ABSTRACT
PPIs are the most popular medications used in treatment of gastric diseases based on acids, and they turn out to be the strongest agents to suppress the acids. But extended use has been associated with hypochlorhydria and micronutrient deficiency Vitamin C being one. The efficacy of altered gastric acidity on the absorption of ascorbic acid and increasing its oxidation and subsequent reduced stomach and plasma levels of Vitamin C is studied in this narrative review, which is based on available clinical trials, case reports and mechanistic studies in the past 2 decades. Clinically, it cause dysfunctioning of collagen synthesis, immune deficiency, oxidative stress and in severe condition, scurvy. The vulnerable groups affected are the elderly, the malnourished, the post bariatric surgical and the Helicobacter pylori infected. Diet can help returning levels of the Vitamin C to normal, but close monitoring and actual nutritional planning are inevitable. This review gives relevance to increased clinical awareness, the individualization of supplementation strategy, and further research to reach some established regulations as to the prevention of nutrient deficiencies in long-term PPI users.
INTRODUCTION
Scurvy is a systemic illness caused by chronic ascorbic acid deficiency, which impairs collagen synthesis, immune function and iron metabolism – eventually leading to gum bleeding, brittle capillaries and impaired wound healing (Khalifeet al., 2019). This occurs when the body content of Vitamin C decreases below about 300mg usually after a period of 60 to 90 days of poor intake (Gandhiet al., 2023a). Clinically scurvy presents with symptoms like weakness, bleeding gums, Petechiae, corkscrew hair, joint pains and failure of wound healing (Geber and Murphy, 2012). Scurvy is classically observed among seafarers, but it still occurs in some populations today such as in elderly the restrictive dietary post-bariatric surgery patients, alcoholics or those with psychiatric illness and those living in food deprivation situations (Fenech et al., 2019). Recent Australian and European case reports suggest a resurgence of scurvy even in the economically advanced countries where people face deprivation or poor diet (Agarwalet al., 2015). Humans are especially prone to this deficiency because lack of enzyme responsible for synthesizing Vitamin C in the body (Carr and Maggini, 2017). Vitamin C is essential for the hydroxylation of collagen precursors as well as its antioxidant role and immune function (Ashoret al., 2019). Long term use of PPIs will likely lead to scurvy due to inadequate oral intake of Vitamin C as well as reduced absorption and stability of the vitamin in the gastrointestinal tract.
METHODOLOGY
The evidence base of this systematic review on vitamin C deficiency, and its association with the use of PPI, is the English-language publications of 2004-2024 that were retrieved using PubMed, Scopus, Science Direct, and Google Scholar.
Physiological Roles of Vitamin C: Immunity, Collagen Synthesis, and Antioxidant Function
Vitamin C also known as ascorbic acid and it is important for humans because it helps in making collagen, protects against free radicals and works in the immune system, however it must be acquired through food (Li and Schell horn, 2007). Up to 20% of adults have a light deficiency that can cause fatigue, slow wound healing, bleeding gums and a likelihood to bruise easily. People are still getting true scurvy which causes bleeding. This is especially true for elderly, low-income, restricted diet or post bariatric surgery patients (Velandiaet al., 2008).
GI Handling of Vitamin C
The human stomach has a very acidic lining (1-3) which is important for both digesting protein and making micronutrients available to the body (Fujimori, 2020). In particular stomach acid keeps ascorbate in its reduced, bioactive sate and makes it possible for oxidized dehydroascorbic acid (DHAA) to be reduced to ascorbate and keeps the mucosa healthy (Carabottiet al., 2021). Active secretion of vitamin C into gastric juice which is usually several times higher than plasma levels is significant for its role as an antioxidant and in controlling the conversion of nitrite to nitrosamine which may lessen the risk of cancer (Schorahet al., 1991).
Gastrointestinal physiology is important for getting Vitamin C into the body (Carabottiet al., 2021). The connection between Vitamin C and the stomach helps explain how long-term PPI medication can lead to scurvy which is the main topic of this narrative review Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation (Mowatet al., 2000).
Patterns and Risks of Long-Term Proton Pump Inhibitor Use
PPI’s including omeprazole, pantoprazole and esomeprazole rank among the most prescribed medications globally (Shanikaet al., 2023). They are routinely used to treat gastro esophageal reflux disease, peptic ulcer disease, H. Pylori eradication and NSAID induced (Schnoll-Sussmanet al., 2020). Indeed, PPIs account for approximately 6% of all prescriptions and up to 15% of adults in some regions take them chronically (Jaynes and Kumar, 2019).
Despite strong safety profile short-term accumulating evidence links prolonged PPI use systemic effects including nutrient mal absorption, hypomagnesaemia altered gut microbiome and increased infection risk (Heidelbaugh, 2013a).
Masked Malabsorption by PPIs
Long-term PPI treatment not only suppresses gastric acid secretion but also hampers absorption of numerous essential nutrients leading to a cascade of nutritional consequences (Khaliliet al., 2012). Hypochlorhydria prevents release of calcium from insoluble alts, increasing fracture risk by about 1.3 times, particularly in elderly individuals taking high dose PPIs. Magnesium absorption is also affected by observational reports and case reports demonstrate a 1.6 – 2 times risk increase in hypomagnesaemia leading to symptoms including muscle cramps, arrhythmias and therefore the FDA called a safety alert in 2011 (William and Danziger, 2016). In addition, reduced gastric acidity inhibits pepsin activation blocking the extraction of Vitamin B₁₂ from dietary proteins and elevating the risk of deficiency after two years of continuous PPI use, especially in the elderly or in H. Pylori infected persons (Lamet al., 2013). Iron uptake is blocked by impaired conversion from ferric to ferrous forms and reduced facilitation by ascorbate. Some reports reduced haemoglobin concentrations and increased anemia among PPI users (Koyyada, 2021). Early evidence also suggests that Vitamin D and zinc stores could be affected though the evidence is still inconsistent (Schorah et al., 1991b).
Vitamin C under Siege
This imbalance in diet has an important impact on the metabolism of Vitamin C which is normally secreted in gastric juice in its active form and protected from irreversible inactivation at low pH is rendered labile by the hypochlorhydric conditions created with PPIs (Padayatty and Levine, 2016). Dehydroascorbic acid does not get regenerated and undergoes hydrolysis irreversibly reducing the overall available Vitamin C reduced availability of ascorbate worsens impaired calcium absorption since collagen-based bone matrix synthesis is dependent upon Vitamin C and impairs iron absorption by preventing iron-ascorbate complexes thus aggravating anemia (Carr and Maggini, 2017). This group of deficiencies largely caused by decreased Vitamin C may lead to significant systemic effects including anemia, demineralization, neuromuscular disease and mucosal fragility thus emphasizing the need for overall nutrient surveillance and targeted supplementation in individuals undergoing extended PPI therapy (Jacob and Sotoudeh, 2002).
Mechanistic Link: PPIs and Vitamin C Loss
PPIs irreversibly inhibit the gastric H+/K+ ATPase pump in parietal cells so hypochlorhydria persists and the stomach pH increases <2 to >6 (Mowatet al., 1999).
Vitamin C in the stomach which was initially secreted as biologically active ascorbate becomes chemically unstable at higher pH levels DHAA is unable to revert to active ascorbate and instead degrades to 2, 3-diketogulonic acid, particularly in the presence of trace metals and under normal body temperatures (Mohnet al., 2018). This degradation reduces the bioavailability of Vitamin C in both the stomach and the body reducing its potential as an antioxidant and leading nutrients to be lost before they can be absorbed in the small intestine. Gastric mucosal secretion aggravates the issues: under normal acidity of the stomach it concentrates ascorbate in gastric juice above plasma levels and actively converts DHAA to ascorbate via glutathione-dependent pathways. Hypochlorhydria disrupts the latter which depletes the protective levels of ascorbate very rapidly and compromises the mucosal defences. This involves altering nitrite chemistry and reducing the antioxidant reservoir (O’Connoret al., 1989a).
Dual Threat to Vitamin C: H. pylori and Low Acid
When the pH level of the stomach increases and helicobacter pylori infection occurs a molecular cascade begins to occur (Freedberget al., 2014a). H. Pylori caused gastritis causes parietal cells to function less effectively, increases pH and accelerates the oxidation of ascorbate both in and out of cells. This occurs because bacteria absorb ascorbate and produce oxidative stress (�dum and Andersen, 1995). Research indicates that individuals with H. pylori have significantly lower stomach levels of ascorbate, significantly higher levels of DHAA and don’t completely recover after a dose of Vitamin C (�dum and Andersen, 1995). This is consistent with the notion the hypochlorhydria and microbial stress interact to exacerbate. Fewer Vitamin C prevents the synthesis of collagen. This makes blood vessel weak which manifests as gingival bleeding, petechiae and delayed wound healing- scurvy signs. Furthermore, vitamin C’s capacity to reduce Fe³⁺ to Fe²⁺ is compromised which may result in iron deficiency anemia in individuals who have been on PPI for years (Figure 1) (O’Connor et al., 1989b).
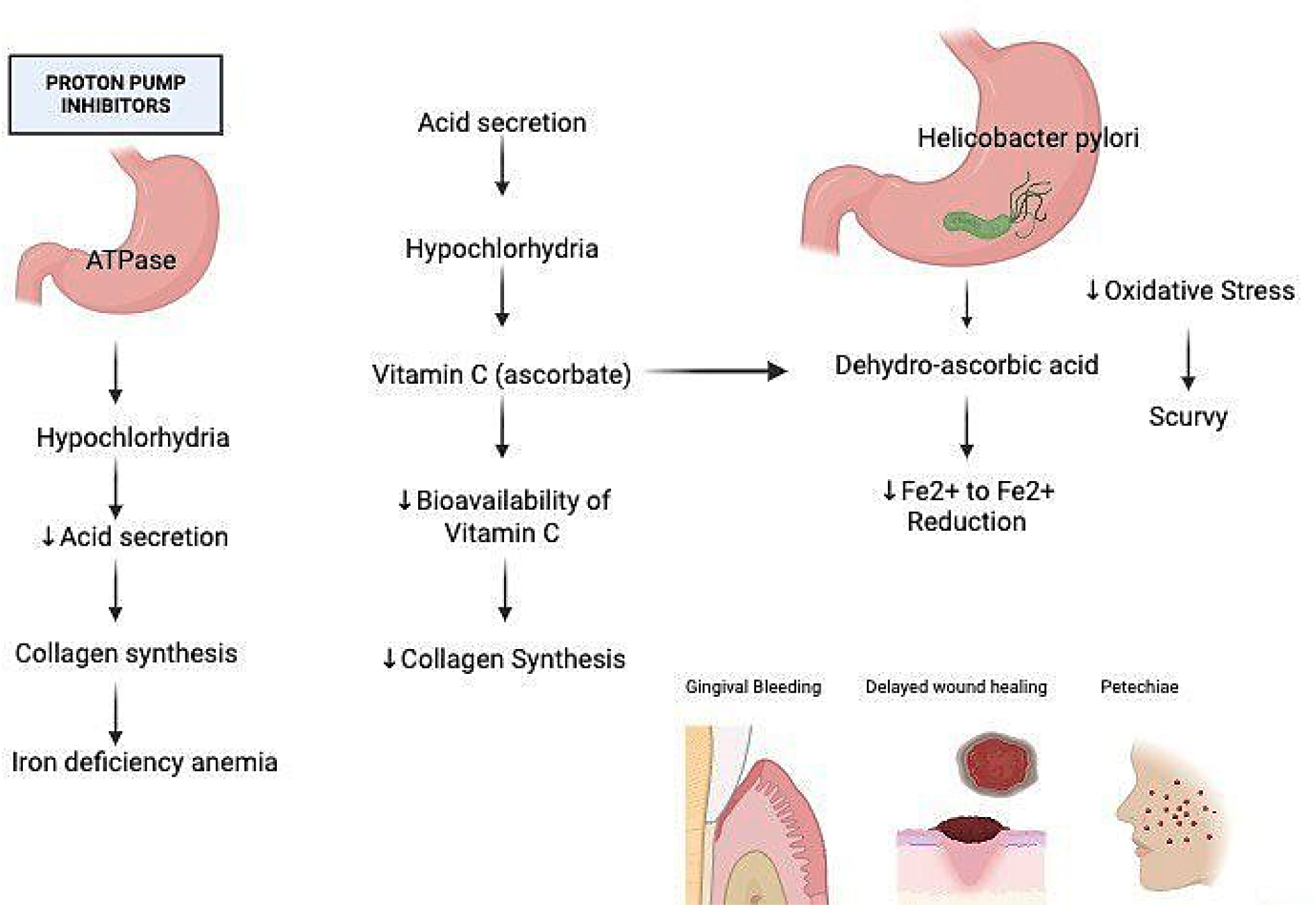
Figure 1:
Biochemical pathway of PPI induced hypochlorhydria leading to ascorbate deficiency.
Clinical Clues: PPIs and C Deficiency
Clinical trials have shown that PPIs cause an immediate decline in Vitamin C levels in the blood gastric environment of healthy subjects (HENRYet al., 2005).
In a controlled trial by Henry et al., in 29 healthy volunteers it was found that four weeks of daily dosing with 40 mg omeprazole caused a significant 12.3% drop in plasma Vitamin C level, irrespective of dietary factors. This effect was seen in both H. Pylori positive and negative subjects (HENRYet al., 2005).
In parallel in another study by Mowat and McColl in fasting samples revealed a significant reduction in intra-gastric Vitamin C level; initial gastric ascorbate level (~3.8 μg/mL) fell to ~0.7 μg/mL under omeprazole therapy (with pH rising from ~1.4 to ~7.2) (Mowat and McColl, 2001). These observations unmistakably show that even a short course of PPI therapy destabilizes Vitamin C reducing its efficacy as an antioxidant in the gastric environment (Henryet al., 2005).
In vitro Proof of PPI Toxicity
Research into the role of Vitamin C also provides this connection. McColl and others found that omeprazole increased the pH of the stomach reduced the levels of antioxidant active ascorbic acid and increased the levels of nitrite in the stomach particularly in individuals infected with H. Pylori (Woodwardet al., 2001). Further in in vitro research demonstrates that omeprazole induces thiol-oxidative stress on gastric epithelial cells that can be prevented by concurrent consumption of Vitamin C (Kohleret al., 2010). This demonstrates that vitamin C provides protection against mucosal effects from PPI. Though there are fewer of these observational studies support the hypothesis that PPI users are low in Vitamin C particularly among those who lack adequate amounts of it in the first place or who have a high rate of infections (HENRYet al., 2005).
Scurvy in the PPI Era
Key case studies of scurvy there is sparse clinical evidence that long term use of PPIs can lead to scurvy but what there is a very strong. A middle aged western Australian man presented with bleeding gums, petechial rash, anemia and plasma Vitamin C undetectable. He was of low socioeconomic status and had a poor diet and had been on omeprazole for several years following bariatric surgery. Supplementation with 1g Vitamin C daily multivitamin corrected the symptoms (Weinsteinet al., 2001).
Fragile Populations: A Rising Concern
A study talked about a teenager with eosinophilic esophagitis who was on long term PPI therapy (omeprazole) and had joint bleeding a rash blood problems and undetectably low serum Vitamin C by mouth. These cases show how hypochlorhydria along with other conditions like inadequate nutrition, mal-absorption and co-morbidities can collectively worsen conditions. Though rare, the clinical expression is similar to that in patients with a history of scurvy (Gallizziet al., 2020). This demonstrates the dangers that are not being mitigated in patients with chronic PPI exposure. Helicobacter pylori infection decreases the acidity of the stomach and enhances oxidative stress, which increases the breakdown of Vitamin C. PPI treated infected patients have reduced gastric ascorbate levels and are more delayed in complete recovery when PPI is given. Long-term inhibition of acid prevents active ascorbate synthesis in the stomach from taking place, making it harder for the body to reduce dehydroascorbic acid and weaken antioxidant protection. PPI long term use greatly impacts the stomach’s function leading to long term hypochlorhydria (gastric pH>6) and this increases the challenge of the stability and variability of Vitamin C. This elevates the body chemistry to induce Vitamin C loss and potential scurvy in susceptible individuals (Heidelbaugh, 2013b). These include individuals of age on dialysis, smokers, alcohol consumers or those with eating disorders. Older age is a risk of factor for achlorhydria and disease states and malnutrition increase the risk of Vitamin C deficiency even among hospitalized and mental patients (up to 30% deficient) (Schleicheret al., 2009).
Clinical Focus: Ascorbate Deficiency from PPIs
The vitamin C may be impaired by chronic PPI therapy. There should always be vigilance in patients on prolonged PPI therapy for Vitamin C deficiency even scurvy (Schleicheret al., 2009). This should particularly be done for those with additional risk factors including restrictive diets, low socioeconomic status, H. Pylori infection, bariatric surgery or being elderly (Heidelbaugh, 2013d). As a part of standard nutritional screening and history-taking individuals should be questioned regarding their consumption of foods rich in Vitamin C, gastrointestinal symptom presence and presence of any other diseases that can worsen their insufficiency, when risk is present, serum or leukocyte ascorbate measurement is an inexpensive and insensitive method to diagnose it (Faddaet al., 2025).
Supplement, Simplify, Stabilize
Regular oral supplementation, typically between 300 to 1000mg daily may cause symptoms to resolve within a few days. Maintenance dose typically a minimum of 100 mg daily may prevent the recurrence of symptoms. A person with mal absorption or acute scurvy such as following bariatric surgery, might require receiving the medication intravenously. It is also important to consider whether continuous PPI therapy is still required. Physicians should consider switching H2- receptor antagonists, switching to on demand or intermittent dosing or discontinuing PPIs when appropriate (Forgacs and Loganayagam, 2008). This approach to de prescribing is consistent with updated stewardship guidance and reduces the overall physiological burden of acid suppression. Monitoring procedures must entail frequent reassessment like nutrient profiles every 6 to 12 months in at risk populations to detect subclinical deficits prior to causing apparent illness.
Healthcare Systems and Society
In addition to Vitamin C monitoring must also examine the related micronutrients like B12, iron, magnesium, zinc and calcium that are usually impaired by inhibiting stomach acid. For healthcare systems the integration of nutrient monitoring into electronic prescribing systems could automatically trigger alerts for long term PPI prescriptions informing doctors to reassess the patient’s risk and consider prescribing Vitamin C as a preventive measure (Heidelbaugh, 2013b). Lastly, public health initiatives can possibly consider socioeconomic issues such as food insecurity and the inaccessibility of fresh fruits and vegetables. The reason is that cost and access issues are a leading reason for cases of PPI related scurvy in populations with economic issues. By incorporating nutrient-centered monitoring and counselling into PPI prescription pathways, physicians can prevent deficient pathways early and maintain their patient’s nutritional status while continuing to control their acid levels effectively (Van BOXELet al., 2009).
Exploring the Unknown: PPIs and Vitamin C
The impact of long term PPI therapy on Vitamin C status is poorly defined because there are many study flaws. Most of the clinical studies are brief, typically consisting of healthy subjects receiving 40mg of omeprazole for four weeks which had a roughly 12% decrease in plasma Vitamin C (Woodwardet al., 2001). These controlled trials are not relevant to clinical patients who often present with comorbidities variable dietary intake and concomitant factors like H. Pylori infection that influence Vitamin C metabolism (McColl, 2009).
Evidence Gaps and Biological Clues
Observational reports on dietary deficiencies associated with PPI use highlight reductions in Vitamin B₁₂, D, magnesium and iron but rarely measure Vitamin C levels leaving extensive gaps in analysis of ascorbate (Heidelbaugh, 2013d). Mechanistic research has clarified that hypochlorhydria tips gastric ascorbate towards instability resulting in lower intragastric and systemic amounts yet the interconnection between gastric depletion and effects of clinical relevance like scurvy remains uninvestigated (McColl, 2009).
Research data suggest an unequivocal need for future longitudinal research observing plasma and gastric Vitamin C levels in patients receiving long-term PPI therapy. Short-term studies showed a decrease of approximately 12% in plasma Vitamin C after 28 days of omeprazole treatment. Adult PPI users with ongoing biochemical monitored augmented by food diaries and symptom surveys should be included in future studies to ascertain the ascorbate levels at which subclinical insufficiency evolves into overt scurvy (Whiteet al., 2002).
Alternative Care, Measured Impact
Comparative studies that compare PPIs to other acid suppressants like H₂ receptor antagonists or potassium-competitive blockers will ascertain whether less intense acid suppression strategies better preserve vitamin C bioavailability (Heidelbaugh, 2013d).
CONCLUSION
The long-term use of Proton Pump Inhibitor (PPI) is effective in the management of acid-spectrum ailments; nevertheless, vitamin C deficiency that can be explained by the case of hypochlorhydria is a major issue. The empirical data shows that the plasma and gastric ascorbate levels decrease significantly during a few weeks, and long-term consequences of the given phenomenon are not thoroughly studied. Ascorbate degradation is encouraged in environments of ongoing hypergastrinemia and high gastric pH, setting up patient’s scurvy, especially with preexisting nutrition predispositions. Even though vitamin C supplementation is easily accessible, there is no active systematic surveillance. It should thus be encouraged that practitioners consider adjunctive employment of vitamin C and the assessment of dietary sufficiency. Prospective, long-term and randomized trials are essential to elucidate the secondary incidence, pathway and implications of this Halloween effect and to guide more prudent nutrient-savvy PPI prescriptions.
Cite this article:
Varshini AJ, Priyadharshini A, Rumana SS, Harinipriya A. Impact of Long-Term PPI Therapy on Vitamin C Levels: Emerging Concerns. J Young Pharm. 2025;17(4):810-5.
ACKNOWLEDGEMENT
I would like to thank SRM College of Pharmacy, SRMIST, Kattankulathur for providing technical resources.
ABBREVIATIONS
| PPI | Proton Pump Inhibitors |
|---|---|
| DHAA | Dehydroascorbic Acid |
| H. pylori | Helicobacter pylori |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| FDA | Food and Drug Administration |
| B₁₂ | Vitamin B12 |
| GI | Gastrointestinal |
| ATPase | Adenosine Triphosphatase |
References
- �dum L., Andersen L. P.. (1995) Investigation of Helicobacter pylori ascorbic acid oxidating activity.. FEMS Immunology and Medical Microbiology 10: 289-294 https://doi.org/10.1111/j.1574-695X.1995.tb00046.x | Google Scholar
- Agarwal A., Shaharyar A., Kumar A., Bhat M. S., Mishra M.. (2015) Scurvy in pediatric age group-A disease often forgotten?. Journal of Clinical Orthopaedics and Trauma 6: 101-107 https://doi.org/10.1016/j.jcot.2014.12.003 | Google Scholar
- Ashor A. W., Brown R., Keenan P. D., Willis N. D., Siervo M., Mathers J. C., et al. (2019) Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses.. Nutrition Research 61: 1-12 https://doi.org/10.1016/j.nutres.2018.08.005 | Google Scholar
- Carabotti M., Annibale B., Lahner E.. (2021) Common pitfalls in the management of patients with micronutrient deficiency: Keep in mind the stomach.. Nutrients 13: 208 https://doi.org/10.3390/nu13010208 | Google Scholar
- Carr A. C., Maggini S.. (2017) Vitamin C and immune function.. Nutrients 9: 1211 https://doi.org/10.3390/nu9111211 | Google Scholar
- Fadda H. M., Shin A., Waseem M. R., Camilleri M.. (2025) Vitamin C reduces gastric pH in pharmacologically induced hypochlorhydria: A potential approach for mitigating pH-dependent drug–drug interactions of weak-base drugs.. Journal of Pharmaceutical Sciences, Article 103809. https://doi.org/10.1016/j.xphs.2025.103809 | Google Scholar
- Fenech M., Amaya I., Valpuesta V., Botella M. A.. (2018) Vitamin C content in fruits: Biosynthesis and regulation.. Frontiers in Plant Science 9: 2006 https://doi.org/10.3389/fpls.2018.02006 | Google Scholar
- Forgacs I., Loganayagam A.. (2008) Overprescribing proton pump inhibitors.. BMJ 336: 2-3 https://doi.org/10.1136/bmj.39406.449456.BE | Google Scholar
- Freedberg D. E., Lebwohl B., Abrams J. A.. (2014a) The impact of proton pump inhibitors on the human gastrointestinal microbiome.. Clinics in Laboratory Medicine 34: 771-785 https://doi.org/10.1016/j.cll.2014.08.008 | Google Scholar
- Fujimori S.. (2020) Gastric acid level of humans must decrease in the future.. World Journal of Gastroenterology 26: 6706-6709 https://doi.org/10.3748/wjg.v26.i43.6706 | Google Scholar
- Gallizzi R., Valenzise M., Passanisi S., Pajno G. B., De Luca F., Zirilli G., et al. (2020) Scurvy may occur even in children with no underlying risk factors: A case report.. Journal of Medical Case Reports 14: 18 https://doi.org/10.1186/s13256-020-2341-z | Google Scholar
- Gandhi M., Elfeky O., Ertugrul H., Chela H. K., Daglilar E.. (2023a) Scurvy: Rediscovering a forgotten disease.. Diseases 11: 78 https://doi.org/10.3390/diseases11020078 | Google Scholar
- Geber J., Murphy E.. (2012) Scurvy in the Great Irish Famine: Evidence of vitamin C deficiency from a mid-19th century skeletal population.. American Journal of Physical Anthropology 148: 512-524 https://doi.org/10.1002/ajpa.22066 | Google Scholar
- Heidelbaugh J. J.. (2013a) Proton pump inhibitors and risk of vitamin and mineral deficiency: Evidence and clinical implications.. Therapeutic Advances in Drug Safety 4: 125-133 https://doi.org/10.1177/2042098613482484 | Google Scholar
- Henry E. B., Carswell A., Wirz A., Fyffe V., Mccoll K. E. L.. (2005) Proton pump inhibitors reduce the bioavailability of dietary vitamin C.. Alimentary Pharmacology and Therapeutics 22: 539-545 https://doi.org/10.1111/j.1365-2036.2005.02568.x | Google Scholar
- Jacob R. A., Sotoudeh G.. (2002) Vitamin C function and status in chronic disease.. Nutrition in Clinical Care 5: 66-74 https://doi.org/10.1046/j.1523-5408.2002.00005.x | Google Scholar
- Jaynes M., Kumar A. B.. (2019) The risks of long-term use of proton pump inhibitors: A critical review.. Therapeutic Advances in Drug Safety 10: Article 2042098618809927 https://doi.org/10.1177/2042098618809927 | Google Scholar
- Khalife R., Grieco A., Khamisa K., Tinmouh A., McCudden C., Saidenberg E., et al. (2019) Scurvy, an old story in a new time: The hematologist’s experience.. Blood Cells, Molecules and Diseases 76: 40-44 https://doi.org/10.1016/j.bcmd.2019.01.004 | Google Scholar
- Khalili H., Huang E. S., Jacobson B. C., Camargo C. A., Feskanich D., Chan A. T., et al. (2012) Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: A prospective cohort study.. BMJ 344: e372-e372 https://doi.org/10.1136/bmj.e372 | Google Scholar
- Kohler J. E., Blass A. L., Liu J., Tai K., Soybel D. I.. (2010) Antioxidant pre-treatment prevents omeprazole-induced toxicity in an model of infectious gastritis.. Free Radical Biology and Medicine 49: 786-791 https://doi.org/10.1016/j.freeradbiomed.2010.05.034 | Google Scholar
- Koyyada A.. (2021) Long-term use of proton pump inhibitors as a risk factor for various adverse manifestations.. Therapie 76: 13-21 https://doi.org/10.1016/j.therap.2020.06.019 | Google Scholar
- Lam J. R., Schneider J. L., Zhao W., Corley D. A.. (2013) Proton pump inhibitor and Histamine 2 receptor antagonist use and vitamin B 12 deficiency.. JAMA 310: 2435-2442 https://doi.org/10.1001/jama.2013.280490 | Google Scholar
- Li Y., Schellhorn H. E.. (2007) New developments and novel therapeutic perspectives for vitamin C.. The Journal of Nutrition 137: 2171-2184 https://doi.org/10.1093/jn/137.10.2171 | Google Scholar
- McColl K. E. L.. (2009) Effect of proton pump inhibitors on vitamins and iron.. The American Journal of Gastroenterology 104: S5-S9 https://doi.org/10.1038/ajg.2009.45 | Google Scholar
- Mohn E. S., Kern H. J., Saltzman E., Mitmesser S. H., McKay D. L.. (2018) Evidence of drug-nutrient interactions with chronic use of commonly prescribed medications: An update.. Pharmaceutics 10: 36 https://doi.org/10.3390/pharmaceutics10010036 | Google Scholar
- Mowat C., Carswell A., Wirz A., McColl K. E. L.. (1999) Omeprazole and dietary nitrate independently affect levels of vitamin C and nitrite in gastric juice.. Gastroenterology 116: 813-822 https://doi.org/10.1016/S0016-5085(99)70064-8 | Google Scholar
- Mowat C., McColl K. E. L.. (2001) Alterations in intragastric nitrite and vitamin C levels during acid inhibitory therapy.. Best Practice & Research. Clinical Gastroenterology 15: 523-537 https://doi.org/10.1053/bega.2000.0196 | Google Scholar
- Mowat C., Williams C., Gillen D., Hossack M., Gilmour D., Carswell A., Wirz A., Preston T., McColl K. E. L., et al. (2000) Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation.. Gastroenterology 119: 339-347 https://doi.org/10.1053/gast.2000.9367 | Google Scholar
- O’Connor H. J., Schorah C. J., Habibzedah N., Axon A. T., Cockel R.. (1989a) Vitamin C in the human stomach: Relation to gastric pH, gastroduodenal disease, and possible sources.. Gut 30: 436-442 https://doi.org/10.1136/gut.30.4.436 | Google Scholar
- Padayatty S. J., Levine M.. (2016) Vitamin C: The known and the unknown and Goldilocks.. Oral Diseases 22: 463-493 https://doi.org/10.1111/odi.12446 | Google Scholar
- Schleicher R. L., Carroll M. D., Ford E. S., Lacher D. A.. (2009) Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES).. The American Journal of Clinical Nutrition 90: 1252-1263 https://doi.org/10.3945/ajcn.2008.27016 | Google Scholar
- Schnoll-Sussman F., Niec R., Katz P. O.. (2020) Proton pump inhibitors: The good, bad, and ugly.. Gastrointestinal Endoscopy Clinics of North America 30: 239-251 https://doi.org/10.1016/j.giec.2019.12.005 | Google Scholar
- Schorah C. J., Sobala G. M., Sanderson M., Collis N., Primrose J. N.. (1991a) Gastric juice ascorbic acid: Effects of disease and implications for gastric carcinogenesis.. The American Journal of Clinical Nutrition 53: 287S-293S https://doi.org/10.1093/ajcn/53.1.287S | Google Scholar
- Shanika L. G. T., Reynolds A., Pattison S., Braund R.. (2023) Proton pump inhibitor use: Systematic review of global trends and practices.. European Journal of Clinical Pharmacology 79: 1159-1172 https://doi.org/10.1007/s00228-023-03534-z | Google Scholar
- Van Boxel O. S., Hagenaars M. P., Smout A. J. P. M., Siersema P. D.. (2009) Socio-demographic factors influence chronic proton pump inhibitor use by a large population in the Netherlands.. Alimentary Pharmacology and Therapeutics 29: 571-579 https://doi.org/10.1111/j.1365-2036.2008.03900.x | Google Scholar
- Velandia B., Centor R. M., McConnell V., Shah M.. (2008) Scurvy is still present in developed countries.. Journal of General Internal Medicine 23: 1281-1284 https://doi.org/10.1007/s11606-008-0577-1 | Google Scholar
- Weinstein M., Babyn P., Zlotkin S.. (2001) An orange a day keeps the doctor away: Scurvy in the year 2000.. Pediatrics 108: E55 https://doi.org/10.1542/peds.108.3.e55 | Google Scholar
- White K. L. M., Chalmers D. M., Martin I. G., Everett S. M., Neville P. M., Naylor G., Sutcliffe A. E., Dixon M. F., Turner P. C., Schorah C. J., et al. (2002) Dietary antioxidants and DNA damage in patients on long-term acid-suppression therapy: A randomized controlled study.. The British Journal of Nutrition 88: 265-271 https://doi.org/10.1079/BJN2002619 | Google Scholar
- William J. H., Danziger J.. (2016) Proton-pump inhibitor-induced hypomagnesemia: Current research and proposed mechanisms.. World Journal of Nephrology 5: 152-157 https://doi.org/10.5527/wjn.v5.i2.152 | Google Scholar
- Woodward M., Tunstall-Pedoe H., McColl K.. (2001) Helicobacter pylori infection reduces systemic availability of dietary vitamin C.. European Journal of Gastroenterology and Hepatology 13: 233-237 https://doi.org/10.1097/00042737-200103000-00003 | Google Scholar
