurring fungal nail illness, necessitates prolonged treatment accompanied by consistent adherence to the recommended therapeutic regimen. This study concentrated on the preparation of innovative compatible nail lacquer. The developed lacquers underwent a comprehensive evaluation encompassing various physicochemical and molecular analyses and antimicrobial studies. The aforementioned studies led to the conclusion that the drug and the excipients utilized in the optimized formulations exhibited compatibility with one another. All formulations demonstrated effective film formation, optimal drying time, smooth flow characteristics, and appropriate volatile content requirements. The findings derived from the in vitro studies suggest that formulations F2 and F3 exhibited the most favorable permeation and antifungal characteristics among all the optimized formulations. The developed and optimized ketoconazole nail lacquers were safe and effective against causative agents and can be used as a transungual drug delivery system for the treatment of onychomycosis.
Cite this article:
Pradhan PT, Panigrahi A, Ray J, Dash RS, Pattnaik G, Sahu A, et al. Development and Characterization of Ketoconazole-Loaded Nail Lacquer for the Topical Treatment of Onychomycosis. J Young Pharm. 2025;17(3):604-11.
References
- Aggarwal R., Targhotra M., Sahoo P. K., Chauhan M. K.. (2020) Efinaconazole nail lacquer for the transungual drug delivery: Formulation, optimization, characterization and evaluation. Journal of Drug Delivery Science and Technology 60: Article 101998 https://doi.org/10.1016/j.jddst.2020.101998 | Google Scholar
- Ali F., Habibullah S., Mohanty B., Behera A., Giri Y., Nayak B. S., et al. (2022) Novel emulgel of acetazolamide for ocular drug delivery. Journal of Applied Pharmaceutical Science 13: 127-135 https://doi.org/10.7324/JAPS.2023.53382 | Google Scholar
- Baran R., Bristow I., Dawber R. P., Haneke E., Tosti A.Baran R., Bristow I., Dawber R. P. R., Haneke E., Tosti A., et al. (1996) A text atlas of nail disorders : 98-124 https://doi.org/10.3109/9780203427200-9 | Google Scholar
- Borman A. M., Summerbell R. C.. (2015) Trichophyton, Microsporum, Epidermophyton, and agents of superficial mycoses. Manual of clinical microbiology : 2128-2152 https://doi.org/10.1128/9781555817381.ch123 | Google Scholar
- Bryson P. H., Draelos Z. D.. (2022) Cosmetic dermatology: Products and procedures : 280-288 https://doi.org/10.1002/9781119676881.ch29 | Google Scholar
- Chandra R., Kumar S., Aggarwal A.. (2012) Evaluation of nail lacquer. Indo Global Journal of Pharmaceutical Sciences 2: 379-382 https://doi.org/10.35652/IGJPS.2012.43 | Google Scholar
- de Berker D., Ruben B. S., Baran R.Baran R., de Berker D., Holzberg M., Piraccini B. M., Richert B., Thomas L., et al. (2019) Baran and Dawber’s diseases of the nails and their management : 1-58 https://doi.org/10.1002/9781119323396.ch1 | Google Scholar
- Dehari D., Mehata A. K., Priya V., Parbat D., Kumar D., Srivastava A. K., Singh S., Agrawal A. K., et al. (2022) Luliconazole nail lacquer for the treatment of onychomycosis: Formulation, characterization and and evaluation. AAPS PharmSciTech 23: 175 https://doi.org/10.1208/s12249-022-02324-7 | Google Scholar
- Dhingra G., Ahmad N., Tanwar S., Goyal S., Chaturvedi V., Tanwar K., et al. (2022) Nail disorders: An updated review. International Journal of Pharmaceutical Sciences Review and Research : 135-144 https://doi.org/10.47583/ijpsrr.2022.v75i02.022 | Google Scholar
- Elkeeb R., AliKhan A., Elkeeb L., Hui X., Maibach H. I.. (2010) Transungual drug delivery: Current status. International Journal of Pharmaceutics 384: 1-8 https://doi.org/10.1016/j.ijpharm.2009.10.002 | Google Scholar
- Giri Y., Habibullah S., Dixit P. K., Mahalik G., Mohanty B., Behera A., et al. (2024) Development of microemulgel formulations with varied permeation enhancers for transungual delivery of luliconazole in onychomycosis management. Colloids and Surfaces. B, Biointerfaces 234: Article 113718 https://doi.org/10.1016/j.colsurfb.2023.113718 | Google Scholar
- Gupta A. K., Stec N., Summerbell R. C., Shear N. H., Piguet V., Tosti A., Piraccini B. M., et al. (2020) Onychomycosis: A review. Journal of the European Academy of Dermatology and Venereology 34: 1972-1990 https://doi.org/10.1111/jdv.16394 | Google Scholar
- Jena B. R., Panda S. P., Kulandaivelu U., Alavala R. R., Rao G. S. N. K., Swain S., Pattnaik G., Ghose D., et al. (2021) AQbD driven development of an RP-HPLC method for the quantitation of abiraterone acetate for its pharmaceutical formulations in the presence of degradants. Turkish Journal of Pharmaceutical Sciences 18: 718-729 https://doi.org/10.4274/tjps.galenos.2021.74150 | Google Scholar
- Kaur S., Kumar M., Bhatia A., Chopra S.. (2024) A recent review on transungual drug delivery system. Journal of Dispersion Science and Technology 21: 1-20 https://doi.org/10.1080/01932691.2024.2334012 | Google Scholar
- Khattab A., Shalaby S.. (2018) Optimized ciclopirox-based eudragit RLPO nail lacquer: Effect of endopeptidase enzyme as permeation enhancer on transungual drug delivery and efficiency against onychomycosis. AAPS PharmSciTech 19: 1048-1060 https://doi.org/10.1208/s12249-017-0917-8 | Google Scholar
- Kushwaha A. S., Repka M. A., Narasimha Murthy S.. (2017) A novel apremilast nail lacquer formulation for the treatment of nail psoriasis. AAPS PharmSciTech 18: 2949-2956 https://doi.org/10.1208/s12249-017-0776-3 | Google Scholar
- Laubé F., Poupon A., Zinck P., Müller-Goymann C., Reichl S., Nardello-Rataj V., et al. (2019) Physicochemical investigations of native nails and synthetic models for a better understanding of surface adhesion of nail lacquers. European Journal of Pharmaceutical Sciences 131: 208-217 https://doi.org/10.1016/j.ejps.2019.02.014 | Google Scholar
- Lee D. H., Lim S., Kwak S. S., Kim J.. (2024) Advancements in skin‐mediated drug delivery: Mechanisms, techniques, and applications. Advanced Healthcare Materials 13: Article e2302375 https://doi.org/10.1002/adhm.202302375 | Google Scholar
- Maksimov A. Y., Balandina S. Y., Topanov P. A., Mashevskaya I. V., Chaudhary S.. (2021) Organic antifungal drugs and targets of their action. Current Topics in Medicinal Chemistry 21: 705-736 https://doi.org/10.2174/1568026621666210108122622 | Google Scholar
- Murdan S., Bari A., Ahmed S., Hossin B., Kerai L.. (2017) Nail lacquer films’ surface energies and water-resistance and adhesion do not predict their residence. British Journal of Pharmacy 2: 42-54 https://doi.org/10.5920/bjpharm.2017.03 | Google Scholar
- Puri V., Savla R., Chen K., Robinson K., Virani A., Michniak-Kohn B., et al. (2022) Antifungal nail lacquer for enhanced transungual delivery of econazole nitrate. Pharmaceutics 14: 2204 https://doi.org/10.3390/pharmaceutics14102204 | Google Scholar
- Rahal A.. (Master’s thesis https://doi.org/10.3390/pharmaceutics14102204 | Google Scholar
- Sandewicz R. W.. (2016) Formulation of nail care products. Handbook of formulating dermal applications: A definitive practical guide : 539-588 https://doi.org/10.3390/pharmaceutics14102204 | Google Scholar
- Shivakumar H. N., Vaka S. R. K., Madhav N. V. S., Chandra H., Murthy S. N.. (2010) Bilayered nail lacquer of terbinafine hydrochloride for treatment of onychomycosis. Journal of Pharmaceutical Sciences 99: 4267-4276 https://doi.org/10.1002/jps.22150 | Google Scholar
- Thatai P., Kaur K., Sapra B.. (2018)
In vitro evaluation of transungual formulation of ketoconazole for the Management of onychomycosis. Drug Delivery Letters 8: 140-152 https://doi.org/10.2174/2210303108666180313141228 | Google Scholar - Vejnovic I., Simmler L., Betz G.. (2010) Investigation of different formulations for drug delivery through the nail plate. International Journal of Pharmaceutics 386: 185-194 https://doi.org/10.1016/j.ijpharm.2009.11.019 | Google Scholar
- Yuan L., Pan M., Shi K., Hu D., Li Y., Chen Y., Qian Z., et al. (2022) Nanocarriers for promoting skin delivery of therapeutic agents. Applied Materials Today 27: Article 101438 https://doi.org/10.1016/j.apmt.2022.101438 | Google Scholar
- Zhang Y.-B., Xu D., Bai L., Zhou Y.-M., Zhang H., Cui Y.-L., et al. (2022) A review of non-invasive drug delivery through respiratory routes. Pharmaceutics 14: 1974 https://doi.org/10.3390/pharmaceutics14091974 | Google Scholar
