ABSTRACT
Background
A heterocyclic hydrocarbon having a 5, 5-diphenylimidazolidine heterocyclic ring that possesses distinctive fundamental structural characteristics. It is a fused ring of aromatic di-benzene and imidazolidine. The flexible heterocyclic molecules in 5, 5-diphenylimidazolidine that have two nitrogen atoms. The biological activity of the 5, 5-diphenylimidazolidine ring and its derivatives is significant and encouraging. We produce a variety of 4-(chloroethoxy)-3- [2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid and its derivatives in this investigation. In literature survey and molecular docking; it was confirmed that 5, 5-diphenylimidazolidine-2,4-dione gives anticonvulsant effects. The pharmacological samples were examined for their ability to prevent convulsions using the strychnine-induced convulsion method.
Materials and Methods
Benzoin; Benzil; Urea; Glacial Acetic Acid; 4- Amino Benzoic Acid; Con. HNO3; Formic Acid; 2- Nitro Aniline; 4- Nitro Aniline; Aniline; Acetyl Chloride; Formic Acid; 4- amino Phenol are used for the synthesis.IR, NMR and MS are used for interpretation.
Results
Our research led us to the conclusion that a variety of compounds have strong anticonvulsant properties. The compound 4-(2-chloro-N-(2-phenoxyethyl) aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD5)- (scheme II A); 4-(3-chloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD6)- (scheme II A); 4-(2,5-dichloro-N-(2-phenoxyethyl)aniline)- 3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD7) (scheme II A) and 4-(N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD3) (scheme II A) gives strong anti-convulsant effects against phenytoin drug.
Conclusion
The title compounds and their derivatives were examined for their ability to treat convulsions. Studies of the relationship between structure and activity revealed that compounds containing 5, 5-diphenylimidazolidine derivatives that have an electron-withdrawing group have higher activity than those that have an electron-donating group.
INTRODUCTION
The five 5-diphenylimidazolidine nucleuses were discovered in 1954. It comprises a di-benzene and imidazolidine ring joined. Its structure is comparable to that of the drug phenytoin.1 The heterocyclic nucleus of 5, 5-diphenylimidazolidine are significant due to its many medicinal applications. In 1952, researcher Brecker produced the first 5, 5-diphenylimidazolidine.2 Figure 1 shows the presence of 5-diphenylimidazolidine. Today’s preferred moiety, 5, 5-diphenylimidazolidine, exhibits a variety of pharmacological properties.
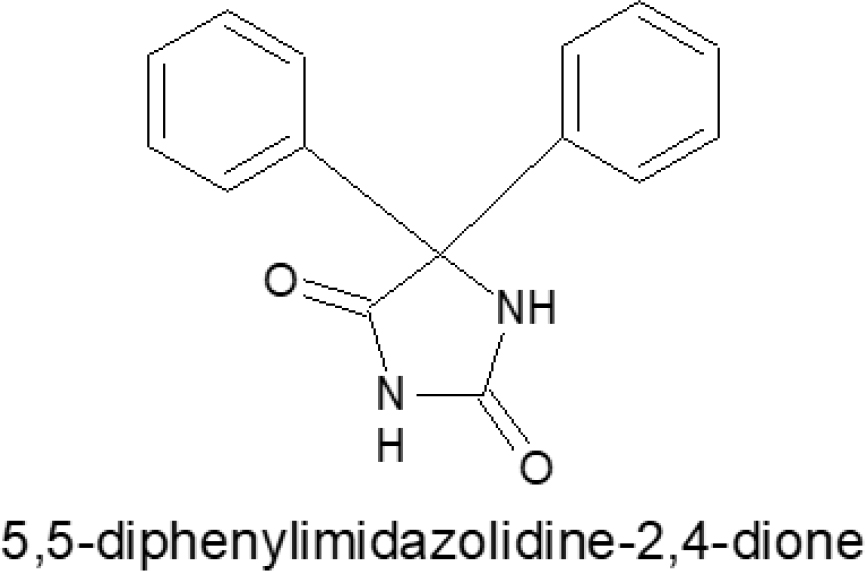
Figure 1:
5, 5-diphenylimidazolidine heterocyclic nucleus.
The molecular weight of 5, 5-diphenylimidazolidine-2,4-dione is 252.273 and the chemical formula is C15H14N2O2. Since 1960, a compound containing one imidazolidine and two benzene rings has been frequently used in pharmacology. Many drugs’ active components are constructed of 5, 5-diphenylimidazolidine-2, 4-dione rings, which have excellent basic characteristics due to the presence of two nitrogen atoms in their structure. Epidemiological studies show that 60 million people worldwide suffer from epilepsy, a common brain condition.3–5 This figure rises by roughly 20,000 new cases per year. Despite the availability of more than 40 different anti-epileptic drugs in the Indian market, only 30% of individuals with uncontrolled seizures have been cured.6–9 As a result, antiepileptic chemical research is now brisk. The primary aim is the investigation of new anticonvulsant medicines. In vivo identification of 5, 5-diphenylimidazolidine-2, and 4-dione derivatives was done based on the kind of seizure.10 An aryl binding site containing an aryl/alkyl group, a hydrogen bonding domain, and an electron donor group are all required requirements for possible anticonvulsant activity, and they all influence the anticonvulsant activity of different derivatives.11 Many heterocyclic building blocks have biological importance due to their structural similarity to nucleobases and other heterocyclic building blocks, such as the 5, 5-diphenylimidazolidine-2, 4-dione derivative, which suppresses angiogenesis in vitro as well as in vivo biological activity. As a result, the World Health Organization and new scientists around the world have made significant efforts to treat such convulsions, and various research groups have made efforts to identify novel anticonvulsant medications. In contrast, 5, 5-diphenylimidazolidine-2, 4-dione and its derivatives comprise one of the most important groups of organic heterocyclic compounds having anticonvulsant therapeutic activity.12 Green chemistry is the development of chemical products and processes that avoid the usage and production of harmful compounds. One of the goals of green chemistry is to synthesize physiologically active moieties with high percentage yield and purity using green solvents such as water. Few medicines for CNS action require a high purity and safety profile for pertaining biological activity. One of the most commonly utilised medications in the treatment of epilepsy is phenytoin. However, because to its limited solubility in water, both as free acid and as sodium salt, delivery to patients is difficult and rarely satisfactory. Phenytoin is administered orally as a sodium salt in a high alkaline solution, as it requires a PH of 10 to 12 to remain in solution. The first anticonvulsant medication, phenytoin (5, 5-diphenylimidazolidine-2,4-dione), is frequently mentioned as a classic example of an anticonvulsant functioning as a sodium channel blocker. In general, it is synthesised by condensation of Benzil and urea in the presence of base (30% w/v NaOH) utilising ethanol as a solvent, which works as a CNS stimulant.13 The removal of solvents following synthesis is the most complex and uncertain step. When a solvent other than water is utilised, transformation in polymorphism plays a key role. If a solvent other than water is employed, an additional 30% cost is estimated.
MATERIALS AND METHODS
Materials
4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid and its derivatives was prepared by using 5,5-diphenylimidazolidine-2,4-dione, 2-hydroxy-5-sulfobenzoic acid, 1,2-dichloroethane, 2-nitro aniline, 4-nitro aniline, aniline, acetyl chloride, formic acid etc. Some chemicals are available at colleges; some chemicals are obtained from Modern Chemicals in Nashik.
Methods
By using a traditional approach, all diphenyl imidazolidine derivatives were created. By using the open tube capillary method, melting points were measured and determined. The chemicals’ purity was examined using Thin Layer Chromatography (TLC) techniques. IR spectra were collected using KBr pellets and a Perkin Elmer Spectrum FTIR spectrometer. It was shown in Schemes I A and II B illustrate the synthesis pathway for 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid and its derivatives.
Experimental Work
(Scheme I A)
Synthesis of 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid.
(Scheme II B)
Synthesis of 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (SPD) (SPD1 – SPD7).
Synthetic procedures
Synthesis of 5,5-diphenylimidazolidine -2,4-dione (SPB)- (scheme I A)
Take 5.3 g of 2-hydroxy-1,2-diphenylethanone in 100 mL RBF The add 3.0 g of urea in that RBF Then add 15 mL 30% aq. NaOH (Sodium Hydroxide) Lastly add 75 mL of C2H5OH(ethanol) Attach the reflux condenser and boil under reflux using a heating mantle for at least 2 hr Cool to room temperature Pour the reaction mixture product into 125 mL of water and mix thoroughly Allow to stand for 10 min Then filter under the suction pump to remove an insoluble by-product. Render the product filtrate with strongly acidic acid with concentrated HCl. Cool in Ice water and immediately filter off PPT.
Synthesis of 3-[(2,4-dioxo-5,5-diphenylimidazolidin-1-yl) carbonyl]-4-hydroxy benzene sulfonic acid (SPC)-(scheme I A)
Take 1 g of 5,5-diphenylimidazolidine -2,4-dione into the round bottom flask, add 5 mL of 2-hydroxy-5-sulfobenzoic acid into the RBF of 100 mL. Heat the reaction mixture at about 80-100°C for 4 hr (Refluxing the reaction mixture) Cool the reaction mixture by adding 10 mL of crushed ice. Again add ice-cold water. Filter the reaction mixture and collect the product, it gives 3-[(2,4-dioxo- 5,5-diphenylimidazolidin-1-yl) carbonyl]-4-hydroxy benzene sulfonic acid. Record Melting point, Theoretical yield, Practical yield, and % of Practical yield.
Synthesis of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid (SPD)-(scheme I A)
Take 1 g of 3-[(2,4-dioxo-5,5-diphenylimidazolidin-1-yl) carbonyl]-4-hydroxy benzene sulfonic acid into the round bottom flask, add 5 mL 1,2-dichloroethane into the RBF of 100 mL. Reflux the reaction mixture at about 80-100°C for 3 hr. Cool the reaction mixture by adding 5 mL of crushed ice water. Filter the reaction mixture and collect the product, it gives 4-(chloroethoxy)-3- [2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid. Record Melting point, Theoretical yield, Practical yield and % of Practical yield.
4-(2-phenoxyethyl formate)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD1)-(scheme II A)
In a round bottom flask take 2 g of 4-(chloroethane)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid, 10 mL of formic acid; the reaction mixture heat under reflux condition for 3 hr after completion of reaction remove the RBF from hot water bath allow to cool the reaction mixture then add 50 mL of ice cold water into the reaction mixture then allow to stand it for 5 min. filter the product and recrystallized with ethanol to give 4-(2-phenoxyethyl formate)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid.

Scheme I A:
Synthesis of 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid.
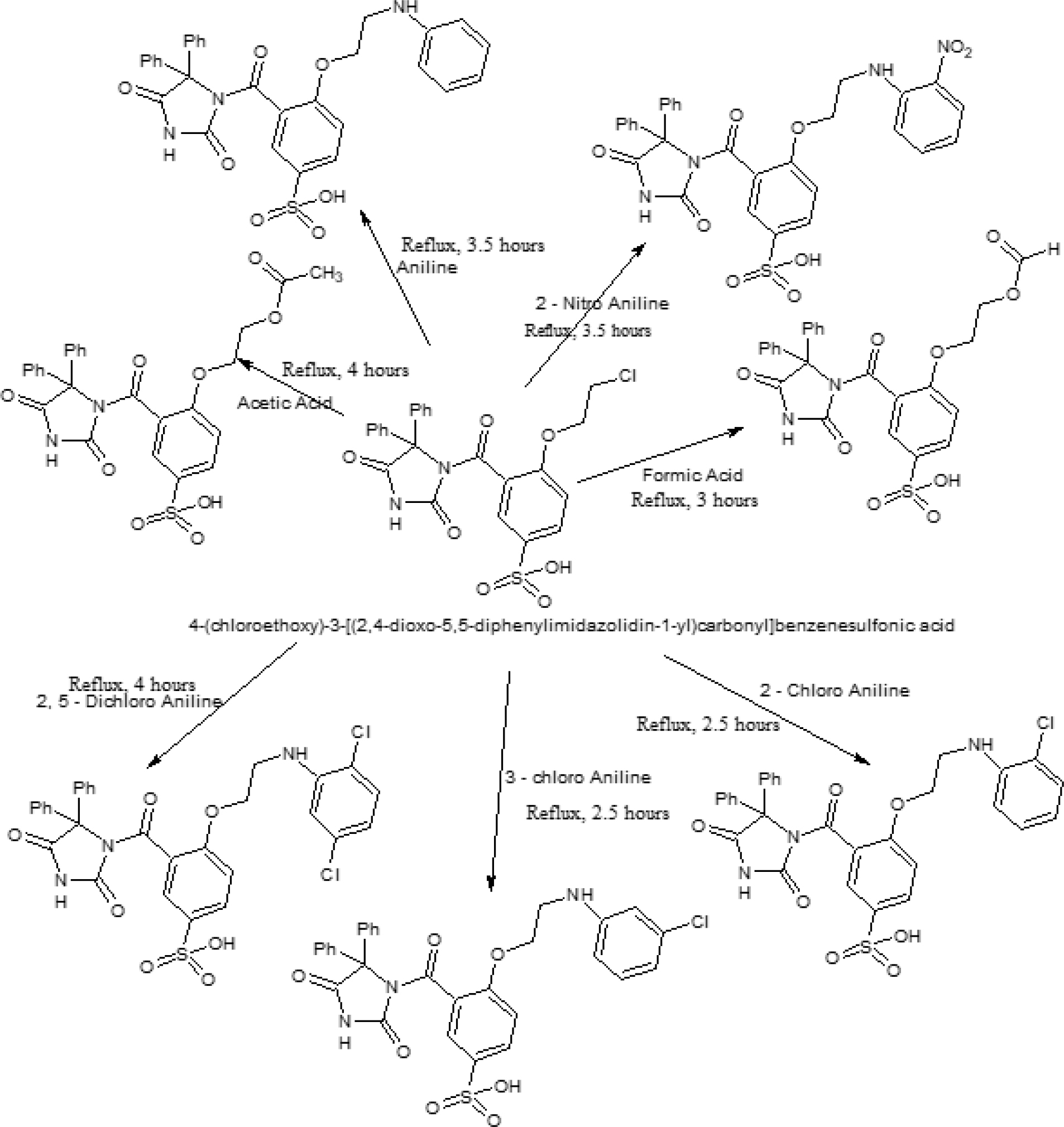
Scheme II B:
Synthesis of 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (SPD) (SPD1 – SPD7).
4-(2-phenoxyethyl acetate)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD2)-(scheme II A)
In a round bottom flask take 2 g of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid, 10 mL of acetic acid; the reaction mixture heat under reflux condition for 4 hr; after completion of reaction removes the RBF from hot water bath allow to cool the reaction mixture then add 50 mL of ice cold water into the reaction mixture then allow to stand it for 10 min. filter the product and recrystallized with ethanol to give 4-(2-phenoxyethyl acetate)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid.
4-(N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD3)-(scheme II A)
In a round bottom flask take 2 g of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid, 10 mL of Aniline; the reaction mixture heat under reflux condition for 3.5 hr after completion of reaction remove the RBF from hot water bath allow to cool the reaction mixture then add 50 mL
of ice cold water into the reaction mixture then allow to stand it for 10 min. filter the product and recrystallized with ethanol to give 4-(N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid.
4-(2-nitro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD4)- (scheme II A)
In a round bottom flask take 2 g of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid, 10 mL of 2-Nitro Aniline; the reaction mixture heat under reflux condition for 3.5 hr; after completion of reaction removes the RBF from hot water bath allow to cool the reaction mixture then add 50 mL of ice cold water into the reaction mixture then allow to stand it for 10 min. filter the product and recrystallized with ethanol to give 4-(2-nitro-N-(2-phenoxyethyl)aniline)-3- [2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid.
4-(2-chloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD5)- (scheme II A)
In a round bottom flask take 2 g of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid, 10 mL of 2-chloro Aniline; the reaction mixture heat under reflux condition for 2.5 hr after completion of reaction remove the RBF from hot water bath allow to cool the reaction mixture then add 50 mL of ice cold water into the reaction mixture then allow to stand it for 10 min. filter the product and recrystallized with ethanol to give 4-(2-chloro-N-(2-phenoxyethyl)aniline)-3- [2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid.
4-(3-chloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD6)- (scheme II A)
In a round bottom flask take 2 g of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid, 10 mL of 3-chloro Aniline; the reaction mixture heat under reflux condition for 2.5 hr; after completion of reaction remove the RBF from hot water bath allow to cool the reaction mixture then add 50 mL of ice cold water into the reaction mixture then allow to stand it for 10 min. filter the product and recrystallized with ethanol to give 4-(3-chloro-N-(2-phenoxyethyl)aniline)-3- [2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid.
4-(2,5-dichloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD7)- (scheme II A)
In a round bottom flask take 2 g of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid, 10 mL of 2,5-dichloro Aniline; the reaction mixture heat under reflux condition for 4 hr after completion of reaction remove the RBF from hot water bath allow to cool the reaction mixture then add 50 mL of ice cold water into the reaction mixture then allow to stand it for 10 min. filter the product and recrystallized with ethanol to give 4-(2,5-dichloro-N-(2-phenoxyethyl)aniline)-3- [2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid.
RESULTS
Characterization
Physical Data like % yield, Molecular weight and Melting Point etc. of various derivatives of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene Sulfonic acid Derivatives (SPD) (SPD1 – SPD7) are given in Table 1.
| Sl. No. | Compounds | Colors of compounds | Molecular formula | Melting point | %yields | Molecular weight |
|---|---|---|---|---|---|---|
| 1 | SPD | White | C24H19ClN2O7S | 278°C | 79% | 514.93 |
| 2 | SPD 1 | White | C25H20N2O9S | 312°C | 95% | 524.50 |
| 3 | SPD 2 | Yellow | C26H22N2O9S | 310°C | 95% | 538.53 |
| 4 | SPD 3 | Brown | C30H25N3O7S | 320°C | 90% | 571.60 |
| 5 | SPD 4 | Brown | C30H24N4O9S | 305°C | 82% | 616.60 |
| 6 | SPD 5 | White | C30H24ClN3O7S | 317°C | 75% | 606.04 |
| 7 | SPD 6 | White | C30H24ClN3O7S | 323°C | 68% | 606.04 |
| 8 | SPD 7 | Light green | C30H23Cl2N3O7S | 293°C | 80% | 640.49 |
Spectral Data
Synthesis of 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid (SPD)- (scheme I A)
FTIR (KBr) ν cm-1: 1671.98 C=C Stretch (Aromatic), 1085.74 C-C Stretch (Aromatic), 1294.0 C-N Stretch (Aromatic), 3247.54 N-H Stretch (Aromatic), 1718.26 C=O Stretch (Aryl ketone), 1341.54 S=O Stretch (sulfonic acid), 693.28 C-Cl Stretch (Aliphatic),1998.25 C-H bend (Aromatic); 1H NMR (400 MHz, DMSO): δ 11.5 Ar N-H (s, 1H), δ 8.7-7.1 Ar C-H (m, 15H), δ 6.3 CH2 Group (s, 2H), δ 6.5 CH2 Group (s, 2H), δ 5.1 O-H (s, 1H); Mol.Wt: 534
4-(2-phenoxyethyl formate)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD1)- (scheme II A)
FTIR (KBr) ν cm-1: 1679.69 C=C Stretch (Aromatic), 1232.29 C-C Stretch (Aromatic), 2804.96 C-N Stretch (Aromatic), 3267.79 N-H Stretch (Aromatic), 1718.26 C=O Stretch (Aryl ketone), 1349.28 S=O Stretch (sulfonic acid), 1957.39 C-H bend (Aromatic); 1H NMR (400 MHz, DMSO): δ 10.6 N-H (s, 1H), δ 8.6-7.1 Ar C-H (m, 19H), δ 6.3 CH2 Group (s, 2H), δ 6.7 CH2 (s, 2H), δ 5.1 O-H (s, 1H); Mol.Wt: 428
4-(2-phenoxyethyl acetate)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD2)- (scheme II A)
FTIR (KBr) ν cm-1: 1655.26 C=C Stretch (Aromatic), 1232.29 C-C Stretch (Aromatic), 2828.10 C-N Stretch (Aromatic), 3229.22 N-H Stretch (Aromatic), 1742.26 C=O Stretch (Aryl ketone), 1949.68 S=O Stretch (sulfonic acid), 1H NMR (400 MHz, DMSO): δ 10.1 Ar N-H (s, 1H), δ 7.8-6.6 Ar C-H (m, 16 H), δ 6.0 CH2 Group (d, 4H), δ 5.5 C-OH (s, 1H), 3.1 CH3 Group (s, 3H), Mol.Wt: 534
4-(N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD3)- (scheme II A)
FTIR (KBr) ν cm-11620.29 C=C Stretch (Aromatic), 1047.15 C-C Stretch (Aromatic), 2835.81 C-N Stretch (Aromatic), 3401.82 N-H Stretch (Aromatic), 1728.87 C=O Stretch (Aryl ketone), 2003.68 S=O Stretch (sulfonic acid); 1H NMR (400 MHz, DMSO): δ 12.3 N-H (s, 1H), δ 8.5-7.0 Ar C-H (m, 15H), δ 6.3 CH2 Group (s, 4H), δ 5.4 C-OH (s, 1H); Mol.Wt: 442
4-(2-nitro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD4)- (scheme II A)
FTIR (KBr) ν cm-1: 1671.98 C=C Stretch (Aromatic), 1132.01 C-C Stretch (Aromatic), 1266.86 C-N Stretch (Aromatic), 3321.78 N-H Stretch (Aromatic), 1720.10 C=O Stretch (Aryl ketone), 1995.96 S=O Stretch (Sulfonic Acid); 1H NMR (400 MHz, DMSO): δ 12.0 N-H (S, 1H), δ 8.7-7.1 Ar C-H (m, 15H), δ 6.1 CH2 Group (s, 4H), δ 5.4 C-OH (s, 1H); Mol.Wt: 514
4-(2-chloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD5)- (scheme II A)
FTIR (KBr) ν cm-1: 1610.27 C=C Stretch (Aromatic), 1085.73 C-C Stretch (Aromatic), 1278.57 C-N Stretch (Aromatic), 3306.36 N-H Stretch (Aromatic), 1717.55 C=O Stretch (Aryl ketone), 2026.86 S=O Stretch (Sulfonic Acid), 908.30 C-Cl (Aliphatic); 1H NMR (400 MHz, DMSO): δ 11.5 Ar N-H (s, 1H), δ 8.717-7.158 Ar C-H (m, 19H), δ 6.0 CH2 Group (s, 4H), δ 5.1 C-OH (s, 1H); Mol.Wt: 465
4-(3-chloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD6)- (scheme II A)
FTIR (KBr) ν cm-1: 1610.27 C=C Stretch (Aromatic), 1087.11 C-C Stretch (Aromatic), 1363.43 C-N Stretch (Aromatic), 3105.81 N-H Stretch (Aromatic), 1726.95 C=O Stretch (Aryl ketone), 2003.38 S=O Stretch (sulfonic acid), 916.22 C-Cl (Aliphatic); 1H NMR (400 MHz, DMSO): δ δ 10.6 N-H (s, 1H), δ 8.5-7.1 Ar C-H (m, 18H), δ 6.1 CH2 Group (d, 4H), δ 5.4 C-OH (s, 1H); Mol.Wt: 505.
4-(2,5-dichloro-N-(2-phenoxy ethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid: (SPD7)- (scheme II A)
FTIR (KBr) ν cm-1: 1602.56 C=C Stretch (Aromatic), 1032.01 C-C Stretch (Aromatic), 1363.43 C-N Stretch (Aromatic), 3105.80 N-H Stretch (Aromatic), 1726.26 C=O Stretch (Aryl ketone), 1995.96 S=O Stretch (sulfonic acid), 901.55 C-Cl (Aliphatic); 1H NMR (400 MHz, DMSO): δ δ 10.1 N-H (s, 1H), δ 8.7-7.1 Ar C-H (m, 15H), δ 6.3 CH2 Group (d, 4H), δ 5.1 C-OH (s, 1H); Mol.Wt: 534.
Biological evaluation
Anticonvulsant activity
There are numerous 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (SPD) (SPD1 – SPD7) are effective against tonic-clonic (grand mal) generalized seizures.13 Strychnine can be purchased on the market as a crystalline powder that is white, odorless, and bitter. Strychnine was administered intravenously (direct injection into a vein), orally (eaten by mouth, breathed in), or combined with a solution. A small amount of strychnine, a potent poison, is all that is required to cause convulsions.
Strychnine Induced Convulsion Method
Six groups of Wistar Rats will be divided. Each group has six animals (n = 6) and receives treatment for ten days. The first group will be given distilled water as a control treatment, and the second group will be given normal medication. It uses 100 mg/kg of phenytoin. The third group will receive a lower dose of novel substituted 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (a), the fourth group will receive a middle dose of novel substituted 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (b), and the fifth group will receive a higher dose of novel substituted 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (c). Each animal will be observed individually for convulsive behavior for the next 30 min. In this method we are used; we used 36 Wistar rats and they had body weights of around 150-250 g. In this Strychnine Induced Convulsion Method; Wistar rats were divided into 6 groups Group 1 is Vehicle control; Group 2 is Negative control (Strychnine 85 mg/kg); Group 3 is Standard (Phenytoin 100 mg/kg); Group 4 is Novel substituted 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid (SPD) (SPD1 – SPD7) derivatives (a) Lower dose; Group 5 is Novel substituted 4-(chloroethoxy)-3- [2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid (SPD) (SPD1 – SPD7) derivatives (b) Middle dose; Group 6 is Novel substituted 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid (SPD) (SPD1 – SPD7) derivatives (c) Higher dose. The grouping of Anticonvulsant Activity testing of the Wistar rats were shown in Table 2.
| No. of Groups | No. of Rats |
|---|---|
| Vehicle Control (Water). | 6 |
| Negative Control (Strychnine 85 mg/kg). | 6 |
| Standard (Phenytoin 100 mg/kg). | 6 |
| 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (a) Lower dose. | 6 |
| 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (b) Middle dose. | 6 |
| 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid Derivatives (c) Higher dose. | 6 |
| Total | 36 |
DISCUSSION
Anticonvulsant action was observed in hydantoin derivatives. An exhaustive literature search revealed that no such hydantoin derivatives in the suggested scheme had previously been described. The compounds were made by reacting substituted anilines with urea in the presence of hydrochloric acid and glacial acid in dry ethanol. Hot water was used to separate the phenyl urea and diphenyl urea mixture. Diphenyl urea remains undissolved while phenyl urea dissolves in hot water. The crystallized substituted Phenyl urea derivatives were collected, filtered, and dried in an oven. The Novel substituted syntheses 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene sulfonic acid (SPD) Derivatives from (SPD1 – SPD7) were produced in accordance with scheme II B. It was prepared by 1 g of 3-[(2,4-dioxo-5,5-Diphenylimidazolidin-1-yl) carbonyl]-4-hydroxy benzene sulfonic acid into the round bottom flask, add 5 mL 1,2-Dichloroethane into the RBF of 100 mL. Reflux the reaction mixture at about 80-100°C for 3 hr. Cool the reaction mixture by adding 5 mL of crushed ice water. Filter the reaction mixture and collect the product, it gives 4-(chloroethoxy)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] benzene Sulfonic Acid. 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] benzene Sulfonic Acid (SPD) was reacted with various reagents to produce various products. The newly synthesized 4-(chloroethoxy)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] Benzene Sulfonic Acid (SPD) derivatives were subjected to anticonvulsant screening by using standard model Strychnine Induced Convulsion. Compound (SPD1 – SPD7) containing halogen aromatic group derivative, showed more protection against induced seizures at lower dose levels (30 mg/kg) and higher dose level (200 mg/ kg). Compound (SPD1 – SPD7) containing aliphatic group derivative, showed less protection against induced seizures at higher dose levels (30 mg/kg). Phenytoin is a medication used in the management and treatment of epilepsy, generalized tonic-clonic seizures, complex partial seizures, and status epilepticus. It is in the anticonvulsants class of drugs. According to the results obtained in the Strychnine Induced Convulsion Method, at least 75% of protection (three mice protected out of four tested) was demonstrated for compounds like 4-(2-chloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD5)- (scheme II A); 4-(3-chloro-N-(2-phenoxyethyl)aniline)- 3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD6)- (scheme II A) containing chlorine halogen group in their structure (2-C1-Aniline)-SPD5 and (3-C1–Aniline)- SPD6 (Table 3). Notably, 4-(2,5-dichloro-N-(2-phenoxyethyl) aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD7) (scheme II A) and 4-(N-(2-phenoxyethyl) aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] Sulfonic acid (SPD3) (scheme II A) derivatives provided maximal (100%) protection. Other substances showed weak (25%) at a dose of 100 mg/kg. An equally important, well-established, and commonly used preclinical seizure model in the discovery which is effective in human focal epilepsy. Therefore, in the next step of the pharmacological characterization, all final compounds obtained were studied in this seizure model. As a result, compounds 4-(2,5-dichloro-N-(2-phenoxyethyl) aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] Sulfonic acid (SPD7) (scheme II A) and 4-(N-(2-phenoxyethyl) aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD3) (scheme II A) displayed a potent anticonvulsant efficacy providing more than 75% protection, whereas 4-(2-chloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] Sulfonic acid (SPD5)- (scheme II A); 4-(3-chloro-N-(2-phenoxyethyl)aniline)- 3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD6)- (scheme II A) protected at least 75% of protection (three mice protected out of four tested animals). A more detailed pharmacological characterization of compounds, which demonstrated the most beneficial anticonvulsant and safety profile, proved its activity in the Strychnine Induced Convulsion Method. It should be emphasized that the Strychnine Induced Convulsion Method is currently one of the most important screening models for the identification and characterization of novel compounds with a potential efficacy in pharmacoresistant epilepsy. In summary, the obtained in vivo data enabled to identify compound code like SPD7, SPD5 and SPD6 as a potent and broad-spectrum anticonvulsant for future preclinical development (especially after oral administration). It should be emphasized that the key chemical modification performed in the current studies allowed to obtain water-soluble salts which were close analogues of hybrid anticonvulsants reported previously. The results of Anticonvulsant Activity testing of the prepared compounds were shown in Table 3.
| Compounds | Doses (mg/kg) | Onset of Convulsions (sec) | Duration of Convulsions (sec) | Avg. % Protection | Recovery/Death |
|---|---|---|---|---|---|
| SPD | 50 | 93 | 148 | 67.52% | Recovery |
| 100 | 104 | 52 | |||
| 200 | 122 | 26 | |||
| SPD1 | 50 | 84 | 119 | 62.63% | Recovery |
| 100 | 98 | 90 | |||
| 200 | 114 | 46 | |||
| SPD2 | 50 | 86 | 183 | 64.16% | Recovery |
| 100 | 100 | 103 | |||
| 200 | 127 | 41 | |||
| SPD3 | 50 | 82 | 139 | 100% | Recovery |
| 100 | 91 | 89 | |||
| 200 | 107 | 53 | |||
| SPD4 | 50 | 81 | 119 | 75.63% | Recovery |
| 100 | 90 | 80 | |||
| 200 | 106 | 66 | |||
| SPD5 | 50 | 63 | 163 | 76.38% | Recovery |
| 100 | 79 | 101 | |||
| 200 | 96 | 63 | |||
| SPD6 | 50 | 58 | 142 | 79.10% | Recovery |
| 100 | 70 | 99 | |||
| 200 | 88 | 73 | |||
| SPD7 | 50 | 69 | 150 | 100% | Recovery |
| 100 | 78 | 91 | |||
| 200 | 96 | 52 | |||
| Std. Phenytoin | 100 | 119 | 11 | 100% | Recovery |
CONCLUSION
According to the proposed scheme, new hydantoin compounds were synthesized with high percentage yields. Different physical, analytical, and spectral data (1H NMR and FT-IR) validated and characterized the structures, yielding positive results. Because hydantoin compounds have strong anticonvulsant properties, it was determined to test them for anticonvulsant pharmacological action. On the Wistar Rat, all of the compounds demonstrated anticonvulsant action. In the Central Instrumentation Facility, FTIR, NMR spectroscopy, and MS were used to confirm the structures of synthesized chemicals Pune University; Savitribai Phule; and Pune. Wistar rats weighing 150-200 g were used to test the biological effects of anti-convulsant. In this study, derivatives demonstrated more potent anticonvulsant effects against various convulsion kinds. It was discovered that some of the synthesized chemicals have strong anti-convulsant properties. When compared to other 5,5-diphenylimidazolidine-2,4-dione derivatives, synthetic molecules were more active. Hence, the compound 4-(2-chloro-N-(2-phenoxyethyl)aniline)-3-[2,4-dioxo- 5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD5)- (scheme II A); 4-(3-chloro-N-(2-phenoxyethyl)aniline)- 3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD6)- (scheme II A); 4-(2,5-dichloro-N-(2-phenoxyethyl) aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD7) (scheme II A) and 4-(N-(2-phenoxyethyl) aniline)-3-[2,4-dioxo-5,5-diphenylimidazolidine-1-yl) carbonyl] sulfonic acid (SPD3) (scheme II A) gives strong anti-convulsant effects against phenytoin drug. Furthermore, compounds demonstrated anticonvulsant activity in a Strychnine-induced convulsion animal. Because it can be easily synthesised in high yields by condensation of various substituted aliphatic/aromatic amines in a heated media, 5,5-diphenylimidazolidine is widely employed in the synthesis of many chemical compounds. Many heterocyclic and non-heterocyclic compounds and complexes are rich in 5,5-diphenylimidazolidine because of their broad chemical and therapeutic applications. This work aims to describe the rationalized knowledge of 5,5-diphenylimidazolidine derivatives that have anticancer, anti-TB, antibacterial, anticonvulsant, antioxidants, anti-inflammatory, antidiabetic, and anti-HIV activities. Hybridization with various pharmacophores, prodrugs, and metal complexes can be suggested to create an appealing scaffold for innovative, safe, effective, and cost-effective treatment methods. In the field of medicinal chemistry, 5,5-diphenylimidazolidine, a versatile nucleus, must serve as a future therapeutic lead for developing various biological agents through structural modifications on 5,5-diphenylimidazolidine scaffolds, which can be used to develop potentially active agents in future investigations. Finally, we established a simple and effective technique for the production of 5,5-diphenylimidazolidine base derivatives using phenytoin under mild reaction conditions with competitive and high yield. The current technology has the benefits of operational simplicity, high efficiency, no side product creation, an easy workup procedure, and a shorter reaction time, making it appropriate for large-scale manufacture of 5,5-diphenylimidazolidine bases derivatives.
Cite this article
Bhor RJ, Patare SS, Shirole RB, Kadam KS, Kolhe MH, Ghogare R. Synthetic and in vivo Antiepileptic Activity of “Benzene Sulfonic Acid and its Derivatives” . J Young Pharm. 2024;16(1):24-32.
ACKNOWLEDGEMENT
The authors are thankful to Dr. S.B. Bhawar, Pravara Rural College of Pharmacy, Pravaranagar.
ABBREVIATIONS
| FTIR | Fourier transform infrared spectroscopy |
|---|---|
| NMR spectroscopy | Nuclear magnetic spectroscopy |
| MS | Mass spectroscopy |
| KBr | Potassium Bromide |
| % yield | Percentage yields |
| MP | Melting point |
| mg/kg | Milligram/kilograms |
| sec | Seconds |
| δ | Chemical shift |
| Mol.Wt | Molecular Weight |
| gm | Gram |
References
- French JA, Staley BA. AED Treatment through Different Ages: As Our Brains Change, Should Our Drug Choices Also. Epilepsy Currents. 2012;12(1):22-7. [Google Scholar]
- Anger T, Madge DJ, Mulla M, Riddall D. Medicinal Chemistry of Neuronal Voltage-Gated Sodium Channel Blockers. Journal of Medicinal Chemistry. 2001;44:115-37. [PubMed] | [CrossRef] | [Google Scholar]
- Ragavendran JV, Sriram D, Kotapati S, Stables J, Yogeeswari P. Newer GABA Derivatives for the Treatment of Epilepsy Including Febrile Seizures: A Bioisosteric Approach. European Journal of Medicinal Chemistry. 2008;43:2650-5. [PubMed] | [CrossRef] | [Google Scholar]
- Ohgoh M, Hanada T. Mode of Seizure Inhibition by Sodium Channel Blockers, an SV2A Ligand, and an AMPA Receptor Antagonist in a Rat Amygdala Kindling Model. Epilepsy Research. 2019;154:42-9. [PubMed] | [CrossRef] | [Google Scholar]
- Brown DJ. The Chemistry of Heterocyclic Compounds, The Pyrimidines. 2009 [PubMed] | [CrossRef] | [Google Scholar]
- Padwa A, Woolhouse AD, Katritzky AR, Rees CW. Oxford. Comprehensive heterocyclic chemistry. Comprehensive heterocyclic chemistry. 1984;3:57 [PubMed] | [CrossRef] | [Google Scholar]
- Bettendorff L, Wirtzfeld B, Makarchikov AF, Mazzucchelli G, Frédérich M, Gigliobianco T, et al. Discovery of a natural thiamine adenine nucleotide. Nat Chem Biol. 2007;3(4):211-2. [PubMed] | [CrossRef] | [Google Scholar]
- Zempleni J, Galloway JR, McCormick DB. Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans. Am J Clin Nutr. 1996;63(1):54-66. [PubMed] | [CrossRef] | [Google Scholar]
- Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci. 2009;106(36):15424-9. [PubMed] | [CrossRef] | [Google Scholar]
- Azam MA, Kumar BR, Shalini S, Suresh B, Reddy TK, Reddy CD, et al. Synthesis and biological screening of 5-{[(4, 6-disubstituted pyrimidine-2-yl) thio] methyl}-Nphenyl-1, 3, 4-thiadiazol-2-amines. Indian J Pharm Sci. 2008;70(5):672 [PubMed] | [CrossRef] | [Google Scholar]
- Amin KM, El-Zahar MI, Anwar MM, Kamel MM, Mohamed MH. Synthesis and anticancer activity of novel tetralin-6-yl pyridine and tetralin-6-yl pyrimidine derivatives. Acta Pol Pharm. 2009;66:279-91. [PubMed] | [Google Scholar]
- Yadav JS, Kumar SP, Kondaji G, Rao RS, Nagaiah K. A Novel L-Proline Catalyzed Biginelli Reaction: One-Pot Synthesis of 3, 4-Dihydropyrimidin-2 (1H)-ones under Solvent-Free Conditions. Chem Let. 2004;33(9):1168-9. [PubMed] | [Google Scholar]
- Tiwari S, Singh SM, Jain S. Chronic bilateral suppurative otitis media caused by Aspergillus terreus. Mycoses. 1995;38(7-8):297-300. [PubMed] | [Google Scholar]
