ABSTRACT
Emulgel is a blend of gel and emulsion that improves drug penetration across the epidermal barrier and facilitates efficient skin diffusion, particularly for drugs that are poorly soluble in water. It is a potent, thermodynamically robust technique that integrates co-surfactants and surfactants. The properties that emulgel includes are easily spreadable, thixotropic, transparent, greaseless, extended shelf life, detachable, emollient, and bio-friendly. These are utilized in analgesics, anti-inflammatory agents, antifungals, anti-acne products, and cosmetic compositions. This article emphasizes preparation, advancements, applications, clinical studies, and current and future aspects of emulgel for improving transdermal delivery with its regulatory challenges.
INTRODUCTION
Transdermal Drug Delivery System (TDDS) is used when local effects on severe skin disorders cannot be achieved via oral, sublingual, rectal, or parenteral drug delivery techniques. For TDDS, the skin absorbs the drug and delivers it to the site of action, resulting in a more effective therapeutic effect. The skin is one of the most accessible organs for administering drugs, and it can be utilized for both local and systemic treatment. The skin, the largest organ in the body, consists of three main layers: epidermis, dermis, and hypodermis (Figure 1). The epidermis is the outermost layer, consisting of stratified squamous epithelial cells. The dermis, a thick layer of loose connective tissue, is visible to the naked eye and contains important blood vessels. The hypodermis, or subcutaneous tissue, lies underneath the dermis and contains the vascular plexus, lymphatic veins, and a large amount of adipose tissue (Lopez-Ojedaet al., 2021). By removing first-pass metabolism by topical administration, intravenous therapy’s hazards and issues are avoided. Gel formulations are less effective in delivering hydrophobic drugs, but they release active ingredients faster. Because of their high permeability and poor solubility, class II drugs are more difficult to absorb and move through the body than their membrane-passing counterparts. Research has shown that emulgel, a combination of gel and emulsion, may be a preferable alternative for the topical delivery of weakly water-soluble pharmaceuticals (Prusty et al., 2024; Khamkatet al., 2022). Emulgel is a combination of oil-in-water (o/w) or water-in-oil (w/o) emulsions with a gelling agent incorporated. The smooth texture of emulgel ensures better patient compliance due to its ease of spreading on the skin (Olayemi and David, 2023). It has many beneficial qualities for dermatological applications, including thixotropy, non-greasiness, and spreadability. It has a higher loading capacity than other transdermal preparations and is affordable (Figure 2) (Talatet al., 2021). Emulgels are classified into three types based on particle size: macroemulsion gel, microemulsion gel, and nanoemulsion gel. Macroemulsions are invisible but visible under a microscope due to larger droplets. Microemulsions are isotropic mixtures of water, surfactant, oil, and co-surfactant with droplets ranging from 10 to 100 nm. Nanoemulsions are transparent oil-water dispersions with globule diameters between 1 and 100 nm, providing improved delivery and transdermal penetration (Charyuluet al., 2021; Ganjuet al., 2024).
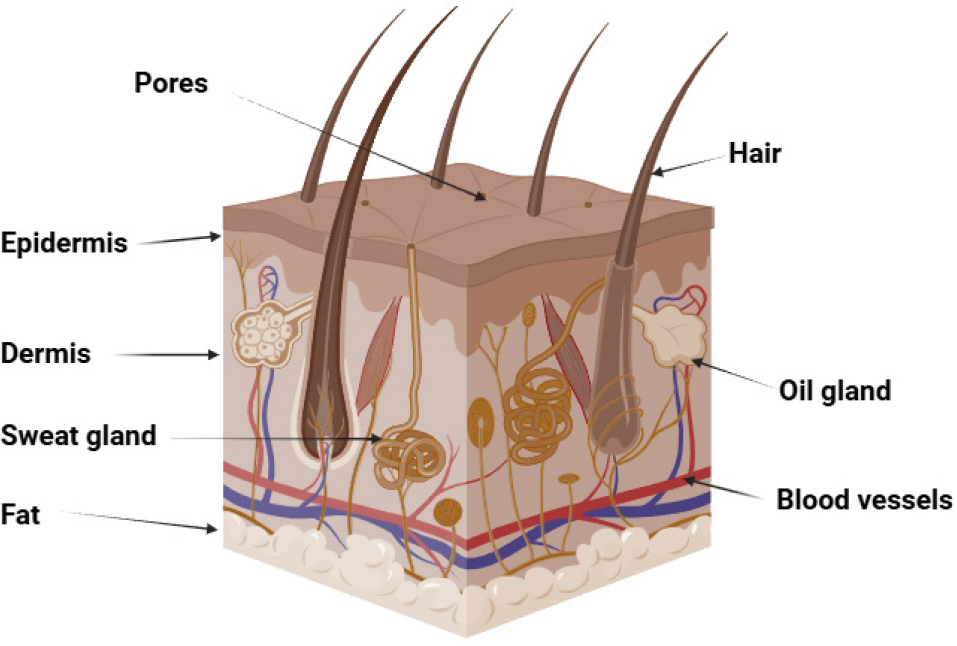
Figure 1:
Structure of human skin.
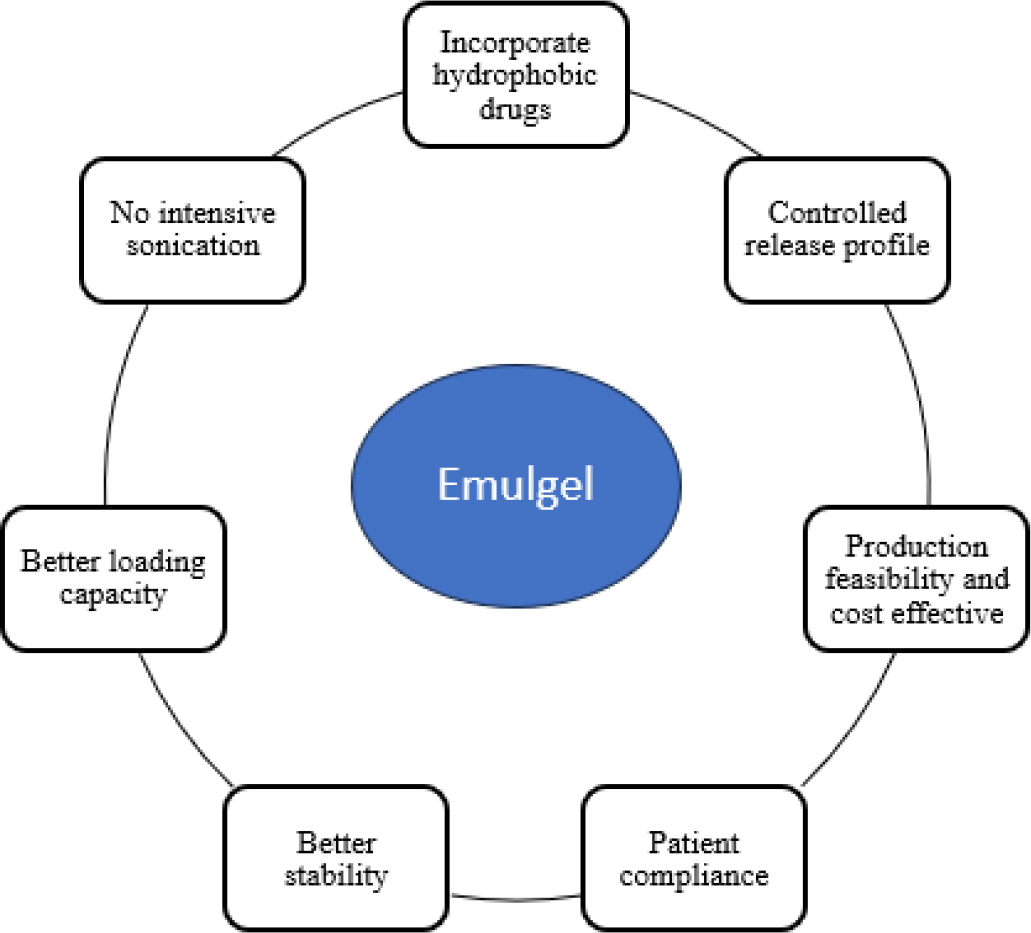
Figure 2:
Advantages of emulgel over other transdermal preparations.
Mechanism of drug absorption of emulgel
Emulgel improves topical medication absorption through follicular, intercellular, and transcellular pathways (Figure 3). Its dual-control release method uses both emulsion and gel qualities. Blood vessels are dispersed throughout the subcutaneous layer, under the dermis and epidermis. The most popular route is the pilosebaceous route, where penetration occurs through the intercellular matrix. Organic solvents like propylene glycol, DMSO, and surfactants can improve drug penetration. Permeation enhancers improve solubility, partitioning, and fluidization of the crystalline structure. Factors influencing drug absorption include lipid content, skin thickness, sweat gland density, hair follicle density, skin pH, skin temperature, hydration, inflammation, and blood flow. Low lipid content increases percutaneous penetration, while thicker skin layers may result in faster absorption. Increased blood flow promotes faster drug diffusion and systemic uptake (Patelet al., 2021; Khamkatet al., 2024).
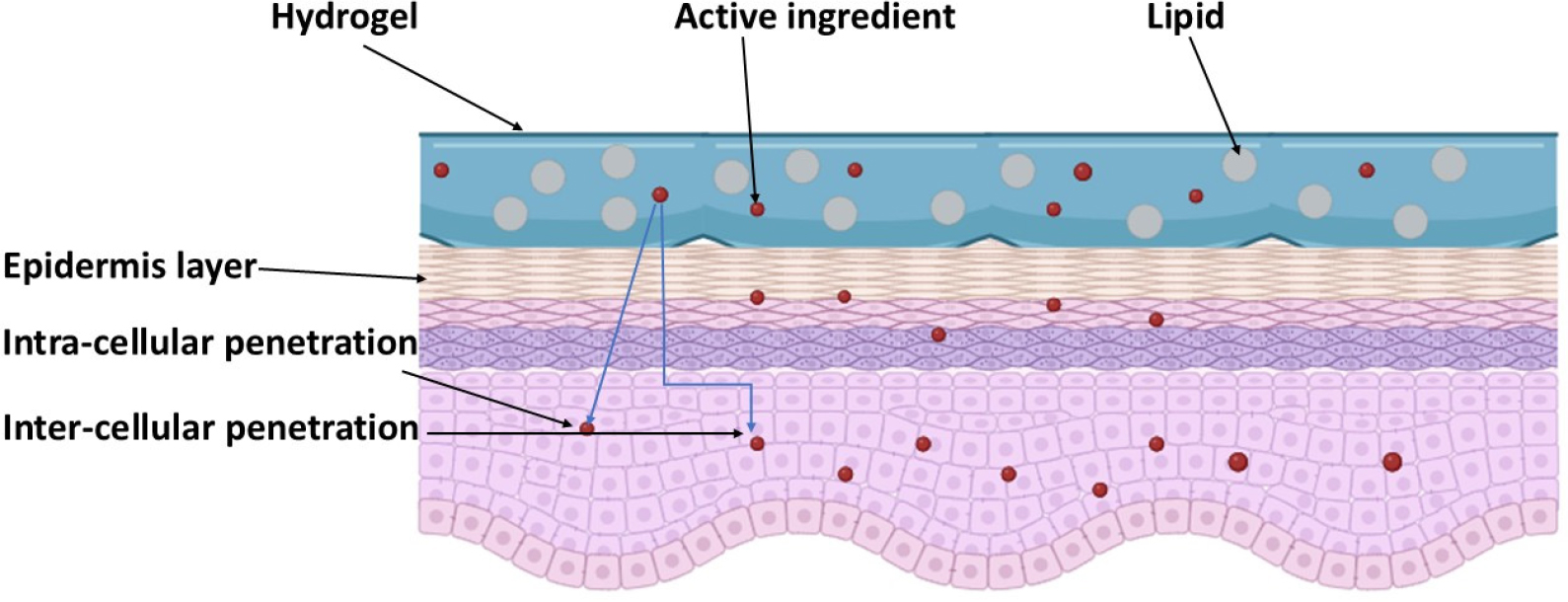
Figure 3:
Mechanism of drug absorption of emulgel.
MATERIALS FOR EMULGEL
Various components used to prepare emulgel are described below. Except for drugs, excipients are used to modify the formulations. The ideal properties of excipients must include the significance of stable chemical and physical substances that are nontoxic, readily available, reasonably priced, and not contraindicated.
Vehicles
A vehicle delivers medication uniformly over the skin, ensuring it reaches the intended location, maintaining therapeutic concentration. Its properties determine absorption, with active components like water, mineral oils, castor oils, fish liver oils, and plant fatty oils.
Emulsifiers
These are utilized to enhance the emulsification process during formulation and maintain product stability throughout its shelf life. Emulsifiers like sodium stearate, span 80, stearic acid, and tween 80 are utilized for preparing emulsions.
Gelling agents
To improve the consistency of gel preparation, gelling chemicals are used. These are made from various types of polymers, including natural ones like guar gum, synthetic polymers like carbomer, poloxamers, alkyl acrylates, and polyvinyl alcohol, and semi-synthetic polymers like cellulose derivatives like Hydroxypropyl Methylcellulose (HPMC), MC, and hydroxyethyl cellulose (Kumbharet al., 2025).
Penetration enhancers
These are compounds that facilitate the skin’s ability to absorb medications. Penetration enhancers, such as surfactants, unsaturated fatty acids, organic solvents, essential oils, propylene glycol derivatives, and urea, are used in various formulations. These agents must be inert, non-allergenic, and compatible with agents.
pH adjusting agents
PREPARATION PROCEDURE OF EMULGELS
The simple method used to prepare emulgel has three main steps. The emulgel is created by combining an emulsion into the gel base after the first two phases of preparing the emulsion and gel base independently (Figure 4). Hydrophilic emulsifying agent is first dissolved in distilled water to create the aqueous phase. Similarly, lipophilic emulsifying agent is dissolved in liquid paraffin to create the oil phase. After being heated independently to 70° to 80°, the oil and aqueous phases are combined while being constantly stirred to create an emulsion. Gelling agent is dissolved at room temperature in distilled water to create the gel phase. Ultimately, an emulgel is created by gently swirling the gel and emulsion phases together in a 1:1 ratio (Figure 5).
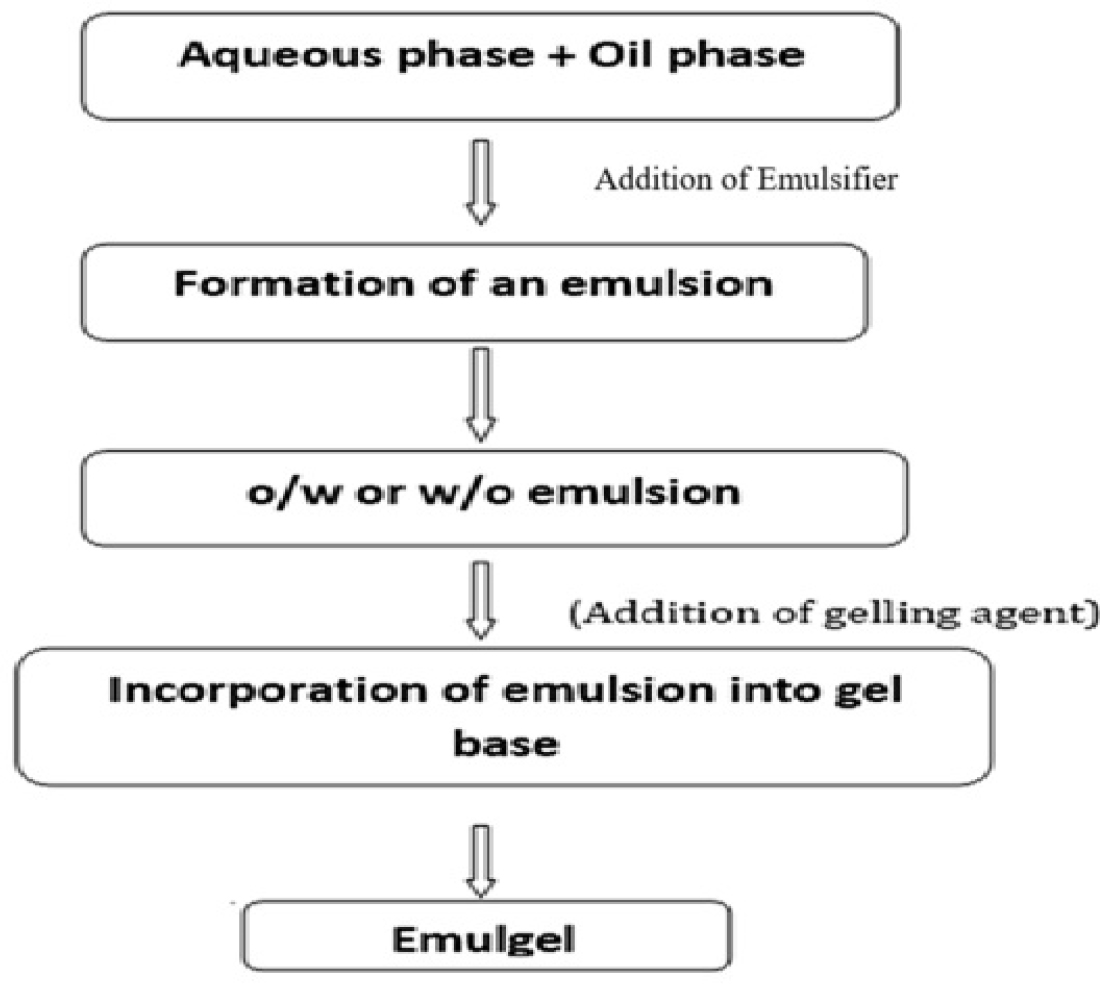
Figure 4:
Fundamental steps for preparing emulgel.
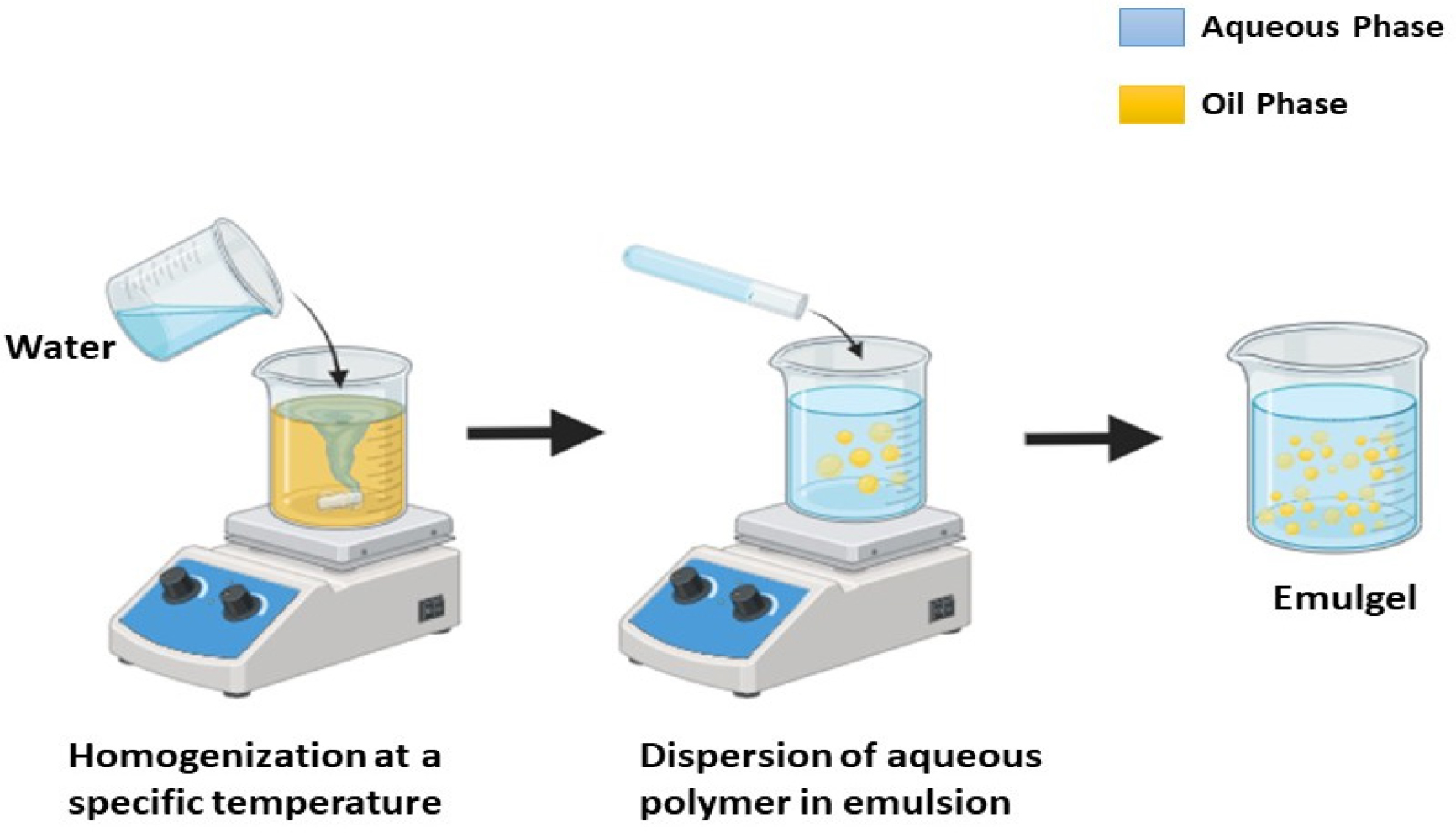
Figure 5:
Schematic representation of emulgel preparation.
APPLICATIONS OF EMULGEL
Emulgels are exploited in the treatment of skin diseases. Table 1 represents marketed emulgel products with different skin applications.
| Sl. No. | Brand Name | Manufacturer | Active Ingredients | Uses | References |
|---|---|---|---|---|---|
| 1. | Diclobar emulgel | Barakat Pharma | Diclofenac diethyl amine | Anti-inflammatory, analgesic | (Patelet al., 2021) |
| 2. | Diclomax Emulgel | Torrent Pharma | Diclofenac sodium | Anti-inflamatory | (Patelet al., 2021) |
| 3. | Levorag emulgel | THD Ltd., | Hibiscus, licorice | Emollient | (Patelet al., 2021) |
| 4. | Voltarol 1.16% emulgel | Haleon Consumer Healthcare | Diclofenac sodium | Anti-inflammatory | (Kumar D, 2016) |
| 5. | Cataflam emulgel | Novartis India Ltd., | Diclofenac diethyl ammonium | NSAIDs | (Malaviet al., 2022) |
| 6. | Accent gel | Zee Laboratories | Aceclofenac | Anti-inflammatory | (Tanaji, 2018) |
| 7. | Avindo gel | Cosme Pharma Lab | Azithromycin | NSAIDs | (Tanaji, 2018) |
| 8. | Cloben gel | Indoco Remedies | Clotrimazole, betamethasone | Antifungal | (Tanaji, 2018) |
| 9. | Nadicin cream | Psycho Remedies | Nadifloxacin | Fluroqinolone antibiotics | (Tanaji, 2018) |
| 10. | Clinagel | Glaxosmithkline Pharmaceuticals Ltd., | Clindamycin phosphate, allantoin | Antibiotics | (Tanaji, 2018) |
| 11 | Voltaren emulgel | GlaxoSmithKline Consumer Healthcare | Diclofenac-diethyl-ammonium | Anti-inflammatory | (Tanaji, 2018) |
| 12 | Miconaz-H-Emulge, Miconazole nitrate, hydrocortisone, Medical union pharmaceuticals | Medical Union Pharmaceuticals | Miconazole nitrate, hydrocortisone | Anti-fungal | (Malaviet al., 2022) |
| 13 | Excex gel | Zee Laboratories | Clindamycin, adapalene | Antibiotic | (Malaviet al., 2022) |
| 14 | Pernox gel | Eris Oaknet Healthcare Pvt. Ltd., | Benzoyl peroxide | Antimicrobial | (Malaviet al., 2022) |
| 15 | Lupigyl gel | Lupin Pharma | Metronidazole, clindamycin | Antibiotic | (Tanaji, 2018) |
| 16 | Topinate gel | Systopic Lab | Clobetasol propionate | Inflammatory and allergic | (Tanaji, 2018) |
| 17 | Kojivit gel | Micro Labs Ltd., | Kojic acid, dipalmitate arbuti | Skin pigmentation concerns | (Tanaji, 2018) |
| 18 | Zorotene gel | Glenmark Pharmaceuticals Ltd., | Tazarotene | Treat acne, Sun damage. | (Tanaji, 2018) |
Treatment of Inflammation
The potential of emulgels to treat inflammation has been well investigated. According to Using oleic acid, Mahajan et al., made dexbiprofen emulgel and discovered that its anti-inflammatory properties were comparable with those of commercially available diclofenac gel (Mahajan and Basarkar, 2019). Burki et al., discovered that rabbit skin containing different concentrations of capsaicin was penetrated by dexibuprofen-capsaicin emulgel; the maximum amount penetrated after 6.5 hr. He discovered that after 6.5 hr, the total quantity of capsaicin that penetrated oil across rabbit skin was 9.83 μg/cm2 when 100 mL of menthol was present, 7.23 μg/cm2 when 75 mL of menthol was present, and 2.23 μg/cm2 when menthol was absent (Burkiet al., 2020). Using carpool 940 and arachis oil as gelling agents, Sri and Arjun made naproxen emulgel. The formulation containing arachis oil (10% w/w), and carbopol 940 (0.5% w/w) was thought to be the best one because it had a greater enhancement ratio than the commercial gel (Sri and Arjun, 2019). For individuals 18 years of age and up, the directly acting drug tapentadol is authorized to treat moderate to severe pain. To create a pseudo ternary phase diagram, Ambhore et al., chose tween 20, light liquid paraffin, and PEG 400 as the surfactant, oil and cosurfactant, respectively (Ambhoreet al., 2017). Ambala et al., used span 80 and tween 20, liquid paraffin, hydroxypropyl methylcellulose, and carbopol 934 to generate ketoprofen emulgels. Compared to HPMC, carbopol performed better as a gelling agent. In 8 hr, the F3 formulation demonstrated a 98.46±2.05% of drug release with good physical appearance and clarity (Ambalaet al., 2015). Mefenamic acid emulgel was created by Khullar et al., using mentha oil and clove oil as penetration enhancers and carbapol 940. The best release of 56% in 240 min and the highest release of 56.23% in 240 min were both demonstrated by the optimised formulation. The hot plate and carrageenan-induced paw oedema tests indicated anti-inflammatory and analgesic properties (Khullaret al., 2012).
Treatment of Acne
Treatment of bacterial infections
Srivastava et al., created a fusidic acid emulgel by combining propylene glycol, span 20, light liquid paraffin, carbopol 934, and peppermint oil. The formulation is appropriate for topical medication delivery because it demonstrated a maximum release of 95.25% in 8 hr (Srivastavaet al., 2020). Metronidazole was made as a topical emulgel by Daood et al., using liquid paraffin, span 20, tween 20, and carbopol 940, and the formulation showed 9% release within 5 hr (Daoodet al., 2019). When Manaswitha et al., analysed ofloxacin emulgels made with oleic acid, they discovered that the oleic acid-formulated emulgels had higher flux than other formulations (Manaswithaet al., 2019). Oseni et al., assessed the antibacterial efficacy of Markhamia tomentosa leaf extract against skin isolates, this study developed an emulgel formulation. The extracts were evaluated against Bacillus subtilis, Staphylococcus aureus, and Staphylococcus epidermidis after being extracted in five different solvents. It was discovered that the extract from distilled water was the most effective against these microbes. The emulgel was smooth, brown, and applied to the skin with ease. The extract was better suited for skin application since it was more viscous (Oseniet al., 2024).
Treatment of wound healing
Khan et al., assessed the effects of topical application of an emulgel based on Ocimum basilicum (OB) on wound healing in an animal model. The prepared OB emulgel released 81.71±1.7% of the medication in 250 min, exhibiting strong physical characteristics and a satisfactory release profile. In in vivo wound healing experiments, OB emulgel demonstrated the highest percentage of wound contraction, exactly like the commercial product. Histopathological analysis showed a discernible improvement in the skin’s histological pattern after 16 days of OB emulgel treatment. The data suggests that 5% OB emulgel could be a new approach to wound healing (Khan et al., 2020).
Treatment of Psoriasis
In a study, Ganarajan and his coworkers contrasted xanthan gum, a gelling agent, with carbopol 934, a synthetic compound. They discovered that xanthan gum outperforms carbopol 934 as a gelling agent. Carbopol, polyethylene glycol, Kollicream3C, and KolliphorCS20 were used as emulsifiers to create the calcipotriol emulgel. PEG, isopropyl alcohol, and penetration enhancers were found to promote medication penetration into the epidermis. Excipient ratios and 32 response surface design were used to optimise the tretinoin emulgel. The optimised emulgel’s anti-acne properties in vitro were comparable to those of the commercially available Sotret® gel (Ganarajanet al., 2018).
Treatment of fungal infections
Luliconazole emulgel with carbopol 934 was selected by Shankar et al., because it had the maximum drug release and antifungal activity (Shankaret al., 2018). Tioconazole-loaded emulgels were made by Sah et al., using span 80, tween 80, light liquid paraffin, and carbopol 934. The findings demonstrated viscosity, extensibility, and spreadability, suggesting that tioconazole emulgels provided a superior platform for topical delivery of hydrophobic medications and improved patient compliance (Sahet al., 2017). As per Yadav et al., Transcutol and eucalyptus oil were combined in various quantities to generate tolnaftate, an antifungal agent with low permeability and poor solubility. Compared to a pure medication emulgel without eucalyptus oil, the formulation’s zone of inhibition showed a considerable improvement in antifungal activity. This was attributed to the emulgel’s greater penetration in fungal cells when eucalyptus oil was present, which produced effective antifungal activity (Yadavet al., 2017). Sawant and Mohite focused on how emulgel might enhance the topical administration of itraconazole. Using Carbopol 934 and Carbopol 940, two gelling agents, emulgel formulations were made. Physical characteristics, globule size, viscosity, drug release, transmission electron microscopy, antifungal activity, skin irritation, and stability were all assessed for the emulgels. When compared to the commercially available cream, the optimised formulation demonstrated greater antifungal effectiveness and drug release. After three months of storage, the emulgel formulation’s stability did not change, and it did not exhibit any oedema or discomfort (Sawant and Mohite, 2015).
CLINICAL STUDIES ON EMULGEL
Gómez-Farto et al., performed a clinical trial between January, 2023 and April, 2024 with 67 patients for the concurrent treatment of Atopic Dermatitis (AD) patients with a formulation that contains Hyaluronic Acid (HA), Calendula officinalis, glycerol, polyphenols and aloe vera. Following their recruitment, they were split into two groups at random, as thirty were placed in the left arm group and thirty in the right arm group. Only eight participants freely decided not to continue the trial, with one patient withdrawing due to annoyance. Therefore, 59 patients in the population were evaluated. 55.9% of the patients with baseline information were female. 64.4% were phototype II patients. One to three times a week, 78% of the patients used creams. For testing on the left arm, 45.8% of patients utilized emulgel. Just 10% of the patients needed no additional care; the others continued receiving their usual course of care. Comorbid conditions such as prurigo nodularis, conjunctivitis, rhinitis, asthma, contact dermatitis, allergies were absent in 21% of the patients. 69% of the patients were exposed to the sun on occasion. Transepidermal Water Loss (TEWL), AD inflammation, epidermal thickness, and allergen-specific Immunoglobulin E (IgE) are all reduced by Epidermal Growth Factor (EGF). Inhibiting the change of intercellular lamellar lipids from a liquid to a solid phase, glycerol speeds up the skin barrier’s repair after disruption, the study revealed. HA is very important for the stratum corneum’s structure and the epidermal barrier. Aloevera decreases the TEWL and increases the SCH, which enhances the skin’s physiological function. After 10 days of emulgel use, the study likewise discovered alterations in the healthy area but no discernible change in the eczematous area’s temperature. The emulgel not only reduces swelling and redness but also stops them from getting worse. The outcomes fall within the typical range for the function of the skin barrier (Gómez-Fartoet al., 2024). Using four vegetable oils, Thakur et al., created a benzoyl peroxide emulgel to treat dryness and irritation of the skin. Span 60 and tween 20 were used as emulsifiers together with sesame oil in the optimised composition. The effectiveness of the 2.5% Thymus vulgaris L. emulgel (TF) was assessed through a single-blind, randomised, split-face, placebo-controlled research. For ninety days, emulgel and a placebo were administered to twenty-five patients with mild to moderate cases of face acne vulgaris. The TF formulation was found to significantly lessen the severity of acne. For mild to moderate acne, the 2.5% Thymus vulgaris L. formulation proved to be both effective and well-tolerated. Using non-invasive skin bioengineering methods, another emulgel with 5% avocado oil and 20% green tea extract was made and tested on twelve female participants. The emulgel was discovered to be stable, effective, and aesthetically pleasing, with good moisturising qualities for acne (Thakuret al., 2012).
CHALLENGES OF EMULGEL
Emulgels are a complicated product that can develop stability problems over time, such as layer separation or the formation of cream-like structures. Because it depends on how they blend with water and oil components and fit with their intended usage, the choice of emulsifier is essential for producing stable emulsions within emulgels. For formulations to be effective, active ingredient compatibility is crucial, particularly when dealing with drugs that are fragile or readily degradable. Selecting a preservative is also a difficult issue because it must stop the growth of microorganisms while making sure the product doesn’t interfere with other ingredients. Additionally, important are rheological characteristics like texture, spreadability, and ease of application. Scalability and regulatory compliance are two more major issues facing the pharmaceutical industry.
FUTURE PROSPECTIVE
Hydrophobic drugs have been difficult to deliver to the biological system. Emulgel can be utilized as a way to transport the hydrophobic drugs into the body (Sreevidya, 2019). To target certain body zones, research is concentrating on creating emulgels that can effectively transport a variety of drugs, including drugs with low and high solubility. The controlled release and targeted administration of medicinal substances may be improved by combining nanotechnology and emulgels. Emulgels made for certain skin types and conditions could offer personalised skincare solutions. The creation of emulgels may be influenced by the movement towards natural and eco-friendly ingredients. To address certain skin issues, advanced active ingredients, including peptides, growth factors, and stem cell extracts, could be included. Traditional oral or injectable medicine delivery methods may be replaced with transdermal distribution.
REGULATORY CHALLENGES
The formulation, stability, biocompatibility, reproducibility, bubble formation, and management of any skin irritation are the regulatory issues faced by emulgels. It can be difficult for the skin to absorb drugs with large particle sizes since some drugs have limited skin permeability. While repeatability guarantees consistent properties, stability is essential for the long-term stability of the emulsion and gel matrix. Choosing the right excipients is essential to guaranteeing both safety and effectiveness. Because emulgels can irritate the skin or trigger allergic reactions, safety and effectiveness are also very important. Preservatives, drug loading capacity, and biocompatibility are other crucial factors. To overcome these obstacles, quality control methods and reverse engineering techniques may be required (Shindeet al., 2022, Bansal, 2023).
CONCLUSION
Emulgels-a combination of emulsion and gel-have enormous potential for use in pharmaceutical formulations because they provide flexibility and adaptability in a range of therapeutic domains. These are helpful in practical healthcare settings due to their ease of use and improved patient adherence. According to various studies, emulgels are a more effective and efficient way to administer drugs than other topical techniques. Emulgels are a common option for delivering hydrophilic and hydrophobic medications because of their exceptional extrusion, spreadability, viscosity, adhesion qualities. They are ideal for loading of hydrophobic medicines onto water-soluble gel bases, making them a viable choice for drug delivery in the future. To administer drugs precisely, future research will focus on improving formulations, finding new emulsifying agents, and using nanotechnology. They might improve treatment results, increase patient adherence, and transform medication delivery systems.
Cite this article:
Khamkat P, Singh S, Chatterjee B, Banerjee S, Maiti S, Mandal S, et al. Advancement and Future Window of Emulgel for Intensifying Transdermal Delivery in the Treatment of Skin Diseases. J Young Pharm. 2025;17(4):816-23.
ACKNOWLEDGEMENT
The authors sincerely thank their respective institutions for their encouragement and support for the quality of the work.
References
- Ali Khan B. A., Ullah S., Khan M. K., Alshahrani S. M., Braga V. A.. (2020) Formulation and evaluation of -based emulgel for wound healing using animal model.. Saudi Pharmaceutical Journal 28: 1842-1850 https://doi.org/10.1016/j.jsps.2020.11.011 | Google Scholar
- Ambala R., Vemula S. K.. (2015) Formulation and characterization of ketoprofen emulgels.. Journal of Applied Pharmaceutical Science 5: 112-117 https://doi.org/10.7324/JAPS.2015.50717 | Google Scholar
- Ambhore N. P., Dandagi P. M., Gadad A. P., Mandora P.. (2017) Formulation and characterization of tapentadol loaded emulgel for topical application.. Indian Journal of Pharmaceutical Education and Research 51: 525-535 https://doi.org/10.5530/ijper.51.4.81 | Google Scholar
- Bansal N.. (2023) Emulgel: An effective drug delivery system.. Research Journal of Pharmacy and Technology 16: 2754-2758 https://doi.org/10.5530/ijper.51.4.81 | Google Scholar
- Burki I. K., Khan M. K., Khan B. A., Uzair B., Braga V. A., Jamil Q. A., et al. (2020) Formulation development, characterization, and evaluation of a novel dexibuprofen-capsaicin skin emulgel with improved anti-inflammatory and analgesic effects.. AAPS PharmSciTech 21: 211 https://doi.org/10.1208/s12249-020-01760-7 | Google Scholar
- Charyulu N. R., Joshi P., Dubey A., Shetty A.. (2021) Emulgel: A boon for enhanced topical drug delivery.. Journal of Young Pharmacists 13: 76-79 https://doi.org/10.5530/jyp.2021.13.17 | Google Scholar
- Daood N. M., E Jassim Z., M Gareeb M., Zeki H.. (2019) Studying the effect of different gelling agent on the preparation and characterization of metronidazole as topical emulgel.. Asian Journal of Pharmaceutical and Clinical Research 12: 571-577 https://doi.org/10.22159/ajpcr.2019.v12i3.31504 | Google Scholar
- Ganarajan G., Sharma D. C., Tangri P., Kothiyal P.. (2018) Design and characterization of apremilast loaded emulgel for topical treatment.. IJPBS 8: 552-562 https://doi.org/10.22159/ajpcr.2019.v12i3.31504 | Google Scholar
- Ganju E., Deshmukh S., Gupta B. K.. (2024) Emulgel towards novel formulation development: A comprehensive review.. International Journal of Medical and Pharmaceutical Sciences 14: 1-6 https://doi.org/10.31782/IJMPS.2024.14101 | Google Scholar
- Gómez-Farto A., Jiménez-Escobar A. L., Pérez-González N., Castán H., Clares B., Arias-Santiago S., Montero-Vílchez T., et al. (2024) Development of an emulgel for the effective treatment of atopic dermatitis: Biocompatibility and clinical investigation.. Gels 10: 370 https://doi.org/10.3390/gels10060370 | Google Scholar
- Khamkat P., Barik V., Mohapatra S., Karati D., Mukherjee S.. (2024) Current approaches on Transfersomal patch: A noninvasive innovative booster for improved transdermal drug delivery.. Current Pharmaceutical Biotechnology. https://doi.org/10.2174/0113892010315069240805074205 | Google Scholar
- Khamkat P., Ghosh A., Mukherjee S.. (2022) Transfersomes: An innovative vesicular carrier for boosted transdermal delivery system.. Research Journal of Pharmacy and Technology 15: 2793-2800 https://doi.org/10.52711/0974-360X.2022.00467 | Google Scholar
- Khullar R., Kumar D., Seth N., Saini S.. (2012) Formulation and evaluation of mefenamic acid emulgel for topical delivery.. Saudi Pharmaceutical Journal 20: 63-67 https://doi.org/10.1016/j.jsps.2011.08.001 | Google Scholar
- Kumar D., Singh J., Antil M., Kumar V.. (2016) Emulgel-novel topical drug delivery system-a comprehensive review.. International Journal of Pharmaceutical Sciences and Research 7: 4733 https://doi.org/10.1016/j.jsps.2011.08.001 | Google Scholar
- Kumbhar P. R., Desai H., Desai V. M., Priya S., Rana V., Singhvi G., et al. (2025) Versatility of emulgel in topical drug delivery transforming its expedition from bench to bedside.. Expert Opinion on Drug Delivery 22: 55-68 https://doi.org/10.1080/17425247.2024.2439457 | Google Scholar
- Lopez-Ojeda W., Pandey A., Alhajj M., Oakley A. M.. (2021) Anatomy, skin (integument). https://doi.org/10.1080/17425247.2024.2439457 | Google Scholar
- Mahajan V. R., Basarkar G. D.. (2019) Formulation design, development and characterization of dexibuprofen emulgel for topical delivery: and evaluation.. Journal of Drug Delivery and Therapeutics 9: 330-342 https://doi.org/10.1080/17425247.2024.2439457 | Google Scholar
- Malavi S., Kumbhar P., Manjappa A., Chopade S., Patil O., Kataria U., Dwivedi J., Disouza J., et al. (2022) Topical emulgel: Basic considerations in development and advanced research.. Indian Journal of Pharmaceutical Sciences 84 https://doi.org/10.36468/pharmaceutical-sciences.1005 | Google Scholar
- Manaswitha A., Sai Swetha P. V. L. D., Devi N. K. D., Naveen Babu K., Ravi Shankar K.. (2019) Oleic acid based emulgel for topical delivery of ofloxacin.. Journal of Drug Delivery and Therapeutics 9: 183-190 https://doi.org/10.22270/jddt.v9i4-A.3451 | Google Scholar
- Olayemi O. J., David C.. (2023) Emulgel: A promising technology for topical delivery of herbal extracts.. British Journal of Pharmacy 8: 1-13 https://doi.org/10.5920/bjpharm.1046 | Google Scholar
- Oseni B. A., Osekita S. T., Ibrahim M. B., Igbokwe N. H., Azubuike C. P.. (2024) Development of emulgel formulation from leaf extract: Characterization and antimicrobial activity against skin isolates.. American Journal of Pharmacotherapy and Pharmaceutical Sciences 3. https://doi.org/10.25259/AJPPS_2024_009 | Google Scholar
- Patel B. M., Kuchekar A. B., Pawar S. R.. (2021) Emulgel approach to formulation development: A review.. Biosciences Biotechnology Research Asia 18: 459-465 https://doi.org/10.13005/bbra/2931 | Google Scholar
- Ranjan P., Jain V., Shende S., Jain P. K.. (2019) Formulation development and evaluation of emulgel of clindamycin phosphate for effective treatment of acne.. Journal of Drug Delivery and Therapeutics 9: 202-207 https://doi.org/10.22270/jddt.v9i4.3026 | Google Scholar
- Sah S. K., Badola A., Mukhopadhyay S.. (2017) Development and evaluation of tioconazole loaded emulgel.. International Journal of Applied Pharmaceutics 9: 83-90 https://doi.org/10.22159/ijap.2017v9i5.20046 | Google Scholar
- Sawant A. A., Mohite S. K.. (2015) Formulation and evaluation of itraconazole emulgel for topical drug delivery.. Asian Journal of Pharmacy and Technology 5: 91-96 https://doi.org/10.5958/2231-5713.2015.00014.8 | Google Scholar
- Shankar D., Gajanan S., Suresh J., Dushyant G.. (2018) Formulation and evaluation of luliconazole emulgel for topical drug delivery.. Int. Res J. Sci. Eng. 3: 85-89 https://doi.org/10.5958/2231-5713.2015.00014.8 | Google Scholar
- Shinde A. A., Velhal A. B., Jadhav P. D., Redasani V. K.. (2022) A review on emulgel: Improvement of topical absorption of drug.. Asian Journal of Research in Pharmaceutical Sciences : 335-340 https://doi.org/10.52711/2231-5659.2022.00057 | Google Scholar
- Sreevidya V. S.. (2019) An overview on emulgel.. International Journal of Pharmaceutical and Phytopharmacological Research 9: 92-97 https://doi.org/10.52711/2231-5659.2022.00057 | Google Scholar
- Sri B. U., Arjun G.. (2019) Formulation and evaluation of naproxen emulgels topical drug delivery systems.. American Journal of PharmacyTech Res 9: 65-75 https://doi.org/10.52711/2231-5659.2022.00057 | Google Scholar
- Srivastava A., Desai S., Jain H., Meshram D. B.. (2020) Formulation and evaluation of fusidic acid emulgel.. Journal of Drug Delivery and Therapeutics 10: 169-175 https://doi.org/10.22270/jddt.v10i3-s.4119 | Google Scholar
- Talat M., Zaman M., Khan R., Jamshaid M., Akhtar M., Mirza A. Z., et al. (2021) Emulgel: An effective drug delivery system.. Drug Development and Industrial Pharmacy 47: 1193-1199 https://doi.org/10.1080/03639045.2021.1993889 | Google Scholar
- Tanaji D. N.. (2018) Emulgel: A comprehensive review for topical delivery of hydrophobic drugs.. Asian Journal of Pharmaceutics (AJP) 12 https://doi.org/10.1080/03639045.2021.1993889 | Google Scholar
- Thakur N. K., Bharti P., Mahant S., Rao R.. (2012) Formulation and characterization of benzoyl peroxide gellified emulsions.. Scientia Pharmaceutica 80: 1045-1060 https://doi.org/10.3797/scipharm.1206-09 | Google Scholar
- Yadav S., Wairkar S., Invally M., Ranade S.. (2017) Topical emulgel of tolnaftate with penetration enhancer: Development, characterisation and antifungal activity.. Indian J. Med Res PharmSci 4: 28-35 https://doi.org/10.3797/scipharm.1206-09 | Google Scholar
