ABSTRACT
Background
The increasing interest in the field of Lipid Self-Emulsifying Technology (LSET) is due to the fact that 40% of new chemical entities exhibit poor oral bioavailability because of their low water solubility. Self-Micro-Emulsifying Drug Delivery Systems (SMEDDS) are isotropic mixtures of oils, surfactants, and cosurfactants, which under gentle agitation in aqueous media form oil-in-water nano-emulsions of oil droplet size <100 nm.
Materials and Methods
Self-emulsification process carried out at varying key compositions in the lipid mixtures; oil, cosurfactant and surfactant. Oil droplet size was measured using laser diffraction and light scattering techniques. Equilibrium phase behavior studies were carried out and phase boundaries were determined mapped out.
Results
At least 40% w/w of cremophor EL is needed to obtain all the way through clear self-emulsifying clear dispersions (L2→L1) on the progressive addition of water. Maximum aqueous solubilization as L2 phase occurred at ≈ 55% w/w for miglyol 812 and {tagat TO/imwitor 988} blends at ratios of either (7:3).
Conclusion
Successful self-micro-emulsifying lipid formulations were developed using droplet size measurements and equilibrium phase studies. Key elements in the oil blends were optimized which includes, type of oil, co-surfactant, surfactant and oil:co-surfactant ratio. Phase dynamic behavior due to the interaction of lipid vehicle with the aqueous media was investigated in an attempt to understand mechanistic of emulsification.
INTRODUCTION
There are more than thirty products based on Lipid Self-Emulsifying Technology (LSET) commercially available in the market (Salva et al., 2017). Reformulation of earlier “Sandimmune” (cyclosporine A) into Neoral ® (Aleksander, 2009) as an archetypical class III A lipid class system has given an impetus to the field of LSET. The increasing interest in the field of LSET is due to the fact that 40% of new chemical entities exhibit poor oral bioavailability because of their low water solubility. There are numerous numbers of active molecules uploaded into lipid self-emulsifying matrix which includes; licochalcone A (Zhuet al., 2021), puerarin (Chenget al., 2016), seocalcitol II (Groveet al., 2006), valsartan (Zhaoet al., 2016), CAT3 (Wanget al., 2021), carvedilol (Mandićet al., 2019) ibuprofen (Nouraeiet al., 2021), methotrexate (Kimet al., 2019), myeloperoxidase (MPO) (Bansode et al., 2106), Artemisone (Zechet al., 2021), Mefenamic (Kumaet al., 2019), curcumin (Aswaret al., 2020), and a new cathepsin K inhibitor (HL235) (Visetvichapornet al., 2020).
Self-micro-emulsifying lipids are isotropic mixtures of oils, surfactants, and cosurfactants, which under gentle agitation in aqueous media form oil-in-water nano-emulsions of oil droplet size < 100 nm. There are four identified types of lipid systems; I, II, III and IV depending on polarity of oil mixture, droplet size and digestibility (Pouton, 2006). These systems provide the drug in an already dissolved form in the oil vehicle which upon contacting with gastrointestinal fluids emulsifies spontaneously producing dispersions with large surface area available for drug diffusion. All Biopharmaceutical drug Class Systems (BCS) including categories, I, II, III and IV (Brouwerset al., 2010; Butleret al., 2010) can benefit from reformulation in oil vehicles (Naser et al., 2018a). In a recent study by (Hasanet al., 2018a) a fast dissolving tablet formulation using theophylline (BCS Class I Compound) was obtained at including only 5% SMEDDS oil vehicle. Constituents of LEST or SMEDDS can be used to mimic bioavailability poorly water-soluble drugs and as intestinal enhancers by weakening the tight junction of paracellular membrane (Buyukozturket al., 2010) or by decreasing efflux of the drug in the GIT due to their effect on to p-glycoprotein transporter (Bansalet al., 2009 and Nornooet al., 2009). Furthermore, potential flavored SMEDDS vehicles were developed for masking organoleptic taste of APIs (Hasanet al., 2015b).
In this investigation, successful self-micro-emulsifying lipid formulations were developed by optimizing key elements in the oil blends which includes, type of oil, co-surfactant, surfacatant and oil:co-surfactant ratio. Phase dynamic behavior due to the interaction of lipid vehicle with the aqueous media was investigated in an attempt to understand mechanistic of emulsification. Moreover, the interaction of lipid vehicles with various pharmaceutical excepients was also characterized.
MATERIALS AND METHODS
Materials
Miglyol 812, medium chain triglyceride, Imwitor 988 (C8/C10 mono/diglycerides at ratios 1:1 and Imwitor 308 (mono: di: triglycerides at ratios of 90: 7: 1) were supplied by Condea Chemie GmbH. Capmul MCM (C8/C10 mono/diglycerides at ratios 1:1) was supplied by Abitec Corporation. Tagat TO (PEG-(25)-glyceryl trioleate) and Tagat S2 (PEG-(20)-glyceryl stearate) were supplied by Goldschmidt AG, Germany. Crillet-3 (polyoxyethylene 20 sorbitan monostearate) was supplied by Croda. Cremophor EL (PEG-(35)-castor oil), Kollidon 30 F, Polyplasdone XL 10, were supplied by BASAF.
Methods
Miscibility of lipid mixtures
Representative percentages of oils, co-surfactants and surfactants of 2 g formulations were weighed in glass vials with tight closures. Mixtures were heated in a water bath at 50ºC for 2 min and then thoroughly vortexed and kept for 24-48 at 25ºC for visual assessment. Continuous single phase mixtures were classified as miscible formulations. Mixtures forming two or more phases were denoted as immiscible systems.
Self-emulsification
An amount of 1gm of each miscible single phase lipid mixture showing was emulsified in 100 mL of Mili Q water in a 500 mL glass beaker. Emulsification was carried out at 25ºC or 37ºC in a thermostatically controlled mechanical shaker set at 100 oscillation per min for 15 min. Areas denoted one phase describes homogeneous oil blends, two phases are immiscible mixtures, SMEDDS are optically clear dispersions while course emulsions refer to turbid aqueous mixtures.
Particle size analysis
Oil droplet diameter for aqueous dispersions of lipid formulations was measured using Quasi-Elastic Light Scattering (QELS, Malvern model LO-C photoncorrelation spectrometer). Experiments were performed in triplicate.
Equilibrium phase studies
Phase behavior studies were conducted using static composition method. Blends of oil, various ratios of surfactant/co-surfactant and water which represent ternary phase diagram were made up at various intervals. Additional compositions at defined intervals were made to demark additional phase boundaries. Various ternary and binary compositions of total weight of 5 g mixtures were made in screw-capped glass vials and heated to 70ºC in a thermostatically controlled water bath for 15 min with intermittent vertexing. Mixtures were left at room temperature without disruption for 24 hr for phase identification.
Solid dispersions of oil mixtures
Oil mixtures representing varying degrees of hydrophilicity were blended with various pharmaceutical excipients including β- Cyclodextrin, Polyplasdone XL 10 and Kollidon 30 F. Physical phase behavior of these blends was studied at increasing concentrations of water. The obtained mixtures were classified as either immiscible (2-phase system), paste like or, powdery in textures.
RESULTS
The emulsification profile of miglyol 812 N, capmul MCM and cremophor EL (HLB value = 14) is shown in Figure 1. Optimum clear SMEDDS dispersion can be obtained at ratios of miglyol 812 N:capmul MCM between 40:60 to 60:40 using only 15% surfactant, see lines a-b, a-c, and a-d. Figure 2 depicts equilibrium phases resulting from dilution of lipid system {miglyol 812/capmul MCM/cremophor EL} with water. At least 40% w/w of cremophor EL is required to obtain all the way through clear self-emulsifying clear dispersions (L2→L1) on the progressive addition of water.The emulsification profile of miglyol 812 (source of triglyceride), capmul MCM (co-surfactant) and tagat S2 (non-ionic surfactant) has produced extended area of microemulsion (SMEDDS), see Figure 3a. Optimum dispersions are obtained at {miglyol 812 N:capmul MCM} ratio of (6:4) (line a-b) using tagat S2 concentration of more than 25% w/w. Similar optimum ratios were obtained using lipid systems including, miglyol 812, capmul MCM and cremophor RH40 or EL (Hasan et al., 2016b). Nonetheless, an area of turbid course emulsion prior SMEDDS region was formed close to the apex towards high concentrations of tagat S2 due to the formation of swollen microemulsion. This area of coarse emulsion adjacent to the SMEDDS region was not observed in the case of using crillet-3 (polyoxyethylene 20 sorbitan monostearate) or using imwitor 308 instead of capmul MCM, Figures 3(b) and 4 respectively. The emulsification ternary plot for miglyol 812 (source of triglyceride), capmul MCM (co-surfactant) and crillet -3 has produced a region of optically clear dispersions of particle size below 100 nm. Optimum dispersions are obtained at crillet concentration of 40% w/w and a ratio of miglyol 812: capmul MCM of 1:1 (line a-b). It is obvious that there is no coarse emulsion region next to SMEDDS area. The emulsification of miglyol 812N (source of triglycerides) / imwitor 308 (co-surfactant)/ crillet-3 (non-ionic surfactant) ternary blend is depicted in Figure 4. Replacing capuml MCM with imwitor 308 has extended SMEDDS region with optimum dispersions obtained at surfactant concentration of 25% w/w.
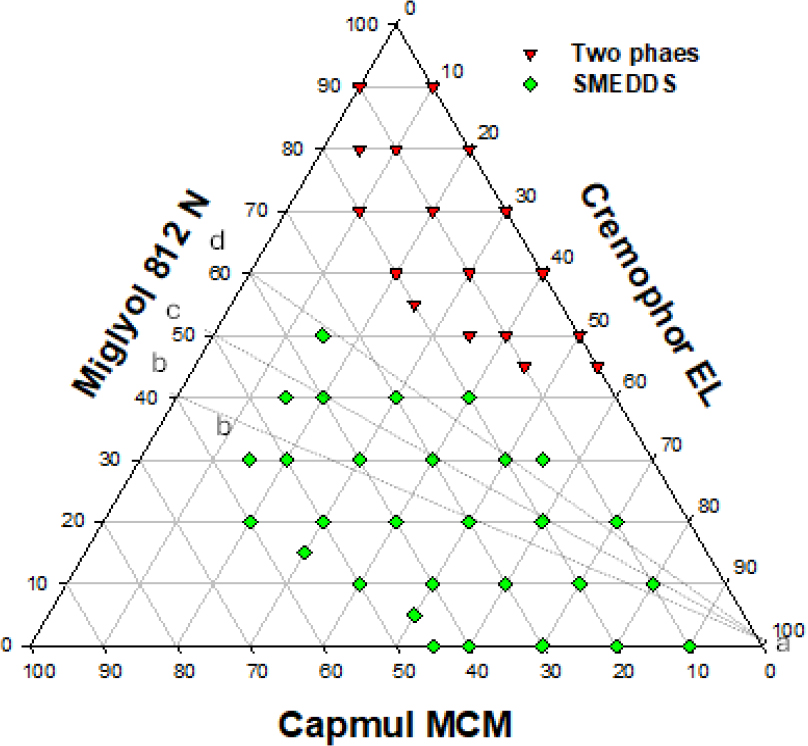
Figure 1:
The emulsification profile of lipidic mix composed of miglyol 812 N, capmul and cremophor EL.
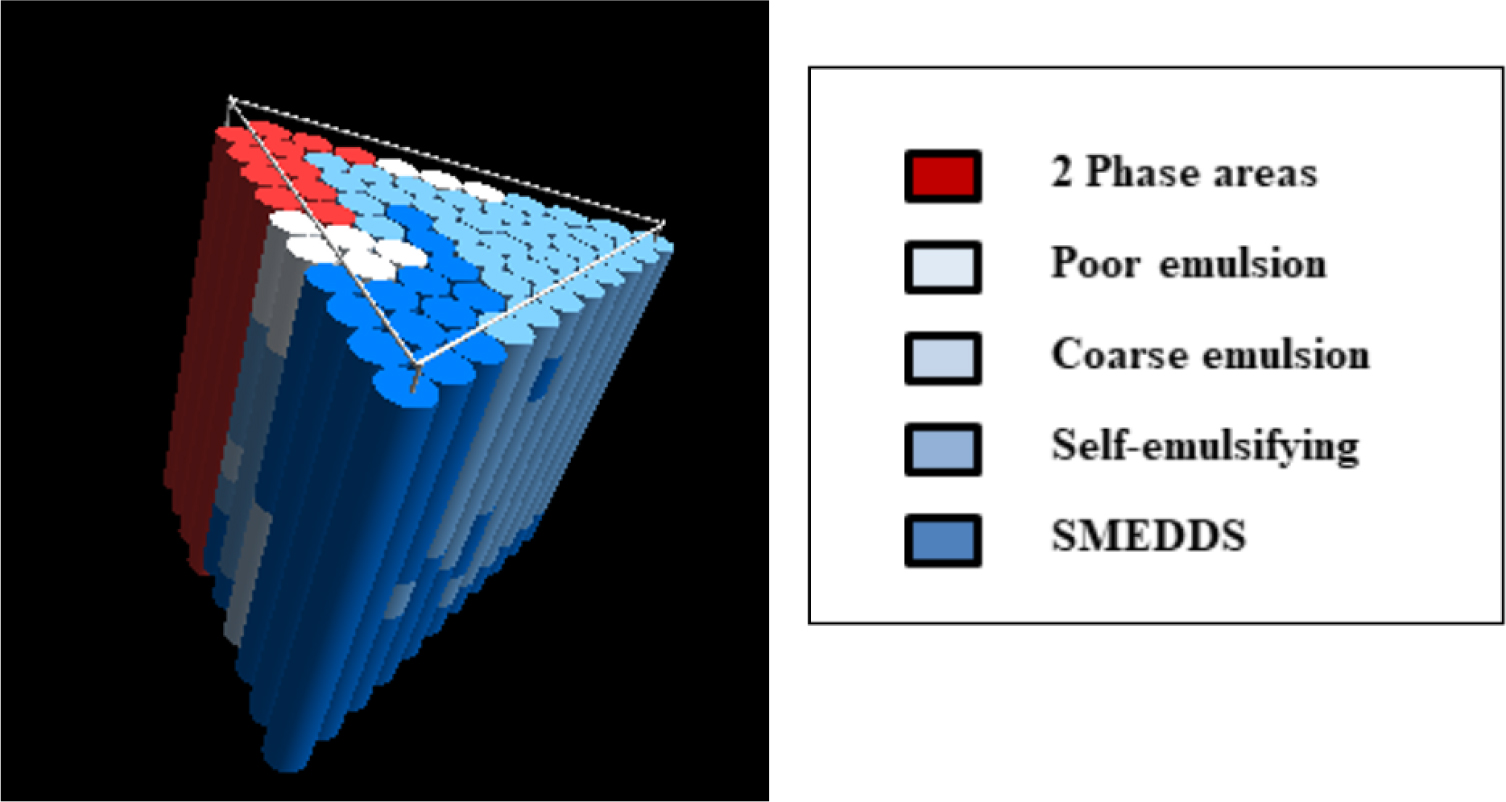
Figure 2:
3D representation of the equilibrium phases resulting from the progressive dilution of water of lipid system composed of miglyol 812, capmul MCM and cremophor EL.
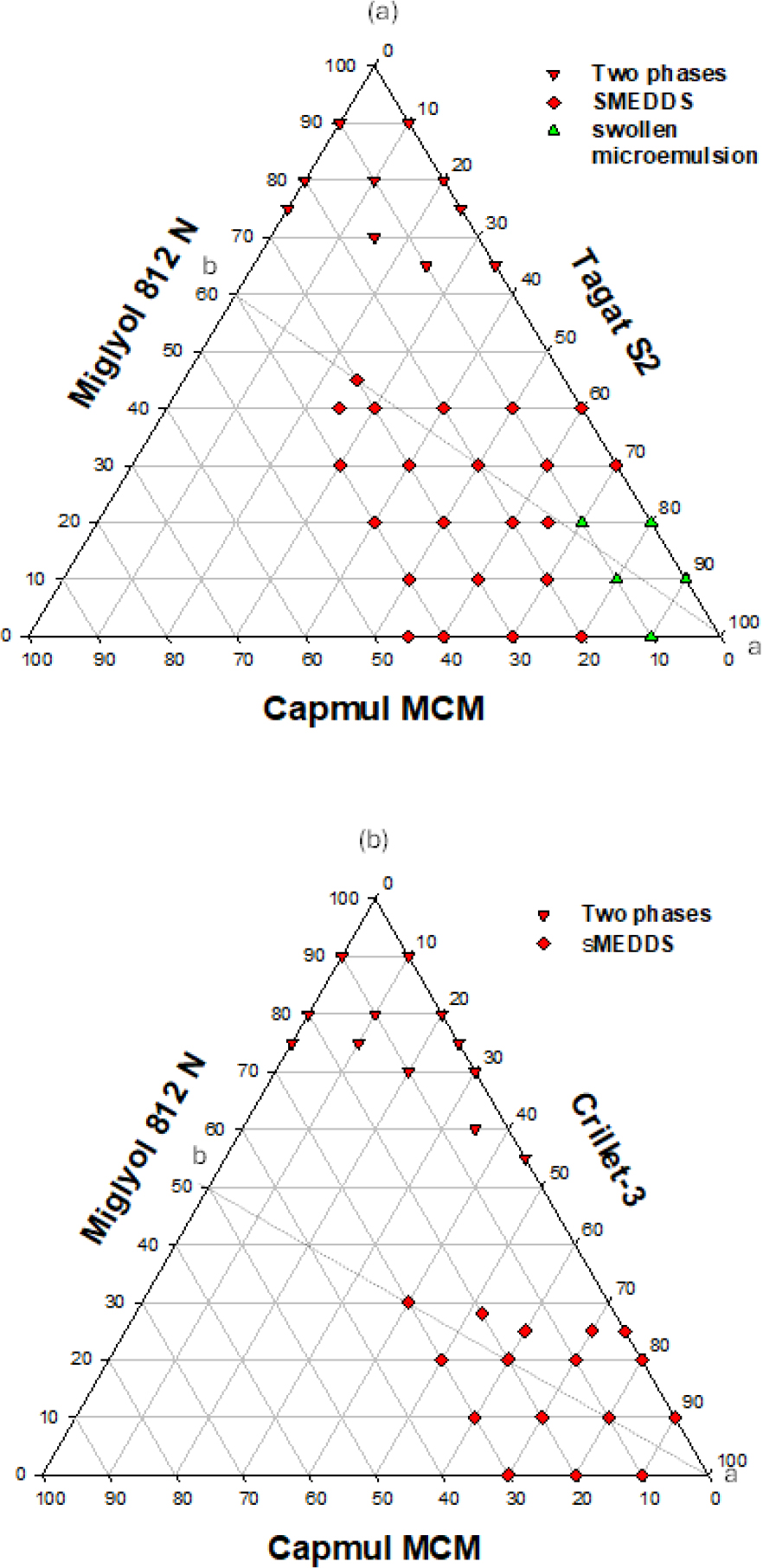
Figure 3:
Emulsification profiles of various lipid compositions; (a) miglyol 812N/ capuml MCM/ tagat S2 or (b) Miglyol 812/ capmul MCM/crillet 3. Areas denoted one phase describes homogeneous oil blends, two phases are immiscible mixtures, SMEDDS are optically clear dispersions while course emulsions refer to turbid aqueous mixtures. Oil formulations were emulsified in water at 37ºC for 15 min.
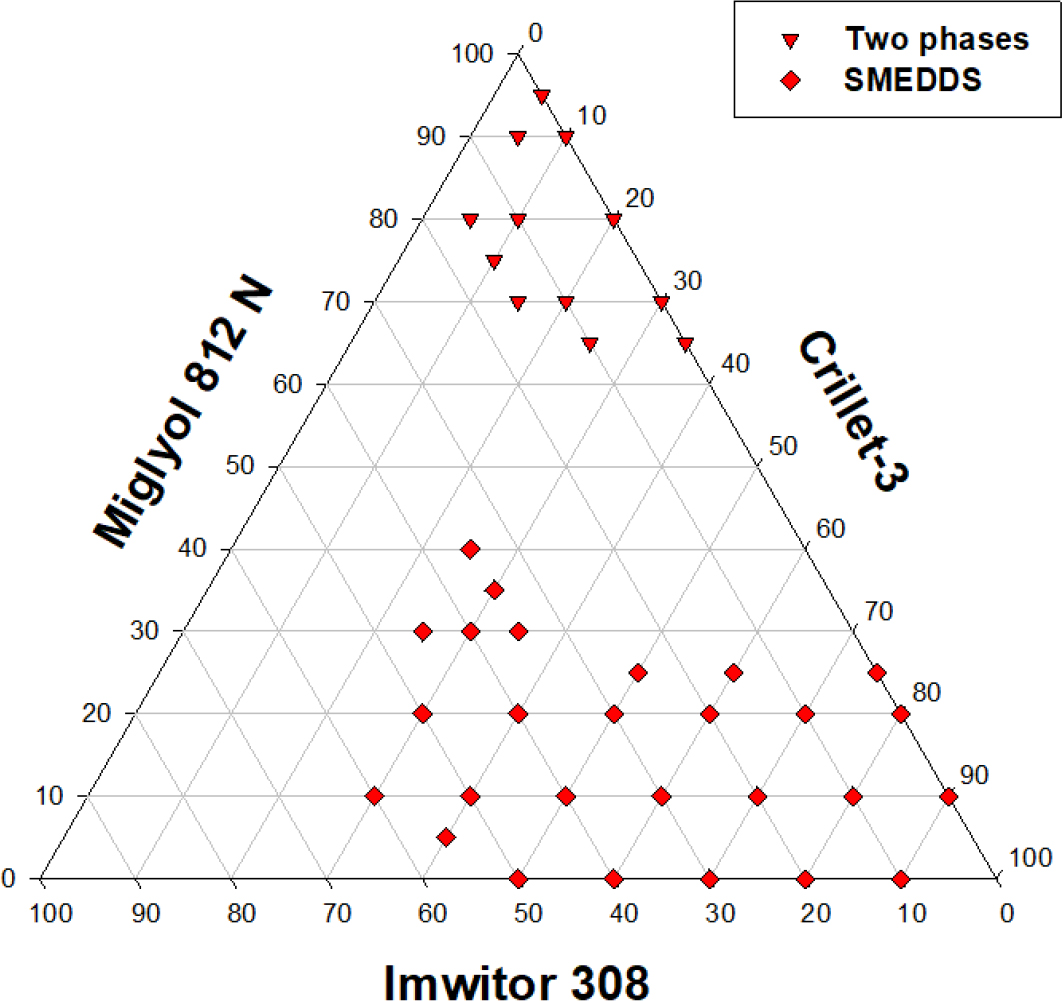
Figure 4:
The effect of including imwitor 308 on the emulsification profiles of miglyol 812 and crillet 3 oil mixtures. Various lipid compositions; (a) miglyol 812N/ imwitor 308/ crillet-3 were emulsified in water at 37ºC for 15 min. Areas denoted one phase describes homogeneous oil blends, two phases are immiscible mixtures, SMEDDS are optically clear dispersions while course emulsions refer to turbid aqueous mixtures.
The aqueous dispersion profile of a type II oil system is shown in Figure 5. A limited area of clear SMEDDS (nano-emulsions) is observed with optimum dispersions obtained at miglyol 812: imwitor ratios of 7:3 using tagat TO concentrations of more than 25% w/w. Dynamic equilibrium phase diagrams for mixtures composed of {miglyol 812/imwitor 988} blends at ratios of either (5:5), or (7:3) and tagat TO diluted with water is depicted in Figures 6a & b, respectively. Clear w/o micro-emulsion is denoted as L2 phase while, turbid emulsion phase is identified as (L1 + L2). For miglyol 812 and {tagat TO/imwitor 988} blends at ratios of (5:5), Figure 6 (a), maximum aqueous solubilization as L2 phase occurred at ≈ 30% w/w. On the other hand, maximum aqueous solubilization as L2 phase occurred at ≈ 55% w/w for miglyol 812 and {tagat TO/imwitor 988} blends at ratios of either (7:3), Figure 6(b). This almost 2 folds increase in the L2 phase region is expected to be due the inclusion of relatively higher concentrations of tagat TO which ensues in increasing polarity of oil droplets.
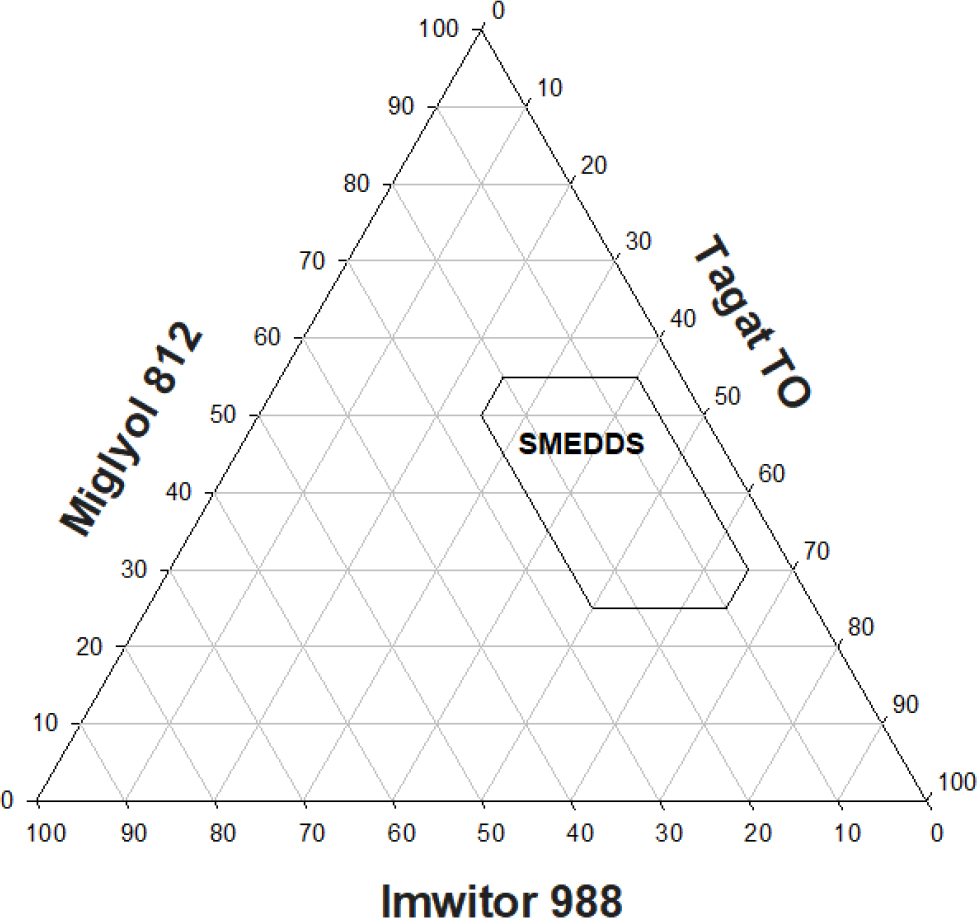
Figure 5:
Emulsification profile lipid system composed of miglyol 812N/ imwitor 988/ tagat TO, Oil formulations were emulsified in water at 37ºC for 15 min. SMEDDS are optically clear dispersions.
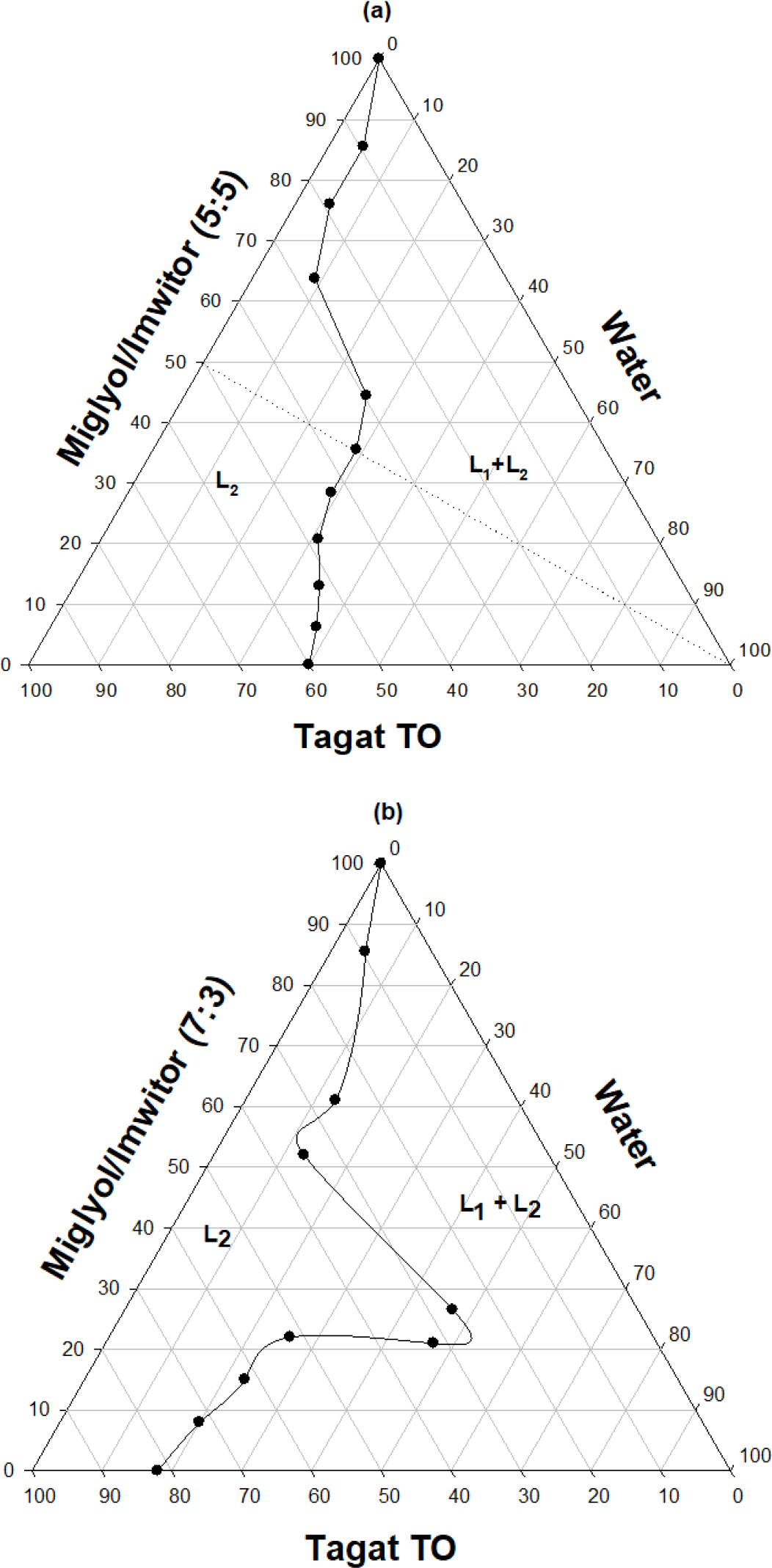
Figure 6:
Dynamic equilibrium phase diagrams for mixtures composed of {miglyol 812/imwitor 988} blends at ratios of either (5:5) or (7:3) and tagat TO diluted with water, Figures 6 (a) and (b), respectively.
The interaction of self-microemulsifying lipid vehicle with various tablet excepients at varying concentration of water is shown in Table 1. The progressive inclusion of kollidon 30 F (polyvinylpyrrolidone PVP) and water in the lipid vehicle has produced immiscible regions at examined ratios. On the hand, the inclusion of varying concentrations of polyplasdone XL 10 (cross-linked PVP) and water in the lipid mix has produced various stable mixtures. The progressive inclusion of polyplasdone XL 10 and water in the lipidic vehicle has transformed mixtures from suspensions, pastes into powdery dosage forms. The neutral crospovidone exhibited better lipophilic interactions with the non-ionic lipid vehicle than Kollidon 30 F (Fransénet al., 2008). Furthermore, the inclusion of cyclodextrin in the oil vehicle has without water produced immiscible mixtures. Nonetheless, at least 20% w/w of cyclodextrin is needed at water concentrations of ≥10% w/w is needed to obtain stable paste physically stable dosage form.
| %(w/w) Type of Excepient | % (w/w) of H2O included in the blended vehicle / Type of formed phase | |||||
|---|---|---|---|---|---|---|
| 10% w/w | 20% w/w | 30% w/w | ||||
| Type of phase formed | ||||||
| kollidone 30 F | 10% | Immiscible | ||||
| 20% | Immiscible | |||||
| 30% | Immiscible | |||||
| Polyplasdone XL 10 | 10% | Suspension | Suspension | Suspension | ||
| 20% | Paste | Paste | Paste | |||
| 30% | Paste | Paste | Paste | |||
| β cyclodextrin | 10% | Immiscible | Immiscible | Immiscible | ||
| 20% | Paste | Paste | Paste | |||
| 30% | Paste | Paste | Paste | |||
DISCUSSION
The lipid mixture which is composed of miglyol 812 N, capmul MCM and cremophor EL is a type III system produces self-micro dispersion of particle size of less than 50 nm depending on oil:cosurfactant ratio and surfactant concentration. This combination of lipdic vehicles produces expanded region of nano-emulsions which can mimic bioavailability of poorly water-soluble drugs. The robustness of this lipid mix is due to the combination of cermophor EL and capumul MCM. Cremophor due to its excellent emulsifying capacity is used for solubilization, protection, and delivery of various hydrophilic and lipophilic Active Pharmaceutical Ingredients (API) (Hasan., 2019d). Cremophor EL (polyoxyethylene-(35)-caster oil) is the liquid form of Cremophor RH 40 (polyoxyethylene-(40)-hydrogenated caster oil). In order to avoid crystallization tendency of lipidic system and thus drugs during industrial processes due to variations in environmental temperatures, cremophor EL is more preferred over Cremophor RH 40. Nonetheless, the use of either cremophor El or RH40 as non-ionic surfactants in the lipid mixture produced similar emulsification profiles of nano-sized dispersions (Hasan, 2014e).
On interaction of water, the following dynamic intermediate phases are formed; L2, L1 + L2, LC and L1 phases (Hasan, 2019d; Hasan, 2019d). As Figure 2 depicts at least 40% w/w of cremophor EL is required to obtain all the way through clear self-emulsifying clear dispersions (L2→L1) on the progressive addition of water. This is in full agreement with a study carried out by Hasan (2019d) using a system composed miglyol 812, imwitor 988 and cremophor RH40. Nonetheless, the study had concluded that incorporating Ibuprofen in the lipid mix has produced various intermediate phases on interaction with water. There is a trend amongst formulation scientists to opt for highly hydrophilic vehicles. In this case, turbid intermediate phases (L1+L2) might not appear during interaction with water and therefore the initial L2 phase could pass instantly through L1 phase. This however may cause hydrophilic constituents to diffuse out into the surrounding aqueous fluids and thus induce drug crystallization in the gut.
Tagat S2 which is polyethylene glycol (PEG) 20 – glyceryl stearate has a relatively low packing parameter, of long carbon tail (C18) with an HLB value of approximately 15. This may compromise the flexibility and the hydration of surfactant film forming at the oil water interface and thus producing turbid dispersions close to the apex towards high concentrations of tagat S2. Nonetheless, the disappearance of coarse emulsion area adjacent to the SMEDDS region in the case of using crillit-3 could be attributed to the fact that crillit-3 is relatively more hydrophilic (HLB~16). Moreover, the inclusion of imwitor 308, a more polar cosurfactant, in the mix increases the fluidity of the surfactant film around oil droplets and hence allows better dispersion. This reflects the fact that crillet-3 has a relatively higher packing parameter due to lower number of Carbons (C12) in the surfactant tail. This results in better chain mobility and hence the formation of flexible surfactant film around oil-water interface which facilitates better dispersions. Imwitor 308 is a mixture of mono, di and triesters (caprylic and capric acid) containing small amounts of unesterified glycerol. Length of the fatty acids and number of free unesterified hydroxyl groups determine degree of surface activity (Raducanet al., 2021). Imwitor 308 has high monoglyceride content, the mono: di: tri ratio is 90: 7: 1, with 1% free glycerol and 1% water content. On the other hand, capmul MCM is an equimolar (1:1) mixtures of mono & di- glycerides. Therefore, the monoglyceride content in Capmul MCM is about 50% whereas the monoglyceride content in imwitor 308 is 90%. As a result, Imwitor 308 is a much more polar compound. Hence, imwitor 308 can profoundly increase, more than capmul MCM, interfacial fluidity of the surfactant film surrounding oil phase causing further decrease in surface tension and thus better dispersions.
Lipid system composed of miglyol 812N/ imwitor 988/ tagat TO is thoroughly studied by Hasan (2021f; 2004e). This system was found to produce nano-sized emulsions with the ability to retain its solvent capacity of active molecules after dispersion, influenced by temperature and ionic strength of the media which can be averted by including lutrol 127 (poloxamer 407) in the lipid matrix. It was found that the expansion of L2 phase due the incorporation of hydrophilic components in the oil mix or the formation of nano-emulsions from lipophilic aqueous dispersible systems is crucial for mechanistics of self-micro-emulsification (Hasan 2014e). In this case, “Diffusion and Stranding” becomes the predominate theory which describes process of self-micro-emulsification. After reaching maximum solubilization (L2) close to the interface, further penetration of water into the systems results in the diffusion of the polar components out of the system. This is accompanied by the formation of regions supersaturated in oil and thus spontaneous nucleation of small oil droplets which eventually lead to the formation of microemulsion optically clear systems. The larger the (L2) area in the phase diagrams the smaller the size of the nucleated oil droplets and vice versa.
CONCLUSION
Robust self-micro-emulsifying lipid formulations representing type II and type III lipid class systems were developed. At least 40% w/w of cremophor EL is needed to obtain all the way through clear self-emulsifying clear dispersions (L2→L1) on the progressive addition of water. The expansion of L2 phase due the incorporation of hydrophilic is crucial for mechanistics of self-micro-emulsification. Maximum aqueous solubilization as L2 phase occurred at ≈ 55% w/w for miglyol 812 and {tagat TO/imwitor 988} blends at ratios of either (7:3). Furthermore, the inclusion of polyplasdone XL 10 (cross-linked PVP) in the lipid mix has produced various stable mixtures in comparison to polyvinylpyrrolidone PVP or β cyclodextrin.
Cite this article:
Hasan NMY. Formulation Design of Sel-Micro-Emulsifying Lipid Systems. J Young Pharm. 2025;17(3):620-6.
ACKNOWLEDGEMENT
The author acknowledges the Faculty of Pharmacy at Bath University for providing excellent facilities for research and for the lipid navigation team for carrying out some of the experiments, also Croda and BASF for sending samples as gifts, and finally the School of Pharmacy at Fakeeh College of Pharmacy for their support.
References
- Aswar M., Bhalekar M., Trimukhe A., Aswar U.. (2020) Self-microemulsifying drug delivery system (SMEDDS) of curcumin attenuates depression in olfactory bulbectomized rats. Heliyon 6: Article e04482 https://doi.org/10.1016/j.heliyon.2020.e04482 | Google Scholar
- Bansal T., Akhtar N., Jaggi M., Khar R. K., Talegaonkar S.. (2009) Novel formulation approaches for optimizing the delivery of anti cancer drugs basedon p-glycoprotein modulation. Drug Discovery Today 21–22: 1067-1074 https://doi.org/10.1016/j.heliyon.2020.e04482 | Google Scholar
- Bansode S. T., Kshirsagar S. J., Madgulkar A. R., Bhalekar M. R., Bandivadekar M. M.. (2016) Design and development of SMEDDS for colon-specific drug delivery. Drug Development and Industrial Pharmacy 42: 611-623 https://doi.org/10.3109/03639045.2015.1062510 | Google Scholar
- Brouwers J., Mols R., Annaert P., Augustijns P.. (2010) Validation of a differential perfusion method with mesenteric blood sampling in rats for intestinal drug interaction profiling. Biopharmaceutics and Drug Disposition 31: 278-285 https://doi.org/10.1002/bdd.710 | Google Scholar
- Butler J. M., Dressman J. B.. (2010) The developability classification system: Application of biopharmaceutics concepts to formulation development. Journal of Pharmaceutical Sciences 99: 4940-4954 https://doi.org/10.1002/jps.22217 | Google Scholar
- Buyukozturk F., Benneyan J. C.. (2010) Carrier Impact of emulsion-baseddrug delivery systems on intestinal permeability and drug release kinetics. Journal of Controlled Release 142: 22-30 https://doi.org/10.1002/jps.22217 | Google Scholar
- Cheng G., Hu R., Ye L., Wang B., Gui Y., Gao S., Li X., Tang J., et al. (2016) Preparation and/ evaluation of puerarin solid self-microemulsifying drug delivery system by spherical crystallization technique. AAPS PharmSciTech 17: 1336-1346 https://doi.org/10.1208/s12249-015-0469-8 | Google Scholar
- Czogalla A.. (2009) Oral cyclosporine A-. https://doi.org/10.1208/s12249-015-0469-8 | Google Scholar
- The current picture of its liposomal and other delivery systems. Cellular and Molecular Biology Letters 14: 139-152 https://doi.org/10.2478/s11658-008-0041-6 | Google Scholar
- Fransén N., Morin M., Björk E., Edsman K.. (2008) Physicochemical interactions between drugs and superdisintegrants. The Journal of Pharmacy and Pharmacology 60: 1583-1589 https://doi.org/10.1211/jpp/60.12.0003 | Google Scholar
- Grove M., Müllertz A., Nielsen J. L., Pedersen G. P.. (2006) Bioavailability of seocalcitol II: Development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. European Journal of Pharmaceutical Sciences 28: 233-242 https://doi.org/10.1016/j.ejps.2006.02.005 | Google Scholar
- Hasan M. Y. N.. (2004e) Array https://doi.org/10.1016/j.ejps.2006.02.005 | Google Scholar
- Hasan N. M. Y.. (2014e) Role of medium-chain fatty acids in the emulsification mechanistics of self-micro-emulsifying lipid formulations. Saudi Pharmaceutical Journal 22: 580-590 https://doi.org/10.1016/j.jsps.2014.02.005 | Google Scholar
- Hasan N. M. Y.. (2016c) Effect of a model lipophilic compound on the phase behaviour of hydrophilic self-micro-emulsifying lipid formulations. Journal of Pharmacy Research 10: 647-654 https://doi.org/10.1016/j.jsps.2014.02.005 | Google Scholar
- Hasan N. M. Y.. (2019d) Role of hydrophilic surfactants in the emulsification mechanistics of type III self-micro-emulsifying drug delivery systems (SMEDDS). International Journal of Applied Pharmaceutics 11: 98-108 https://doi.org/10.22159/ijap.2019v11i3.29732 | Google Scholar
- Hasan N. M. Y.. (2021f) Self-microemulsifying type II lipid class system: Inhibiting salting out effect of electrolytes present in the emulsifying media. International Journal of Biology, Pharmacy and Allied Sciences 10: 1182-1201 https://doi.org/10.22159/ijap.2019v11i3.29732 | Google Scholar
- Hasan N. M. Y., Khaleel M. A., Altwairqi A. S., Alqurashi A. G., Altwairqi A. H.. (2018a) Effect of Self-micro emulsifying lipid formulation on the dissolution and compaction profiles of tablets containing theophylline; a BCS class I compound. Journal of Applied Pharmaceutical Sciences 8: 30-38 https://doi.org/10.22159/ijap.2019v11i3.29732 | Google Scholar
- Hasan N. M. Y., Melfi S. A.. (2015b) Flavored Self Microemulsifying Lipid Formulations for Masking the Organoleptic Taste of Pharmaceutical Actives. Al-Aram, Mohammed SM Al-Wadie, Fahad AK Althobaiti and Majed JA Al-Malki. Journal of Applied Pharmaceutical Sciences 5: 127-134 https://doi.org/10.22159/ijap.2019v11i3.29732 | Google Scholar
- Kim D. S., Cho J. H., Park J. H., Kim J. S., Song E. S., Kwon J., Giri B. R., Jin S. G., Kim K. S., Choi H.-G., Kim D. W., et al. (2019) Self-microemulsifying drug delivery system (SMEDDS) for improved oral delivery and photostability of methotrexate. International Journal of Nanomedicine 14: 4949-4960 https://doi.org/10.2147/IJN.S211014 | Google Scholar
- Kumar M., Singh D., Bedi N.. (2019) Mefenamic acid-loaded solid SMEDDS: An innovative aspect for dose reduction and improved pharmacodynamic profile. Therapeutic Delivery 10: 21-36 https://doi.org/10.4155/tde-2018-0053 | Google Scholar
- Mandić J., Luštrik M., Vrečer F., Gašperlin M., Zvonar Pobirk A.. (2019) Solidification of carvedilol loaded SMEDDS by swirling fluidized bed pellet coating. International Journal of Pharmaceutics 566: 89-100 https://doi.org/10.1016/j.ijpharm.2019.05.055 | Google Scholar
- Nornoo A. O., Zheng H., Lopes L. B., Johnson-Restrepo B., Kannan K., Reed R., et al. (2009) Oral microemulsions of paclitaxel: and pharmacokineticstudies. European Journal of Pharmaceutics and Biopharmaceutics 71: 310-317 https://doi.org/10.1016/j.ejpb.2008.08.015 | Google Scholar
- Nouraei M., Collymore C., Diosady L., Acosta E.. (2021) HLD-NAC design and evaluation of a fully dilutable lecithin-linker SMEDDS for ibuprofen. International Journal of Pharmacy 15: Article 121237 https://doi.org/10.1016/j.ejpb.2008.08.015 | Google Scholar
- Pouton C. W.. (2006) Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. European Journal of Pharmaceutical Sciences 29: 278-287 https://doi.org/10.1016/j.ejps.2006.04.016 | Google Scholar
- Raducan A., Oancea P., Di t. L. M., Stan M., Petcu C., ¸scenco C., Ninciuleanu C.M., Nistor C.L., Cinteza L.O., et al. (2021) Mixed pluronic-Cremophor polymeric micelles as nanocarriers for poorly soluble antibiotics-The influence on the antibacterial activity. T ˘anase, M.A. Pharmaceutics 13: Article 13040435 https://doi.org/10.1016/j.ejps.2006.04.016 | Google Scholar
- Savla R., Browne J., Plassat V., Wasan K. M., Wasan E. K.. (2017) Review and analysis of FDA approved drugs using lipid-based formulations. Drug Development and Industrial Pharmacy 43: 1743-1758 https://doi.org/10.1080/03639045.2017.1342654 | Google Scholar
- Visetvichaporn V., Kim K.-H., Jung K., Cho Y.-S., Kim D.-D.. (2020) Formulation of self-microemulsifying drug delivery system (SMEDDS) by D-optimal mixture design to enhance the oral bioavailability of a new cathepsin K inhibitor (HL235). International Journal of Pharmaceutics 573: Article 118772 https://doi.org/10.1016/j.ijpharm.2019.118772 | Google Scholar
- Wang H., Li L., Ye J., Dong W., Zhang X., Xu Y., Hu J., Wang R., Xia X., Yang Y., Jin D., Wang R., Song Z., Gao L., Liu Y., et al. (2021) Improved safety and anti-glioblastoma efficacy of CAT3-encapsulated SMEDDS through metabolism modification. Molecules 26: Article 484 https://doi.org/10.3390/molecules26020484 | Google Scholar
- Zech J., Salaymeh N., Hunt N. H., Mäder K., Golenser J.. (2021) Efficient treatment of experimental cerebral malaria by an Artemisone-SMEDDS system: Impact of application route and dosing frequency. Antimicrobial Agents and Chemotherapy 65: Article e02106-20 https://doi.org/10.1128/AAC.02106-20 | Google Scholar
- Zhao K., Yuan Y., Wang H., Li P., Bao Z., Li Y., et al. (2016) Preparation and evaluation of valsartan by a novel semi-solid self-microemulsifying delivery system using Gelucire 44/14. Drug Development and Industrial Pharmacy 42: 1545-1552 https://doi.org/10.3109/03639045.2016.1151034 | Google Scholar
- Zhu Z., Liu J., Yang Y., Adu-Frimpong M., Ji H., Toreniyazov E., Wang Q., Yu J., Xu X., et al. (2021) SMEDDS for improved oral bioavailability and anti-hyperuricemic activity of licochalcone A. Journal Microencapsulation 38: 459-471 https://doi.org/10.3109/03639045.2016.1151034 | Google Scholar
