ABSTRACT
Background
Acute Kidney Injury (AKI) is a common condition and can happen due to drugs like Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and which can be reversible by discontinuing NSAIDs. This research aims to examine the risk factors and occurrence of AKI in individuals using NSAIDs, while also investigating the potential correlations between AKI and conditions such as hypertension, gout, and emergency medical situations.
Materials and Methods
A comprehensive total of 125 patients were included based on specific criteria, encompassing both inclusion and exclusion parameters. The medical histories of these patients were meticulously reviewed, with relevant data duly documented, and concludingly subjected to statistical examination.
Results
Among the 125 patients studied, 10 individuals were diagnosed with AKI, resulting in an incidence rate of 8%. Notably, 80% of these AKI cases were observed in the older age group (45-65 years), with 60% being male and 40% female, indicating a higher risk in male patients. Additionally, 40% of the AKI patients had a pre-existing condition of hypertension, which posed a higher susceptibility to AKI development. The diagnosis of AKI was based on laboratory parameter changes, particularly serum creatinine levels, and adhered to the KDIGO scale (Kidney Disease Improving Global Outcomes).
Conclusion
Following the examination of NSAID users, it was concluded that the elderly population and individuals with a history of hypertension face the highest risk of developing AKI, leading to elevated serum creatinine levels. Additionally, male patients exhibited a higher frequency of AKI compared to their female counterparts. Notably, a significant number of patients were found to have taken Diclofenac for an extended duration, which correlated with increased serum creatinine levels and a potential link to AKI development. Consequently, Diclofenac usage warrants consideration as a drug that may contribute to the onset of AKI.
INTRODUCTION
The kidney serves as the primary excretory organ in the body, responsible for eliminating numerous drugs. It receives approximately twenty-five percent of the cardiac output. Given its pivotal role in drug elimination, drugs tend to have a substantial impact on the renal arterioles and glomerular capillaries. Acute Kidney Injury (AKI) arises when the kidneys encounter difficulties in their regular excretory functions. Over-the-counter drugs, when frequently used, can significantly impair kidney function. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are particularly implicated in causing renal damage through two main pathological mechanisms. The first mechanism involves a decrease in plasma flow, resulting in reduced prostaglandin levels, which in turn affect vasodilation at the glomerular level. The other mechanism is Acute Interstitial Nephritis, arising from an immunological response.1 Medications known as NSAIDs, which possess analgesic, antipyretic, and anti-inflammatory properties, are commonly utilized in primary care settings. These drugs are prevalent, with approximately one out of every fifteen patients in the United States currently receiving prescriptions for NSAIDs. Furthermore, low-dose formulations of NSAIDs are available over-the-counter in multiple countries.2 The primary objective of this study is to systematically assess well-established community-based observational studies in order to estimate the risk of AKI caused by NSAIDs in the general population, as well as in patients already diagnosed with Chronic Kidney Disease (CKD). Recent research has highlighted NSAIDs as highly effective medications for managing pain related to renal calculi, even surpassing opioids in effectiveness. Individuals with chronic pain, often associated with rheumatologic conditions like rheumatoid arthritis, osteoarthritis, and other musculoskeletal disorders, constitute the main user group of these drugs. The pharmacological impact of NSAIDs is influenced by the quantity and duration of usage, which can lead to organ-specific involvement, with the kidney being the most vulnerable organ.3 Consequently, this pharmaceutical is known to raise morbidity rates, particularly among the elderly who are concurrently taking various medications (such as antihypertensives, antidepressants, and anticoagulants) that might interact with it. Such individuals are at a heightened risk of developing kidney injury, which could manifest as either temporary or permanent damage. Moreover, individuals with chronic kidney disease face a significantly higher risk of experiencing adverse effects due to long-term use of this medication, as they are three to four times more susceptible to such effects.1 AKI is on the rise in affluent nations. In hospital settings, it is thought to affect approximately 15% of inpatients, and the incidence is even higher in critically ill patients, reaching up to 60%. On the other hand, community-acquired AKI is relatively uncommon, with recent research estimating its prevalence to be around 4.3% of all hospital admissions. However, since not all AKI cases are reported in hospitals, this figure likely underestimates the full impact of community-acquired AKI.4 Epidemiologically, drug-induced kidney diseases present a challenging topic. In US hospitals, 18-27% of all cases of acute renal damage are attributed to drug-related nephrotoxicity. Medications have the potential to affect various components of the kidney, and drugs associated with renal failure are commonly prescribed in clinical settings. The article delves into the primary drugs implicated in such cases and examines six major pathways responsible for drug-induced renal impairment. Notable examples of drugs causing renal failure include NSAIDs, aminoglycosides, amphotericin B, and calcineurin inhibitors, among others. When prescribing and administering medications, the medical community should be vigilant about the patient’s risk factors for nephrotoxicity and be aware of the drug’s inherent potential for causing harm to the kidneys.5,6 The widespread use of NSAIDs by the general population, even for minor pain symptoms, has prompted the need for this study to highlight the potential consequences of such usage. The primary objective is to raise awareness among the general public about the rational and appropriate use of NSAIDs, emphasizing the importance of timing and dosage. It is essential to be cautious about the regular and improper over-the-counter use of NSAIDs as it can lead to adverse outcomes. A recent long-term active pharmacovigilance study conducted in Italy revealed that NSAIDs were responsible for 24.4% of emergency department visits resulting in hospitalization and 8.4% of emergency room visits. Patients admitted to the Orthopaedics and Surgery departments have the highest likelihood of being prescribed NSAIDs. Hence, our study aims to observe the changes in serum creatinine levels in patients admitted to these two respective departments to examine the effect of NSAIDs on kidney function alterations concerning factors like age, gender, and co-morbidities, and assess the changes in laboratory parameters.
MATERIALS AND METHODS
This prospective observational study received approval from the Sumandeep Vidyapeeth Institutional Ethics Committee (SVIEC/ON/Phar/BNPG20/021040). The study focused on patients admitted to the Departments of Orthopaedics, General Medicine, Nephrology, and Surgery at Dhiraj General Hospital, who were prescribed NSAIDs. The sample size of 125 participants was determined according to the instructions provided by the statistician and subsequently enrolled in the study. Patients meeting the inclusion criteria (between 18 and 65 years of age and prescribed NSAIDs) and meeting the exclusion criteria (those unwilling to participate or voluntarily withdrew their consent in the study, patients below 18 or above 65 years of age, lactating and pregnant women) were recruited for further observation to assess the development of AKI due to the continuous administration of NSAIDs. The study participants were thoroughly briefed about the research, and their informed consent was obtained. Relevant subject information was recorded on a pre-designed form. Follow-up appointments were scheduled after the completion of the NSAIDs course to evaluate changes in serum creatinine levels and Glomerular Filtration Rate (GFR). Based on the KDIGO scale criteria, the subjects were subsequently categorized into three stages.
RESULTS
In our study, a total of 125 patients were included, and they were divided into four age groups: 18-35, 36-45, 46-55, and 56-65 years. Our report identified 10 out of 125 patients who developed AKI (Figure 1). Based on the data presented in Table 1, it was observed that a minority of patients developed AKI in each age group, with percentages of 2.12% (n=1), 3.44% (n=1), 13.33% (n=4), and 21.05% (n=4) in the age groups of 18-35, 36-45, 46-55, and 56-65, respectively. The majority of patients in each age group did not experience AKI, with percentages of 97.87% (n=46), 96.55% (n=28), 86.66% (n=26), and 78.94% (n=15) in the age groups of 18-35, 36-45, 46-55, and 56-65, respectively. Consequently, the data illustrates a direct correlation between the development of AKI and increasing age. The analysis reveals that the highest proportion of patients on NSAIDs who experienced AKI belonged to the age group of 46-65 (34.38%). Among the 125 patients on NSAIDs, the distribution by gender revealed that 7.14% (n=6) of male patients developed AKI, while the majority, 92.85% (n=78), did not experience AKI. For the female population, 9.75% (n=4) developed AKI, while 90.24% (n=37) did not. Hence, the data suggests that females faced a higher risk of developing AKI compared to males (Table 1). A total of 4 patients (16%) with hypertension developed AKI, while the majority, 84% (n=21), with hypertension did not experience AKI. For patients with Type-2 diabetes, 10.52% (n=2) developed AKI, whereas 89.47% (n=17) did not. Among those with both hypertension and Type-2 diabetes, 50% (n=2) developed AKI, and the remaining 50% (n=2) did not. In contrast, 3.84% (n=2) of patients without any co-morbidities developed AKI, while the majority, 96.15% (n=50), did not experience AKI (Table 1).
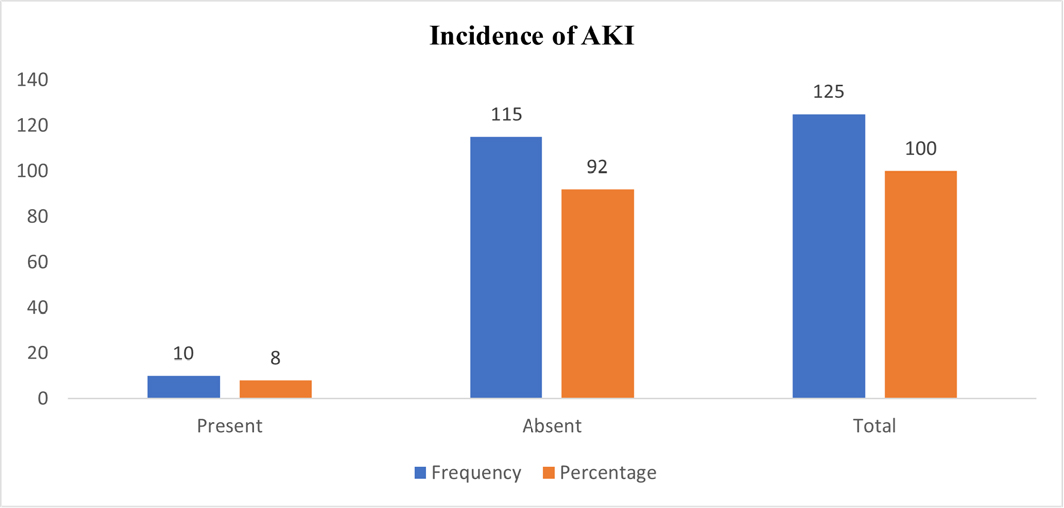
Figure 1:
Incident of AKI in enrolled patients.
| Age | |||||||
|---|---|---|---|---|---|---|---|
| Years | AKI (N) | Percentage (%) | Non-AKI (N) | Percentage (%) | Total | X2 | P value |
| 18-35 | 1 | 2.12 | 46 | 97.87 | 47 | 8.5761 | 0.0354 |
| 36-45 | 1 | 3.44 | 28 | 96.55 | 29 | ||
| 46-55 | 4 | 13.33 | 26 | 86.66 | 30 | ||
| 56-65 | 4 | 21.05 | 15 | 78.94 | 19 | ||
| Gender | |||||||
| Male | 6 | 7.14 | 78 | 92.85 | 84 | 8.5761 | 0.0354 |
| Female | 4 | 9.75 | 37 | 90.24 | 41 | ||
| Comorbidities | |||||||
| K/C/O HTN | 4 | 16 | 21 | 84 | 25 | 15.319 | 0.004 |
| K/C/O DM-2 | 2 | 10.52 | 17 | 89.47 | 19 | ||
| K/C/O DM + HTN | 2 | 50 | 2 | 50 | 4 | ||
| NS | 2 | 3.84 | 50 | 96.15 | 52 | ||
| Other | 0 | 0 | 25 | 100 | 25 | ||
Out of the 125 patients, 10 (8%) developed AKI, while the majority, 115 (92%), did not experience AKI. Table 2 presents the incidence of AKI and non-AKI cases for each medication used in the study. Particularly, Inj. Ketorolac + Tab. Ketorolac, Inj. Ketorolac alone, and Tab. Aceclofenac had the highest percentage of AKI cases, with 66.66%, 50%, and 33.33%, respectively. Conversely, Tab. Diclofenac plus Paracetamol, Inj. Diclofenac + Tab. Diclofenac + Paracetamol, and Inj. Diclofenac + Tab. Aceclofenac showed a lower incidence of AKI, ranging from 2.7% to 13.33%. The remaining medications did not lead to any AKI incidents.
| Medication | AKI (n) | Non AKI (n) | Total |
|---|---|---|---|
| Inj. Diclofenac | 0 (0 %) | 17 (100 %) | 17 |
| Inj. Ketorolac | 1 (50 %) | 1 (50 %) | 2 |
| Tab. Diclofenac + Paracetamol | 1(2.7 %) | 36 (97.28 %) | 37 |
| Tab. Aceclofenac | 1 (33.33 %) | 2 (66.66 %) | 3 |
| Tab. Naproxen + Domperidone | 0 (0 %) | 4 (100 %) | 4 |
| Tab. Etoricoxib | 0 (0 %) | 1 (100 0%) | 1 |
| Tab. Ibuprofen | 0 (0 %) | 1 (100 %) | 1 |
| Inj. Ketorolac + Tab. Ketorolac | 2 (66.66 %) | 1 (33.33 %) | 3 |
| Inj. Diclofenac + Tab. Diclofenac + Paracetamol | 3 (7.31 %) | 38 (92.68 %) | 41 |
| Inj. Diclofenac + Tab. Aceclofenac | 2 (13.33 %) | 13 (86.66 %) | 15 |
| Inj. Diclofenac + Tab. Ibuprofen + Tab. Diclofenac + Paracetamol | 0 (0%) | 1 (100%) | 1 |
| 10 (8 %) | 115 (92 %) | 125 |
As per our result, the patients enrolled in the study initially exhibited either normal or non-elevated serum creatinine levels. However, after the administration of NSAIDs, the mean difference between the on-admission creatinine level and the follow-up creatinine level was 0.43 for those who developed AKI and 0.15 for the non-AKI patients. The data indicates that the 10 patients who developed AKI had a significant mean difference of 0.43, categorizing them as Stage 1 AKI. Similarly, for the eGFR levels, the patients initially had either normal or non-elevated values. After the administration of NSAIDs, the mean difference between the on-admission eGFR level and the follow-up eGFR level was 35.93 for the AKI patients and 16.6 for the non-AKI patients. This significant decrease in eGFR was observed in the 10 patients who developed AKI (Table 3).
| AKI | ||||||
|---|---|---|---|---|---|---|
| Renal parameter | On Admin | On follow up | Mean difference | P value | ||
| Mean | SD | Mean | SD | |||
| Serum creatinine | 0.73 | 0.14 | 1.16 | 0.18 | 0.43 | 0.0001 |
| Urea | 28.6 | 7.8 | 32.4 | 8.07 | 3.8 | 0.2985 |
| eGFR | 105.86 | 27.69 | 69.93 | 18.98 | -35.93 | 0.0033 |
| Non AKI | ||||||
| Serum creatinine | 0.68 | 0.16 | 0.83 | 0.69 | 0.15 | 0.0241 |
| Urea | 27.2 | 9.15 | 28.99 | 8.87 | 1.79 | 0.1334 |
| eGFR | 128.6 | 34.26 | 112 | 30.48 | -16.6 | 0.0001 |
DISCUSSION
AKI poses a widespread public health issue worldwide, contributing to elevated mortality and morbidity rates, and consequently leading to increased healthcare costs. The identification of AKI involves observing an elevation in serum creatinine levels or a decrease in urine output, or both. Kidney damage and functional changes may develop over an extended duration, involving various contributing factors leading to kidney impairment. AKI is not a solitary disease but rather encompasses a diverse range of conditions or co-morbidities, including sepsis, diabetes, hypertension, and others.1 For this research, we enrolled a total of 125 patients who were prescribed NSAIDs during their hospital treatment. The main focus of our study was to observe the incidence of AKI caused by NSAIDs. Among the participants, 84 were male and 41 were female. Additionally, some of the patients had co-morbid conditions such as diabetes and hypertension. The likelihood of developing AKI is higher in the older population compared to the younger population. The patients with comorbid condition such as hypertension, diabetes mellitus and hypertension with diabetes mellitus were enrolled 25, 19 and 4 respectively. Out of the entire population, there were 4 individuals who had both co-morbidities, and they were at the highest risk of developing AKI due to NSAIDs. NSAIDs are widely prescribed medications, frequently utilized by the general population without proper precautions or guidance. Their irrational use can significantly contribute to the development of AKI. Older individuals are particularly vulnerable to AKI as a result of unnecessary NSAID consumption. These drugs are commonly used worldwide.7
Our study’s objective aligns with the research conducted by Konstantinos Kateros, which was a retrospective study aimed at identifying the risk factors for AKI in Orthopaedic Patients who underwent emergency surgeries. Our study also examined risk factors such as age, gender, co-morbidities, and lab parameters, but we focused on patients from both the Orthopaedic and Surgery departments. Konstantinos Kateros’ study included 1025 patients over one year, with 68 patients developing AKI. In comparison, our study comprised 125 patients, of which 10 patients were found to have developed AKI. During our study, we observed that the female population had a higher risk of developing AKI than the male population. The incidence of AKI in the study conducted by Konstantinos Kateros was found to be 8.9% in Orthopaedic patients, while in our study, which included both Orthopaedic and Surgery departments, the incidence was 8%.8
Previous findings indicate that older adults who are prescribed NSAIDs for more than 14 days face a higher risk of AKI and hyperkalemia within the subsequent 30 days, compared to similar patients not prescribed NSAIDs. However, despite these risks, there was no significant increase in short-term mortality. Therefore, for many older adults, the use of prescription NSAIDs might still be considered safe. Nevertheless, healthcare providers should exercise caution and carefully assess the individual patient’s risk before prescribing NSAIDs.9 In our study, we enrolled patients aged between 18 and 65 years, dividing them into four groups based on age: 18-35, 36-45, 46-55, and 56-65. Our findings revealed that the age group ranging from 45-65 had a higher risk of developing AKI while using NSAIDs. Consequently, it suggests that patients aged above 45 years might face an increased risk of AKI due to reduced metabolizing activity or age-related pathophysiological changes in the body. The findings from another randomized study revealed that the use of various NSAIDs can lead to an increase in blood pressure, affecting both non-hypertensive and hypertensive patients. According to this study, significant NSAID use can result in a blood pressure elevation.10 In our study, we also observed individual patients who were non-hypertensive but showed an elevation in blood pressure after NSAID administration. Additionally, some patients with a known history of hypertension exhibited no elevation in blood pressure upon admission but experienced an increase after taking NSAIDs. Therefore, it is crucial for physicians to be vigilant about such changes and closely monitor patients, considering discontinuation of treatment when such alterations are detected. Based on our study, we concluded that patients with AKI and co-morbid conditions like hypertension require precise monitoring while on NSAIDs to avoid potential complications. According to our findings, the patients included in the study had normal or non-elevated serum creatinine levels at the beginning. However, following the administration of NSAIDs, there was a notable difference between the creatinine levels on admission and during the follow-up period. Specifically, those who developed AKI showed a mean difference of 0.43, while the non-AKI patients had a mean difference of 0.15. These results suggest that the 10 patients who developed AKI experienced a significant mean difference of 0.43, classifying them as Stage 1 AKI. Similarly, the initial eGFR levels of the patients were within the normal or non-elevated range. But after the administration of NSAIDs, there was a significant decrease in eGFR for both AKI and non-AKI patients. Specifically, the AKI patients showed a mean difference of 35.93, while the non-AKI patients showed a mean difference of 16.6. This significant decrease in eGFR was observed in the 10 patients who developed AKI. This result is consistent with a previous report.11–13
CONCLUSION
The study examined the risk factors and impact of NSAIDs in AKI development. It was concluded that older patients, especially those aged 45-65, with co-morbidities like hypertension and diabetes, were at higher risk. Females also had a greater likelihood of AKI. Ketorolac and Acelofenac were the most common NSAID associated with AKI. Stage 1 AKI, characterized by a rise in serum creatinine level, was reversible upon NSAID discontinuation. Caution and monitoring are essential when prescribing NSAIDs, particularly for vulnerable patient groups.
Cite this article
Maheshwari RA, Gulati NR, Prajapati PJ, Vekariya NP, Joshi H, Hadia R, et al. An Observational Prospective Study to Find Out the Incidence of Acute Kidney Injury who are Taking Non-Steroidal Anti-inflammatory Drugs. J Young Pharm. 2023;15(4):711-5.
ACKNOWLEDGEMENT
We are sincerely thankful to Sumandeep Vidyapeeth (Deemed to be University) for providing support to carry out the study.
ABBREVIATIONS
| AKI | Acute Kidney Injury |
|---|---|
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| KDIGO | Kidney Disease Improving Global Outcomes |
| CKD | Chronic Kidney Disease |
| eGFR | Estimated Glomerular Filtration Rate |
References
- Zhang X, Donnan PT, Bell S, Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2017;18(1):256 [PubMed] | [CrossRef] | [Google Scholar]
- Lucas GNC, Leitão ACC, Alencar RL, Xavier RMF, Daher EF, da Silva Junior GBD, et al. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. J Bras Nefrol. 2019;41(1):124-30. [PubMed] | [CrossRef] | [Google Scholar]
- [last accessed on Oct 19 2022];Acute kidney failure [online]. Mayoclinic.org. [PubMed] | [CrossRef] | [Google Scholar]
- Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37(2):85-98. [PubMed] | [Google Scholar]
- Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457-68. [PubMed] | [CrossRef] | [Google Scholar]
- Taber SS, Pasko DA. The epidemiology of drug-induced disorders: the kidney. Expert Opin Drug Saf. 2008;7(6):679-90. [PubMed] | [CrossRef] | [Google Scholar]
- Collaborative S. Perioperative nonsteroidal anti-inflammatory drugs (NSAID) administration and acute kidney injury (AKI) in major gastrointestinal surgery: A prospective, multicenter, propensity matched cohort study: A prospective, multicenter, propensity matched cohort study. Ann Surg. 2020 [PubMed] | [CrossRef] | [Google Scholar]
- Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA, et al. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13(1):101 [PubMed] | [CrossRef] | [Google Scholar]
- Nash DM, Markle-Reid M, Brimble KS, McArthur E, Roshanov PS, Fink JC, et al. Nonsteroidal anti-inflammatory drug use and risk of acute kidney injury and hyperkalemia in older adults: a population-based study. Nephrol Dial Transplant. 2019;34(7):1145-54. [PubMed] | [CrossRef] | [Google Scholar]
- Morrison A, Ramey DR, van Adelsberg J, Watson DJ. Systematic review of trials of the effect of continued use of oral non-selective NSAIDs on blood pressure and hypertension. Curr Med Res Opin. 2007;23(10):2395-404. [PubMed] | [CrossRef] | [Google Scholar]
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284(10):1247-55. [PubMed] | [CrossRef] | [Google Scholar]
- Catella-Lawson F, McAdam B, Morrison BW, Kapoor S, Kujubu D, Antes L, et al. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289(2):735-41. [PubMed] | [Google Scholar]
- Whelton A, Schulman G, Wallemark C, Drower EJ, Isakson PC, Verburg KM, et al. Effects of celecoxib and naproxen on renal function in the elderly. Arch Intern Med. 2000;160(10):1465-70. [PubMed] | [CrossRef] | [Google Scholar]
