ABSTRACT
Background
Lipstick is a solid cosmetic designed to impart vibrant colour, hydration, and textural enhancement to the lips. With a growing consumer shift toward natural products. Interest in plant-based colorants and natural emulsifiers has surged. Herbal lipsticks offer enhanced biocompatibility, reduced dermal irritancy. It is available in finishes such as matte, glossy, satin, and metallic, glossy but has unique formulation challenges due to water incorporation. This study aimed to develop and evaluate herbal lipsticks using Beta vulgaris extract as a natural chromophore and diverse natural emulsifiers.
Materials and Methods
Four Formulations (F1-F4) were crafted using beeswax, castor oil, coconut oil, beetroot extract, and natural emulsifiers such as beeswax (E1), lecithin (E2), lanolin (E3), and candelilla wax (E4). The process included solvent-extraction, emulsification, wax melting, molding, and surface flaming. Each formulation was rigorously evaluated for solubility, melting point, pH, colour intensity, mechanical strength, surface anomalies, fragrance retention, water resistance, thixotropy, aging stability, antimicrobial activity, skin compatibility, phytochemical composition, and DSC.
Results
All four herbal lipstick Formulations (F1-F4) showed good solubility in organic solvents and maintained a pH range 5.5 to 6.8. F2 had the highest colour intensity and best spreadability, while F1 showed superior mechanical strength and thermal stability. All formulations were microbiologically safe, stable, and exhibited favorable thixotropic behavior. Phytochemical screening confirmed the presence of flavonoids and phenols.
Conclusion
F2 emerged as the most effective, attributed to lecithin’s emulsification efficiency, offering a promising, elegant natural alternative to conventional lipsticks.
INTRODUCTION
Some recent research suggests that lips serve as a visible indicator of a woman’s fertility. Studies in evolutionary psychology indicate that red lips are associated with higher estrogen levels, signaling health and fertility (Science Note, 2005). Lipstick is a cosmetic preparation that contains pigments, oils, waxes, and emollients to give the lips colour and texture. In Britain, it is commonly referred as “lippy” (Bhagyasree et al., 2024). The colouring of lips is not a new concept. In ancient India, orthodox women used to chew pan a mixture of crushed areca nut, betel leaves, spices, and lime to add colour to their lips (Natnoo, 2018). This practice can still be observed in Indian culture today, especially during festivals such as Holi and Raja (Iftikhar, 2010).
Today, lipsticks are available in various forms, including transparent lipstick, liquid lipstick, lip rouge, lip jelly, lip salve, lip gloss, lip balm, and lip pencils (Sharma, 2008). However, the main concern remains the use of synthetic colours. In the United States, colour additives in cosmetics do not require FDA approval before being marketed. However, in October 2007, the Campaign for Safe Cosmetics reported the presence of lead in several commercially available lipsticks. In response, the FDA issued draft guidance in December 2016, recommending that cosmetic lip products such as lipsticks, glosses, and liners should not contain more than 10 parts per million (ppm) of lead as an impurity. This limit aligns with standards set for food products due to the likelihood of incidental ingestion (U.S. Food and Drug Administration [FDA], 2022). Furthermore, some research articles have highlighted the potential carcinogenic effects of artificial colours (Rasheed et al., 2020). This concern has led to increased demand for herbal lipsticks (Jain et al., 2022).
Despite their growing popularity, herbal lipsticks present several formulation challenges, including inconsistent shades, shorter shelf life, low melting points, and a higher risk of microbial contamination. These issues often arise due to the instability of natural ingredients and the absence of effective binding and stabilizing agents. To address these limitations, the present study introduces a novel approach by incorporating various emulsifying agents such as beeswax, lecithin, lanolin, and candelilla wax.
An emulsifier is a substance that helps to mix two immiscible liquids, like oil and water. Normally, these liquids separate as they are not thermodynamically stable, but an emulsifier enables them to blend uniformly and increase the stability of the formulation (Arian Velayati and Alireza Nouri, 2020). Emulsifiers have two parts, one that attracts water and another that attracts oil. The water-loving part points toward the water, while the oil-loving part points toward the oil. This positioning reduces surface tension and keeps the mixture stable.
These emulsifiers were selected for their ability to improve the texture, spreadability, colour dispersion, and overall stability of the product. A high HLB value (between 10 and 18) indicates a more water-loving emulsifier, suitable for mixing oils into water. In contrast, a low HLB value (between 3 and 8) indicates a more oil-loving emulsifier, better for mixing water into oils (Al-Suwayeh et al., 2020). Among the four developed formulations, the strategic use of natural colorants combined with carefully chosen emulsifiers effectively addresses key formulation challenges. This approach not only enhances the performance of the product but also supports the development of safe, stable, and consumer-friendly herbal lipsticks. This study aimed to develop and evaluate herbal lipsticks using Beta vulgaris extract as a natural chromophore and diverse natural emulsifiers.
MATERIALS AND METHODS
Materials
This formulation is composed of premium ingredients sourced from trusted suppliers. Beeswax is acquired from Akhil Health Care Private Limited, while castor oil is obtained from Unilex Colours and Chemicals Limited. Coconut oil is sourced from Pranav Agro, and lecithin is supplied by Gayathri Global Ingredients. Lanolin is provided by Kuntal Organic LLP, whereas candelilla wax comes from Goldlan Pharmaceutical LLP. Fresh, natural components like beetroot and lemon juice are procured from the local market to ensure quality and purity. Finally, rose essence from H R Aroma contributes a delightful fragrance to the formulation.
Methods
Method of preparation of herbal lipstick involve several steps like extraction of colour, adding of antioxidants and preservatives, dispersion of colour, melting of wax, mixing of wax, moulding and flaming as illustrated in Figures 1, 2A and 2B (Kolekar and Hingane, 2022).
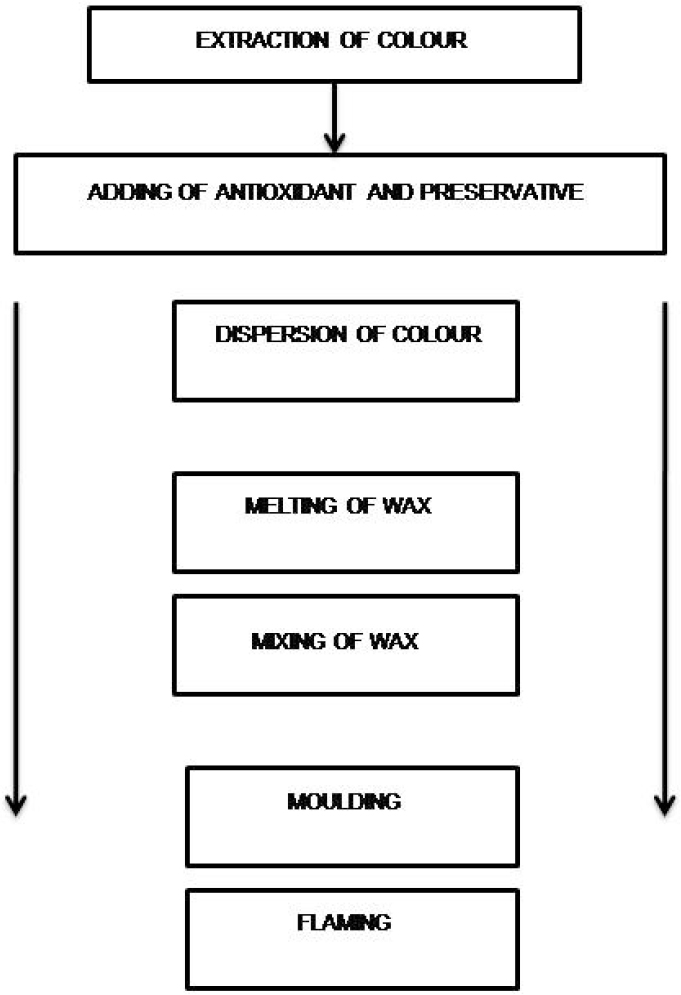
Figure 1:
Schematic layout showing the steps of lipstick.

Figure 2A:
Preparation procedure.
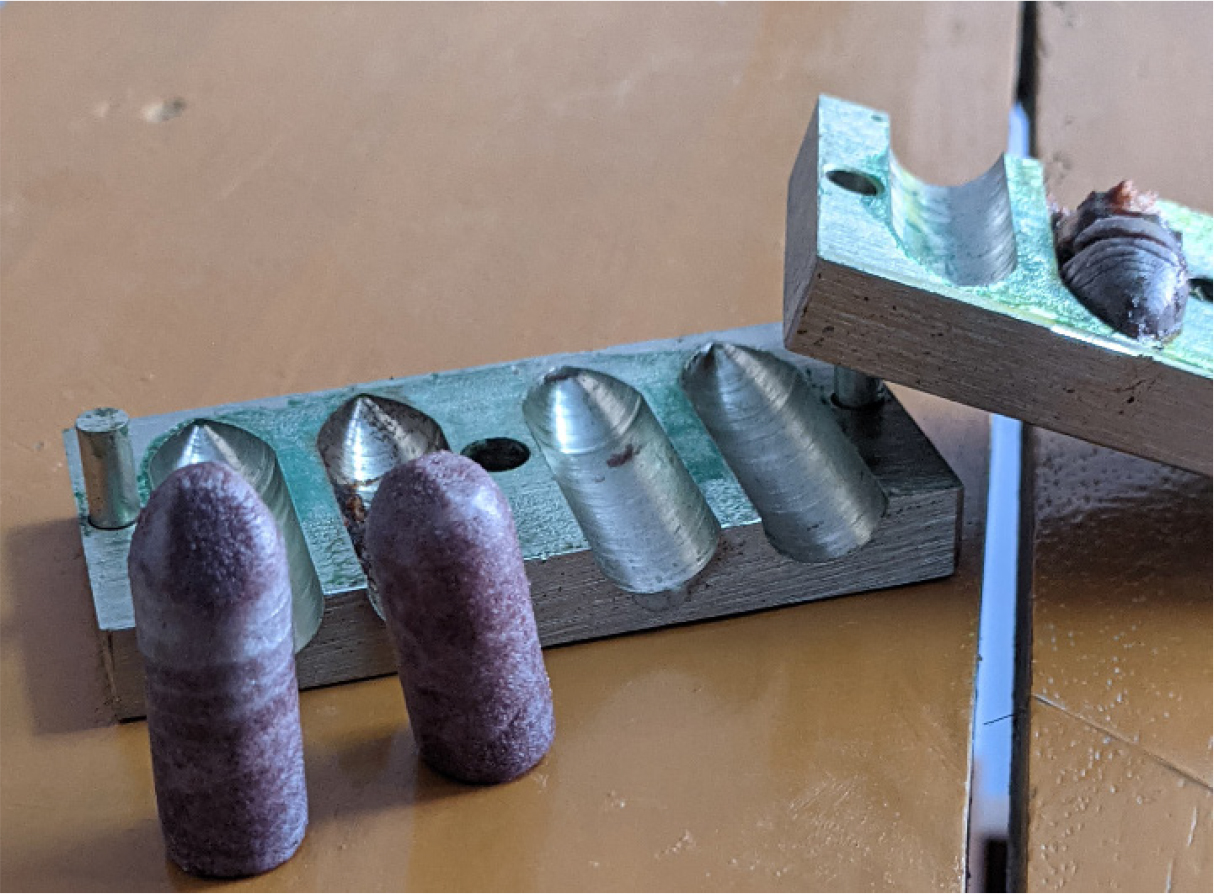
Figure 2B:
Preparation procedure.
- Extraction of colour: Peeled beetroot were cut into small dices and the pigment was extracted by boiling the pieces in distilled water for 9-11 min. The extract was then filtered. The filtrate was subsequently reboiled to concentrate the solution and obtain a thicker consistency.
- Adding of antioxidant and preservative: In the next step, an antioxidant was added into the aqueous phase.
- Dispersion of colour: Once the colour extract was prepared, it was heated in a water bath alongside the oil mixture in separate beakers. The emulsifying agent was added to the oil phase. When both phases reached a temperature of 70ºC, the aqueous phase was gradually added to the oil phase with continuous stirring to ensure proper emulsification.
- Melting of wax: At the next, the wax was melted under controlled heating.
- Mixing of wax: Once the wax was fully melted, it was slowly incorporated into the colour mixture with gentle stirring to prevent air entrapment. Subsequently, the flavoring agent was added.
- Moulding: The prepared mixture was then carefully poured into a mold, leaving a small space at the top. The mold was left undisturbed at room temperature for 20 min to allow the lipstick to solidify
- Flaming:Finally, the lipstick sticks were inserted into containers, and reheating was performed on the exposed ends to achieve a smooth finish (Sharma, 2008). By following the above process, one batch of lipstick was prepared for each formulation with a total batch size of 50 g, based on the composition provided in Table 1 for a single 4.5 g lipstick unit.
| Ingredient (For 4.5 g) | F1 (g) | F2 (g) | F3 (g) | F4 (g) |
|---|---|---|---|---|
| Bees wax | 1.125 | 1.125 | 1.125 | 1.125 |
| Castor oil | 1.125 | 1.125 | 1.125 | 1.125 |
| Coconut oil | 0.562 | 0.562 | 0.562 | 0.562 |
| Bees wax (E1) | 0.562 | — | — | — |
| Lecithin (E2) | — | 0.562 | — | — |
| Lanolin (E3) | — | — | 0.562 | — |
| Candelilla wax (E4) | — | — | — | 0.562 |
| Beet root | 0.843 | 0.843 | 0.843 | 0.843 |
| Lemon juice | 0.140 | 0.140 | 0.140 | 0.140 |
| Rose essence | 0.140 | 0.140 | 0.140 | 0.140 |
Evaluation tests
Evaluation is the tool by which the quality of an formulation can be determine. For herbal lipstick these are melting point, breaking point, force of application, surface anomalies etc.
- Solubility test: Initially, 5 mL of each solvent was poured into separate test tubes. The lipstick sample was then added to each tube, followed by continuous stirring to facilitate dissolution.
- Melting point test: The determination of the melting point is crucial as it indicates the safe storage limit of the product. In the case of the formulated lipstick, the melting point can be measured using the DSC.
- pH test: For the pH measurement determination pH meter method was adopted. Initially the pH meter was calibrated using standard buffer solutions. After calibration, the electrode was immersed in the lipstick dispersion, and the pH value was recorded. This procedure was repeated three times, and the average pH was calculated (Dhadwal et al., 2023).
- Colour intensity test: A spectroscopic method was employed to determine the colour intensity. Initially, 10 mg of each lipstick sample was dissolved in 70% ethanol to obtain a clear solution. The undissolved particles were then removed through filtration, followed by UV-visible spectroscopy analysis. Finally, the absorbance values were compared to assess the colour intensity.
- Breaking point test: The breaking point was used to determine the strength of the lipstick. The lipstick was positioned horizontally in a socket, placed half (1/2) inch from the edge of the support. The weight was gradually increased by a specific amount at 30 sec intervals. The weight at which the lipstick broke was recorded as its breaking point (Hingane et al., 2024).
- Surface anomalies test: The Surface Anomalies Test for lipstick is performed to assess the surface quality of the product, ensuring it is smooth, consistent, and free from visible imperfections. The lipsticks were undergoes visual examination under appropriate lighting to identify any defects such as cracks, air bubbles, oil sweating, uneven texture or colour (Mishra and Dwivedi, 2012).
- Transparency test: It is an in-house method conducted using tissue paper. Initially, lipstick marks were made on a tissue paper. Another tissue paper was then placed over the marked area, followed by the application of pressure to assess the transferability of the lipstick (Pavithra et al., 2022).
- Water resistance test: It had involved applying lipsticks to the skin, followed by exposing the area to running tap water to evaluate its resistance to water.
- Perfume stability test: The perfume stability test for lipstick is performed to assess the durability and consistency of the fragrance over time. The test is carried out in three steps. First one was initial assessment, where the fragrance of the lipstick were evaluated immediately after preparation. Next one is storage conditions, where samples were stored under different conditions. Like that third one is periodic evaluation where the lipsticks were examined at each two-month intervals over a period of six months.
- Skin irritation test: The skin irritation test for lipstick is performed to assess the product’s safety and confirm it does not trigger adverse skin reactions. A small quantity of lipstick was applied to a clean, dry area of skin, such as the inner forearm of volunteers. The applied area was then left undisturbed for 24 to 48 h. This procedure was repeated for each formulation (Mishra and Dwivedi, 2012).
- Aging stability test: The aging stability test for lipstick is performed to assess the product’s stability and performance over time, mimicking the effects of prolonged storage. Lipstick samples were kept in their packaging and stored under various conditions, including room temperature and elevated temperatures (40ºC-45ºC) for a duration of one month (Kolekar and Hingane, 2022).
- Thixotropic behaviour test: To assess the thixotropic behaviour of a lipstick formulation, ensuring smooth application and structural stability, the lipstick samples were gently melted and tested using a rheometer to determine their initial viscosity. The samples were then exposed to continuous shear stress through high-speed stirring or rotational force for 5 min. Once the shear force was removed, the samples were left undisturbed for 30 min to allow them to recover their original structure. Viscosity measurements were taken at regular intervals during the recovery period (Mishra and Dwivedi, 2012).
- Antimicrobial test: The antimicrobial test for lipstick is conducted to evaluate the product’s ability to resist microbial contamination and ensure its safety for consumer use. The inoculated samples were incubated under controlled conditions 30-35ºC and 20-25ºC for a specific 7 days.
Phytochemical screening
Phytochemical screening of Beta vulgaris water extract was performed to detect the presence of different bioactive compounds, including alkaloids, flavonoids, saponins, tannins, and phenolic compounds.
Differential scanning Calorimetry
A DSC-6100 (Seiko Instruments) equipped with a thermal analyzer was used for the analysis. Precisely weighed samples (approximately 2 mg) were placed in sealed aluminum pans and heated under a nitrogen flow of 20 mL/min. The heating was carried out at a scanning rate of 10ºC per min, ranging from 30ºC to 150ºC (Priyadarshan et al., 2024).
RESULTS
The solubility test results for all the Formulations (F1-F4) indicate distinct solvent compatibility. All formulations showed partial solubility in distilled water, while they exhibited good solubility in 70% ethanol, acetone, and chloroform. Moderate solubility in isopropyl alcohol and poor solubility in hexane. Overall, the results showed the formulations are amphiphilic in nature, containing both polar and non-polar ingredients, results presented in Table 2.
| Solvent | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Distilled Water | Partial | Partial | Partial | Partial |
| 70% Ethanol | Good | Good | Good | Good |
| Isopropyl Alcohol | Moderate | Moderate | Moderate | Moderate |
| Acetone | Good | Good | Good | Good |
| Chloroform | Good | Good | Good | Good |
| Hexane | Poor | Poor | Poor | Poor |
Similarly, for the pH test, colour intensity test, breaking point test, surface anomaly evaluation, anti-transparency test, and water resistance test, different results were observed for different formulations. All formulations exhibited pH values within the acceptable dermal range (6.3-6.8), with F1 showing the highest (pH 6.8) and F3 the lowest (pH 6.3). In the colour intensity test, absorbance values ranged from 0.83 to 0.92, where F2 recorded the highest (0.92), indicating the most intense colour, and F1 the lowest (0.83). The breaking point test revealed that F1 had the highest mechanical strength (610 g), while F2 was the softest (450 g). All formulations showed no surface anomalies, reflecting smooth and uniform textures. Regarding anti-transparency test, F1 was rated very good. Similarly, for water resistance test, F1 demonstrated superior performance. The results are presented in Table 3.
| Test | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| pH | 6.8 | 6.5 | 6.3 | 6.7 |
| Colour Intensity (Absorbance of F1 at λmax 535 for 10ppm) | 0.83 | 0.92 | 0.87 | 0.85 |
| Breaking point | 610g | 450g | 520g | 560g |
| Surface anomalies | No Defect | No Defect | No Defect | No Defect |
| Anti-transparency | Comparatively
Very good |
Comparatively
good |
Comparatively
good |
Comparatively
good |
| Water Resistance | Comparatively
Very good |
Comparatively
good |
Comparatively
good |
Comparatively
good |
The perfume stability test showed the fragrance retention for all the formulations. During the initial period of assessment, all the formulations were found to be stable, indicating no immediate loss or change in fragrance. However, upon periodic evaluation, a slight fade in fragrance was observed with all the formulations. The result are given in the Table 4.
| Condition | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Initial Assessment | Stable | Stable | Stable | Stable |
| Storage Conditions | Stable | Stable | Stable | Stable |
| Periodic Evaluation | Slight fade | Slight fade | Slight fade | Slight fade |
In case of skin irritation test, formulations F2, F3, and F4 showed no indication of irritation, showing good dermal compatibility. F1 also did not cause irritation but was noted to cause slight discomfort. The results are given in the Table 5.
| Test | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Skin irritation | No Irritation
But slight discomfort |
No Irritation | No Irritation | No Irritation |
| Aging Stability | Stable | Stable | Stable | Stable |
Initially, all formulations exhibited high viscosities in case of thixotropic behaviour test, with F1 and F4 at 150,000 cP, and F2 and F3 at 145,000 cP. After 10 min of shear, the viscosity of all formulations decreased significantly to 85,000-90,000 cP, indicating that the structure temporarily broke down under stress. Over time, the viscosity gradually recovered, reaching 110,000-120,000 cP after 20 min, and nearly returning to their initial values after 30 min. The result are given in the Table 6.
| Time | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Initial (without Shear) | 150,000cp | 145,000cp | 145,000cp | 150,000cp |
| After 10min | 90,000cp | 85,000cp | 85,000cp | 90,000cp |
| After 20min | 120,000cp | 110,000cp | 110,000cp | 120,000cp |
| After 30min | 145,000cp | 140,000cp | 140,000cp | 145,000cp |
The antimicrobial test on all the formulations (F1-F4) under two different conditions to evaluate their ability to resist microbial contamination. As presented in Table 7, all formulations exhibited no growth.
| Condition | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Condition1 | No growth | No growth | No growth | No growth |
| Condition2 | No growth | No growth | No growth | No growth |
Phytochemical screening of the formulations confirmed the presence of various bioactive compounds. As shown in Table 8, the tests showed positive results (+Ve) for several important phytochemicals.
| Compounds | Test | Observation | Result |
|---|---|---|---|
| Alkaloids | Mayer’s Test | Cream-coloured precipitate | +Ve |
| Flavonoids | Shinoda Test | Pink colour formation | +Ve |
| Saponins | Frothing Test | Froth formation | +Ve |
| Tannins | Ferric chloride Test | Blue colour | +Ve |
| Terpenoids | Salkowski Test | Red | +Ve |
| Phenols | Ferric chloride Test | Dark green | +Ve |
| Glycosides | Keller- Test | Reddish | -Ve |
| Carbohydrates | Molisch’s Test | Purple ring | +Ve |
| Proteins | Biuret Test | Purple color | +Ve |
| Lipids | Spot Test | Oil spot | +Ve |
The Differential Scanning Calorimetry (DSC) analysis showed the thermal behavior and stability of the Formulations (F1-F4). The melting endotherm observed for the formulations were 71.35ºC for F1, 65.19ºC for F2, 67.89ºC for F3, and 68.96ºC for F4, the same is given in Table 9 as displayed in Figure 3.
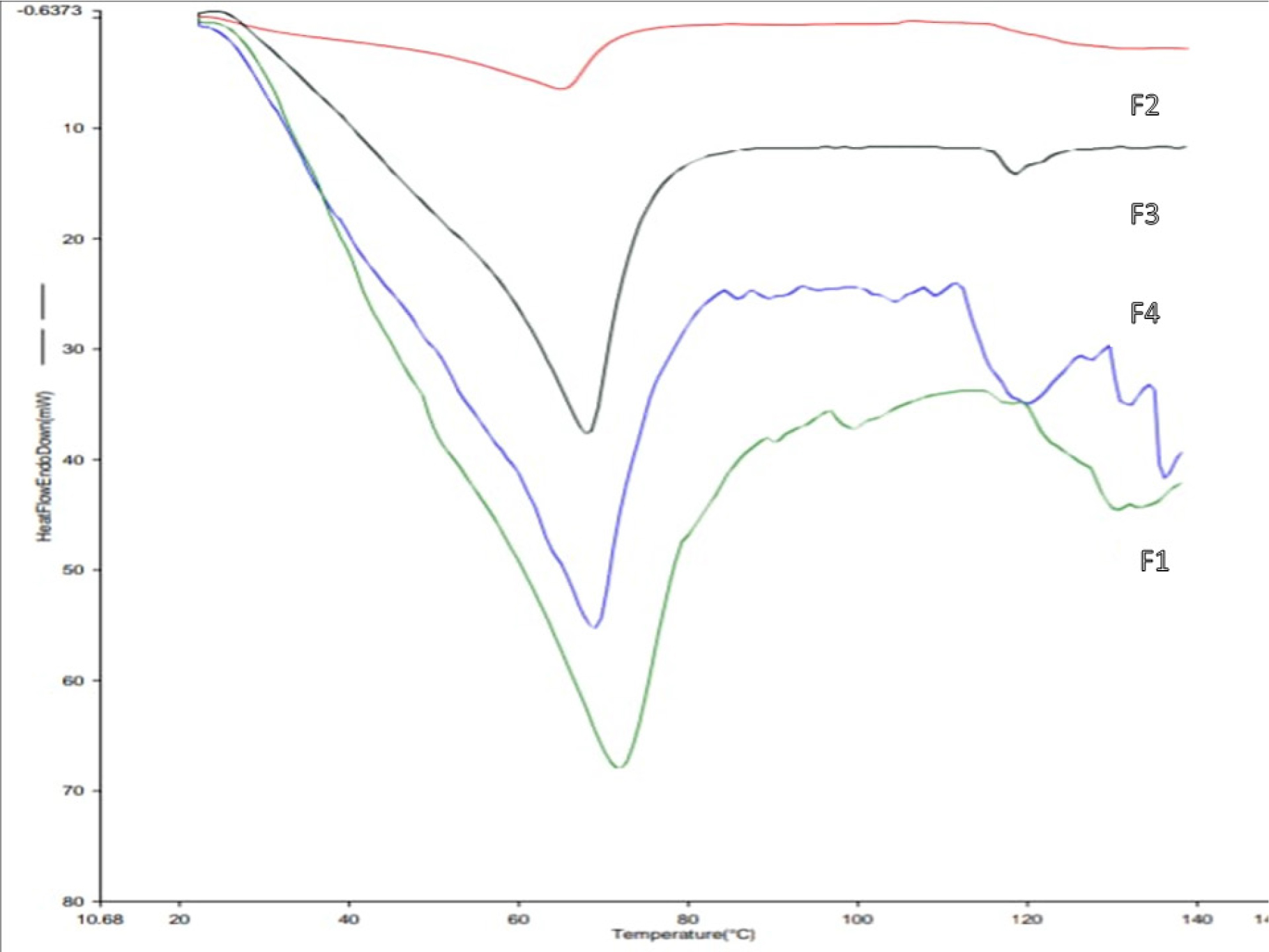
Figure 3:
Thermograms of different lipstick Formulations (F1 to F4).
| F1 | F2 | F3 | F4 |
|---|---|---|---|
| 71.35ºC | 65.19ºC | 67.89ºC | 68.96ºC |
DISCUSSION
The comparative assessment for the Formulations (F1-F4) demonstrated that each exhibited unique physicochemical and functional properties influenced by their respective compositions.
In the pH evaluation test, all the formulations showed pH values within the dermally acceptable range for lip products i.e., from 5.5 to 6.8 (Wiley, 2023). This indicates a low potential for muco-cutaneous irritation and confirms their safety for topical application (Draelos, 2018). In the colour intensity test, F2 shows the highest absorbance, recorded as 0.92 at λmax 535 nm. This result is attributed to the high concentration of betalain pigments present in the F2 formulation (Shrestha and Shrestha, 2024).
The mechanical strength analysis showed that F1 has the highest breaking point (610 g), indicating superior structural integrity. However, this increased hardness may compromise user comfort and ease of application. Formulations F2 with lower breaking strength, offer better application properties. Surface anomaly evaluation confirms that all formulations are free of cracks or deformities, reflecting uniform surface morphology. The transparency tests indicated a high retention time. F1 shows superior opacity, may be due to its higher wax content, which enhances pigment retention and reduces translucency.
Water resistance test shows that all the formulations withstand smudging and maintain their colour when exposed to moisture, which is a favorable trait for long-lasting lip products. The fragrance also remained stable throughout the study period, although a slight decline in fragrance is observed. The skin irritation tests confirmed dermatological safety, with no adverse reactions observed in volunteers except with mild discomfort in case of F1. This may be due to higher wax concentration, possibly affecting skin feel.
Stability is observed with all the formulations, with each maintaining its physical and organoleptic properties under standard storage conditions. This reflects long shelf-life performance and dependable product quality.
All the formulations exhibited thixotropic behavior of suitable rheological characteristics for cosmetic use. When subjected to shear stress, such as during application, their viscosity decreases significantly (from 145,000-150,000 cp to 85,000-90,000 cp), allowing for easy and smooth spreading (Mishra and Dwivedi, 2012). Notably, F1 and F4 fully recovered their original viscosity within 30 min, demonstrating efficient re-solidification, a key factor in preventing smudging after application.
The microbial limit test, performed according to USP, confirmed the microbiological safety of all the formulations, with no detectable bacterial or fungal growth (United States Pharmacopeia [USP], 2020). The phyto-chemical analysis identified the presence of flavonoids, phenolic compounds, and tannins bio-actives with known antioxidant, antimicrobial, and anti-inflammatory properties (Bainsal et al., 2021). These findings suggested added therapeutic potential, extending the product’s benefits beyond cosmetic use.
The DSC analysis provided the insight into thermal behavior. F1 exhibits the highest thermal peak (71.35ºC), confirming its excellent thermal stability. F2 has the lowest peak (65.19ºC), supporting its enhanced spreadability. F3 and F4 exhibit intermediate thermal events, suggesting a balance between application properties and heat resistance as displayed in Figure 3. All formulations demonstrated acceptable thermal profiles for commercial applications.
Collectively, these results highlight the versatility of natural emulsifiers and beetroot extract in developing herbal lipsticks with a favorable physicochemical, aesthetic, and safety attributes.
CONCLUSION
Based on the comprehensive evaluation of all the Formulations (F1-F4), each demonstrated acceptable performance with respect to physicochemical, microbiological, and stability evaluation parameters. However, certain formulations exhibited superior characteristics in specific tests. Among all the formulations, F1 caused slight discomfort in the skin irritation test, but exhibited the highest thermal peak (71.35ºC), confirming its excellent thermal stability. The formulation F2 had the most skin-friendly (pH 6.5) and showed no irritation, making it preferable for sensitive skin, while similarly, F2 exhibited the highest colour intensity (absorbance 0.92), suggesting enhanced pigment dispersion-likely due to the presence of an emulsifier with a low HLB value. It also showed the lowest breaking point (450 g), indicating a softer consistency. Furthermore, it demonstrated excellent thixotropic recovery, with viscosity effectively restoring over time, ensuring consistent spreadability and performance during application. The DSC data further support the good spreadability of the F2 formulation, with a peak temperature of 65.19ºC, indicating lower rigidity. All Formulations (F1-F4) passed the microbiological safety test, exhibited good perfume stability, had no surface anomalies, and remained within acceptable limits for other evaluation parameters, confirming their suitability for safe cosmetic use. Hence, each formulation had unique features. Considering all, in conclusion formulation F2 is emerged as the best formulation; offering high colour intensity, good spreadability, and satisfactory performance basing on evaluation criteria, making it the most promising herbal lipstick formulation among the four.
Cite this article:
Nahak A, Padhi AN, Mahapatra AK, Sahu A, Kakinada SK, Prusty A. Harnessing Beet Root Pigments as Natural Colorant for a Glossy Herbal Lipstick: Formulation and Evaluation. J Young Pharm. 2025;17(3):627-35.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the support and resources provided by R.C.P.H.S.
ABBREVIATIONS
| DSC | Differential Scanning Calorimetry |
|---|---|
| FDA | Food and Drug Administration |
| HLB | Hydrophilic Lipophilic Balance |
| UV | Ultraviolet. |
References
- Alam, S., Algahtani, M. S., Ahmad, M. Z., & Ahmad, J. (2020, June). Investigation utilizing the HLB concept for the development of moisturizing cream and lotion: In vitro characterization and stability evaluation. Cosmetics, 7(2), 43. https://doi.org/10.3390/cosmetics7020043
- Bainsal, N., Kaur, S., & Mallan, S. (2021). Pharmacognostical, physicochemical and phytochemical studies of different varieties of beet root grown in Punjab. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 12(4).
- Bhagyasree, M., Aswani, D., & Umadevi, P. (2024). Formulation and evaluation of herbal lipsticks. International Journal of Creative Research Thoughts (IJCRT), 12(11), d657.
- Dhadwal, A., Thakur, C., Aniket, S., & Kumari, P. (2023). Formulation and evaluation of herbal lipstick by using natural ingredients. Ymer, 23(6), 501–516.
- Draelos, Z. D. (2018). Cosmetics and dermatologic problems and solutions (3rd ed.). CRC Press.
- Hingane, L. D., Bhanudas, V. S., Madhukar, S. R., & Gitesh, L. A. (2024, November). Formulation and evaluation of herbal lipstick. International Journal of Advanced Research in Science, Communication and Technology (IJARSCT), 4(3).
- Iftikhar, R. (2010). Cultural contribution of Mughal ladies. Research Journal of South Asian Studies, 25(2), 323–339.
- Jain, S., Jain, S., Pillai, S., Mandloi, R. S., & Namdev, N. (2022). Formulation and evaluation of herbal lipstick by using beetroot extract. Research Journal of Pharmacognosy and Phytochemistry, 14(1), 23–25.
- Kolekar, A. S., & Hingane, L. D. (2022, June). Formulation and evaluation of herbal lipstick. International Journal of Creative Research Thoughts (IJCRT), 10(6).
- Mishra, P., & Dwivedi, S. (2012). Formulation and evaluation of lipstick containing herbal ingredients. Asian Journal of Medical and Pharmaceutical Researches, 2(3), 58–60.
- Natnoo, S. A. (2018). Betel-Leaf (Pan) culture: A study of Mughal India. SSRG International Journal of Humanities and Social Science, 5(1), 39.
- Pavithra, T. K., Kumar, B. S. A. BS, Mouna, A., & Munegowda, S. (2022). Formulation and evaluation of herbal lipstick containing alkanet root as a natural colorant. Revista de Chimie, 73(4), 36–40. https://doi.org/10.37358/RC.22.4.8545
- Priyadarshan, K. R., Sahu, A., Mahapatra, A. K., Chowdary, K. A., Nahak, A., & Patra, R. K. (2024). Rivaroxaban solid dispersions for dissolution enhancement and formulation of mouth-disintegrating tablets. Journal of Applied Pharmaceutical Research, 12(4), 75–82. https://doi.org/10.69857/joapr.v12i4.647
- Rasheed, N., Rahman, S. A., & Hafsa, S. (2020). Formulation and evaluation of herbal lipsticks. Research Journal of Pharmacy and Technology, 13(4), 1693–1700. https://doi.org/10.5958/0974-360X.2020.00306.6
- Science note. (2005, November 28). Why do men find big lips and little noses so sexy? I’ll paint you a picture. Times Book Company.
- Sharma, P. P. (2008). Cosmetics formulation, manufacturing, and quality control (4th ed.). Vandana Publication Pvt.
- Shrestha, Y. K., & Shrestha, S. K. (2024). Fundamentals of colorimetry. In A. K. Samanta (Ed.), Advances in colorimetry (pp. —-). IntechOpen.
- United States Food and Drug Administration. (2022, November 22). Limiting lead in lipstick and other cosmetics. US FDA.
- United States Pharmacopeia (USP). (2020). USP 43-NF, 38, General Chapters <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests; and <62> Tests for Specified Microorganisms.
- Velayati, A., & Nouri, A. (2020). Emulsification and emulsion flow in thermal recovery operations with a focus on SAGD operations: A critical review. Fuel, 267, Article 117141. https://doi.org/10.1016/j.fuel.2020.117141
- Wiley. (2023, August). The pH of the lip surface. Skin Research and Technology, 29(8).
