ABSTRACT
Background
Favipiravir has a low bioavailability, a fast elimination molecule, and severe gastrointestinal side effects. The bioavailability followed by therapeutic efficacy could be improving by sustained release formulation such as nanoparticle. The anti-viral drug favipiravir is made of nanoparticle matrixes through solvent evaporation.
Materials and Methods
In this study, the Box-Behnken design with three factors and three levels was heavily utilised to optimise parameters like concentration of polymer (A), sonication frequency (B), and time of sonication (C). Particle size, practical yield, and entrapment effectiveness were three dependent variables that were measured as responses. The independent as well as dependent variables were related using mathematical equations and response surface plots.
Results
The designed model formula has a particle size of 343.14 nm, entrapment efficiency of 83.74 percent, and a practical yield of 85.39 percent with respective A, B, and C levels of 750, 37.5, and 40. The results of the observed responses were very similar to those expected by the process that had been optimised. Morphological analysis, and In vitro release study was used to characterise the prepared nanoparticle. Until 24 hr later, the prepared nanoparticle demonstrated good sustained drug release.
Conclusion
The factorial design allows large-scale favipiravir nanoparticle synthesis with homogeneous particle size distribution, excellent entrapment efficiency, and practical yield.
INTRODUCTION
Research and its application in the area of nanoscience and nanotechnology have grown at an unprecedented rate in recent time. There is forecasting view that the utilization of nanotechnology in medicine will result in significant improvements in diseases diagnosis and treatment. Nanoparticles are incredibly tiny particles that act as a single entity when it comes to their properties and modes of transport.1 It’s mainly applicable for the separation and purification of biological molecules and cells,1 MRI contrast enhancement,2 drug and gene delivery,3 tissue engineering,4 tumour destruction,5 and bio-detection of pathogens and proteins6,7 are just a few of the many applications for nanoparticle research, which is currently the most studied branch of science.8 Due to characteristics like size, surface characteristics, and ease of adaptability for both active and passive targeting, nanoparticles are widely used in drug delivery systems. These particles can be employed for both targeted release and controlled drug release during transportation. By changing the distribution and clearance of the drug, they can improve therapeutic efficacy while significantly reducing side effects.9
Favipiravir (FAVI), which has recently been accepted for use in the treatment of COVID-19, has been approved by Japan for use in the treatment of viral infections like influenza and Ebola. Notably, it suppresses the viral RNA-dependent polymerase enzyme, preventing viral replication. Recent research reports claim that FAVI revealed antiviral activity for RNA viruses to a greater extent than other antiviral medications. FAVI is widely available as an oral tablet under the brand names10 Araflu, Fabiflu, and Avigan. The recommended dose of FAVI is 1800 mg bid on day one and 800 mg bid every two to fourteen days in many nations, including India. High patient non-adherence has been linked to high toxicity and unfavourable effects.11 The Biopharmaceutical Classification System (BCS) classifies FAV as a class III drug with a shorter half-life of 2-5.5 hr. Therefore, novel drug delivery would be required to decrease the dosing frequency and dose size.
In this study, (Poly-Methyl-Metha-Acrylate) PMMA was used as a biodegradable polymer to prepare FAVI nanoparticles. FAVI has a low bioavailability, a fast elimination molecule, and severe gastrointestinal side effects. A sustained release formulation, like a nanoparticle, could increase bioavailability and therapeutic efficacy. In this study, the Box-Behnken model was used to create FAVI nanoparticles and find the best formula.
MATERIALS AND METHODS
The chemicals FAVI compound, PMMA (Poly-Methyl-Metha-Acrylate), Poloxamer 407, and ethanol were procured from Sigma Aldrich, India. The chemicals and solvents employed in the study were of analytical grade.
Optimization of FAVI nanoparticles
The Box-Behnken design allowed for a much comprehensive analysis of the relationship between the independent as well as dependent variables. By varying the levels of polymer concentration, Sonication frequency, and time.12 This experimental design provided valuable insights into optimising the formulation process for achieving desired outcomes in terms of particle size, practical yield, and Entrapment Efficiency (EE). The concentration of polymer varied from 300-1200 mg, the sonication frequency ranged from 20 kHz to 60 kHz, and the time of sonication varied from 15 min to 60 min. The results showed that increasing the concentration of polymer led to larger particle sizes but improved entrapment efficiency and practical yield. Higher sonication frequencies resulted in smaller particle sizes, while longer sonication times further reduced particle sizes but had diminishing effects on entrapment efficiency and practical yield. This experimental design provided valuable insights into optimizing the formulation process for achieving desired outcomes in terms of particle size, practical yield, and entrapment efficiency. The concentration of polymer varied from 300-1200 mg, the sonication frequency ranged from 20 kHz to 60 kHz, and the time of sonication varied from 15 min to 60 min. The results showed that increasing the concentration of polymer led to larger particle sizes but improved entrapment efficiency and practical yield. Higher sonication frequencies resulted in smaller particle sizes, while longer sonication times further reduced particle sizes but had diminishing effects on EE and practical yield.
In the first stage, initial experiments were conducted to identify the primary components and ascertain the suitable ranges within which the optimal conditions are situated. The study examined the impact of three variables, namely the concentration of polymer, sonication frequency and time of sonication, on the size, EE, and practical yield. Based on initial screening, it was determined that the concentration of polymer, sonication frequency, and time of sonication were the key variables in the particular range of 300-1200 mg, 20-60 kHz, and 15-60 min, respectively. A 3-level, 3-factor Box-Behnken design13 was utilized in this work to investigate the impact of independent factors (particle size, practical yield, and entrapment efficiency) on each dependent variable, based on the findings from the preliminary trials. This particular design has the potential to be well-suited for the purpose of investigating quadratic response surfaces and creating models based on second-order polynomials. The design consists of replicated core points and midpoints of edges of the multi-dimensional cube that define the area of focus. The study was carried out according to the established design, and the outcome values for the dependent variables were documented in Table 1. The analysis of the surfaces of response variables within the study domain was performed by using Stat-Ease Design Expert software version V9.0.1.
| Factor 1 | Factor 2 | Factor 3 | Response Y1 | Response Y2 | Response Y3 | |
|---|---|---|---|---|---|---|
| Run | A: Polymer | B: Sonication time | C: Sonication Frequency | Particle size | Entrapment efficiency | Practical yield |
| mg | min | kHz | nm | % | % | |
| 1 | 750 | 37.5 | 40 | 352.12 | 86.42 | 86.3 |
| 2 | 750 | 15 | 20 | 354.24 | 84.22 | 86.4 |
| 3 | 750 | 60 | 20 | 350.4 | 86.06 | 86.6 |
| 4 | 300 | 15 | 40 | 356.62 | 86.42 | 88.64 |
| 5 | 300 | 37.5 | 60 | 356.46 | 78.62 | 88.12 |
| 6 | 300 | 60 | 40 | 358.26 | 89.48 | 88.64 |
| 7 | 1200 | 37.5 | 20 | 367.34 | 86.34 | 78.6 |
| 8 | 300 | 37.5 | 20 | 352.42 | 72.64 | 88.24 |
| 9 | 750 | 15 | 60 | 351.24 | 80.64 | 86.24 |
| 10 | 1200 | 60 | 40 | 360.12 | 82.64 | 72.4 |
| 11 | 750 | 37.5 | 40 | 352.46 | 86.04 | 86.82 |
| 12 | 750 | 37.5 | 40 | 354.22 | 86.4 | 86.42 |
| 13 | 750 | 37.5 | 40 | 356.42 | 82.54 | 81.25 |
| 14 | 1200 | 15 | 40 | 261.42 | 84.64 | 85.02 |
| 15 | 1200 | 37.5 | 60 | 251.4 | 85.06 | 88.32 |
| 16 | 750 | 37.5 | 40 | 356.12 | 86.32 | 87.02 |
| 17 | 750 | 37.5 | 40 | 342.14 | 83.62 | 86.74 |
Preparation of nanoparticle
FAVI nanoparticles were made through solvent evaporation.14 FAVI and PMMA were dissolved in ethanol and dichloromethane, respectively. Then the FAVI-PMMA combination was dropwise added to a 2% w/v poloxamer 407 solution as a quasiemulsifier in an Ultrasonic Probe Sonicator (Qsonica, USA) at 20-60 kHz with varying time intervals. Add 200 mL of water and stir with a magnetic stirrer for 12 hr to complete precipitation. After removing organic solvent with a Roto-evaporator (Heidolph Instruments, Germany), the nanoparticle suspension was freeze-dried at 20°C to make a dry powder.
Characterization of prepared nanoparticles
Particle size
A Zetasizer 300 HS (Malvern Instruments, UK) was used to measure the particle size of the nanoparticle. The samples were diluted and placed in the sample holder. The auto-correlation function of the intensity of light scattered was used to compute the diameter of the nanoparticle. The measurements were made in triplicate.
Entrapment efficiency (%)
Entrapment efficiency was measured by measuring the amount of drug entrapped in the nanoparticle matrix. The FAVI samples were analysed by the (High Performance Liquid Chromatography) HPLC (Shimadzu India Pvt. Ltd., India) method at 225 nm,15 which was obtained after ultracentrifugation of nanoparticle suspensions. The following formula was used to compute the EE (%):
Where, W1 is quantity of entrapped drugs, W2 is the total quantity of drugs used.
Morphology of nanoparticles
The Scanning Electron Microscope (SEM) was used to examine the morphology of FAVI nanoparticles. A small amount of nanoparticles were dispersed on a metal stub, which was then coated with gold using a Hitachi 1010 ion sputter and magnified using a Hitachi 3000 N scanning electron microscope (JSM 5610 LV SEM, JEOL, Japan). The photos were taken at a 20 kV acceleration voltage and a chamber pressure of 0.6 mmHg.
In vitro release study
In vitro drug release of particle was determined by using cellulose Dialysis tube16 (Himedia Laboratory, India). Nanoparticle (drug equivalent of 2 mg) was mixed in 5 mL of water and it loaded into Dialysis tube composed of cellulose (Molecular weight cut-off 35 kDa). Then the Dialysis tube was immersed in 300 mL dissolution medium at 37°C under a magnetic stirring of 75 rpm. An adequate volume of the samples was eluted at different time interval and replaced with same volume of the fresh medium. The eluted sample was centrifuged and the supernatant containing drug was estimated by using HPLC technique.
RESULTS
Optimization of formulation process variables
The solvent evaporation method is a commonly employed methodology for the synthesis of nanoparticles. This method offers several potential benefits in the synthesis of biodegradable materials, such as accelerated nanoparticle formation, enhanced selectivity of the resulting products, a narrow distribution of particle sizes, and a high yield. This work presents the initial successful achievement in the synthesis of nanoparticles of Favipiravir. Based on initial inquiries, it has been determined that the concentration of polymer (A), the frequency of sonication (B), and the duration of sonication (C) are the primary factors that significantly impact the size of particles, the practical yield, and the efficiency of entrapment.
The implementation of the surface response methodology works on the Box-Behnken design necessitated the execution of seventeen tests. The factor combinations in the experimental design resulted in varying responses, as shown in Table 1. The obtained results provide unambiguous evidence of a highly dependence with the dependent variables and the chosen independent variables, as demonstrated by the significant variation seen throughout the 17 batches. The data was analysis using Design Expert software V9.0.1 in order to provide an ANOVA result, regression equation and coefficients. The mathematical link between the researched variables is expressed by multiple linear regression data. The equations presented above depict the quantitative relationship between the variables of concentration of polymer (A), sonication frequency (B), and time of sonication (C), as well as their combined effects on particle size (Y1), EE (Y2), and practical yield (Y3). The coefficients values of A, B, and C are associated with the impact of the variables on the dependent variables Y1, Y2, and Y3. Interaction values are shown by coefficients that have more than one factor, while coefficients with higher order value indicate a quadratic relationship. A positive symbol signifies the presence of a complementary impact, where the negative symbol denotes the existence of an antagonistic effect. The researchers employed a backward elimination approach is to fit the data into the quadratic model. The statistical significance of all polynomial equations was determined using ANOVA (Tables 2–4) with a significance level of p<0.014, as given by the software. The mathematical models produced to estimate the Y1 was showed as statistically significant, which was indicated by a value (F) of 4.82 (p<0.0005) and a high R2 of 0.9942. The particle size is significantly influenced by all the independent variables and quadratic terms of AB, AC, and BC. This is evident from the P-values, which are less than 0.05, indicating the statistical significance were showed in Table 3. The findings of the analysis demonstrate that the impact of variable A could be more pronounced in comparison to variables B and C.
| Sources of variations | Sum of squares | d f | Mean square | F value | p -Prob value>F | R R |
|---|---|---|---|---|---|---|
| Model | 13019.88 | 6 | 2169.98 | 4.82 | 0.0145 | Significant |
| A-Pol·ymer | 4208.11 | 1 | 4208.11 | 9.35 | 0.0121 | |
| B-Sonication time | 954.85 | 1 | 954.85 | 2.12 | 0.1759 | |
| C-Sonication Frequency | 1896.05 | 1 | 1896.05 | 4.21 | 0.0672 | |
| AB | 2355.16 | 1 | 2355.16 | 5.23 | 0.0452 | |
| AC | 3598.80 | 1 | 3598.80 | 8.00 | 0.0179 | |
| BC | 6.92 | 1 | 6.92 | 0.015 | 0.9038 | |
| Residual | 4499.96 | 10 | 450.00 | |||
| Lack of Fit | 4484.02 | 6 | 747.34 | 187.47 | < 0.0001 | Significant |
| Sources of variations | Sum of squares | d f | Mean square | F value | p-value Prob >F | R 2 |
|---|---|---|---|---|---|---|
| Model | 0.000 | 0 | ||||
| Residual | 241.76 | 16 | 15.11 | |||
| Lack of Fit | 230.38 | 12 | 19.20 | 6.75 | 0.0397 | significant |
| Sources of variations | Sum of squares | d f | Mean square | F value | p -value Prob >F | R R |
|---|---|---|---|---|---|---|
| Model | 136.54 | 3 | 45.51 | 3.88 | 0.0350 | Significant |
| A-Polymer | 107.31 | 1 | 107.31 | 9.15 | 0.0098 | |
| B-Sonication time | 17.76 | 1 | 17.76 | 1.51 | 0.2403 | |
| C-Sonication Frequency | 11.47 | 1 | 11.47 | 0.98 | 0.3407 | |
| Residual | 152.48 | 13 | 11.73 | |||
| Lack of Fit | 128.90 | 9 | 14.32 | 2.43 | 0.2037 | Significant |
The perturbation and 3D response of plots were employed to provide a more comprehensive understanding of the impact of both the primary as well as interacting impacts of the independent variables on the size. Figure 1 displays the perturbation plot, illustrating the primary effects of variables on the particle size (Y1) of nanoparticles. The shown Figure 1 demonstrates that variable A exerts the primary and significant influence on Y1, whereas variable B exhibits a moderate impact on Y1. Subsequently, variable C is observed to have a less effect on Y1. The association between the independent and dependent variables was further clarified through the utilization of response surface plots. Figures 2 and 3 depict the interaction influence of variables A and B on the size (Y1) while maintaining a constant level of variable C. At lower concentrations of A (polymer), the value of Y1 exhibits an increase from 352 nm to 358 nm. Likewise, when A is elevated, the value of Y1 exhibits an increase from 251 nm to 367 nm. The particle size study of the nanoparticles yielded measurements in the range to 251-367 nm, as presented in Table 2. The equation used to determine particle size demonstrated a strong correlation coefficient of 1.000, indicating a high degree of association between the variables. Additionally, the model F-value of 187.47 suggests that the model is statistically significant, further supporting its validity. Model terms are considered important when the data of “Prob>F” are >0.0500. The findings of the analysis demonstrate that the influence of variable C, specifically the sonication frequency, is more pronounced compared to variables A and B. All the three variables exhibit a negative impact on particle size, indicating an inverse relationship between the factors and response. The impact of both the primary and interacting impacts of the independent factors on the size was further clarified through the utilization of three-dimensional response and perturbation plots.
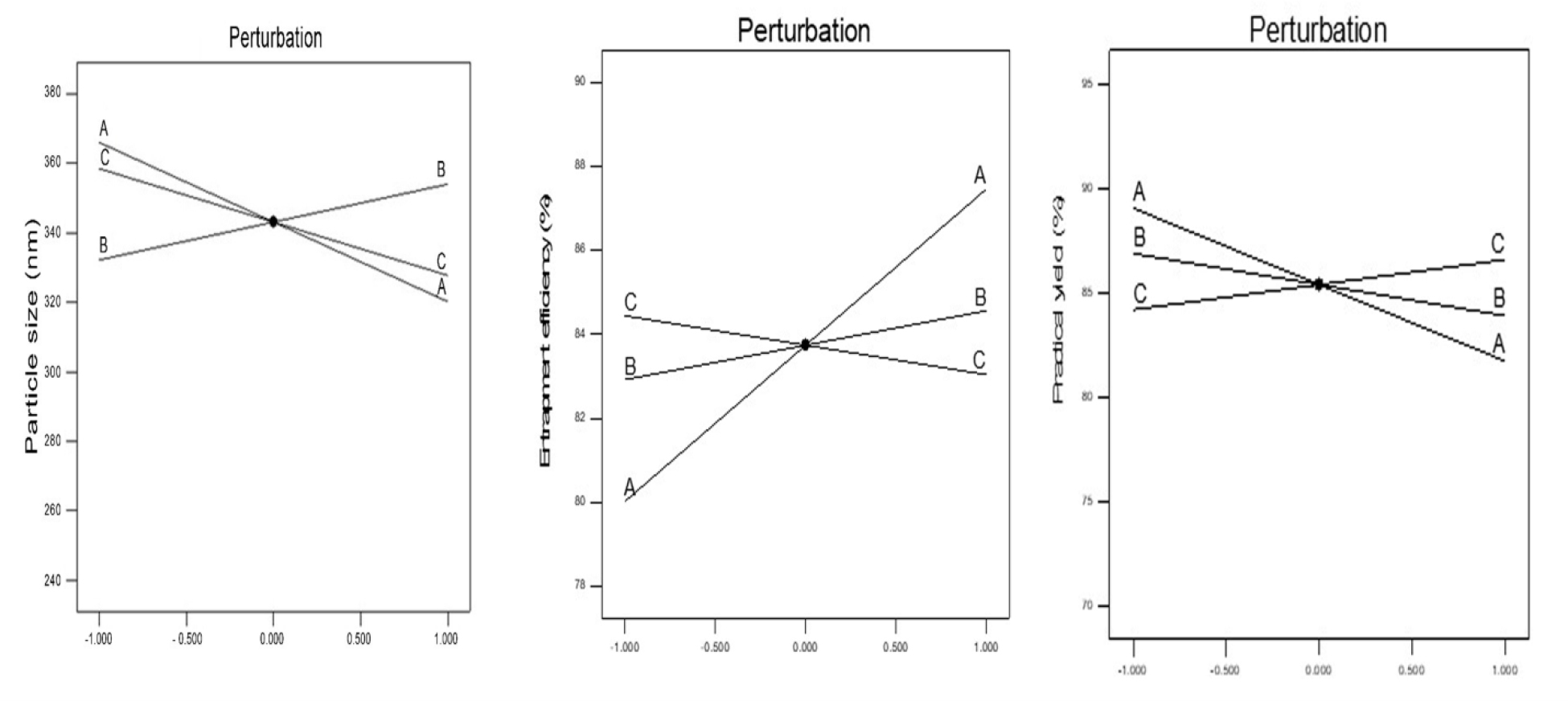
Figure 1:
Perturbation plots showing the main effect of polymer concentration (A), sonication frequency (B) and sonication time(C) on particle size (Y1), entrapment efficiency (Y2) and practical yield (Y3).
The figure presented in Figure 1 illustrates the separate effects of all the variables on the EE. The analysis reveals that there are interacting effects among all the factors in relation to the response variable Y2. Figure 2 and Figure 3 present the 2D contour plots and 3D response surfaces respectively, which demonstrate how independent variables interact with the response variable Y2. One variable was held constant in these numbers, while the other two were changed within a defined range. The contour plots and response surfaces provide insight into the characteristics and magnitude of the interaction among many elements. At lower concentrations of A, the reduction in Y2 was seen to be from 89% to 72%. In a similar vein, it was shown that at elevated doses of A, the reduction in Y2 was measured to be 86% to 82%. Figure 1 illustrates gives the relationship between variables A as well as C on practical yield, specifically at a constant level of C. Similarly, Figures 2 and 3 depict the relationship between variables A as well as B on practical yield, but at a constant level of B. At lower concentrations of A, the percentage of Y3 decreased to 88.12% from 88.64%. In a similar vein, it was shown that at elevated levels of variable A, the percentage of Y3 decreased from 88% to 78%. Once the polynomial equations that establish the relationship between the independent and dependent variables were derived, the procedure was subsequently refined to enhance the accuracy and efficiency of the obtained responses. The utilization of the desirability approach in numerical optimization was employed to identify the optimal configurations of the process variables in order to get the desired results. The conditions were optimized through the imposition of constraints on both the dependent and independent variables. A procedure of optimization was used to identify the best quantities of variables A, B, and C that would maximize the values of Y1, Y2, and Y3.
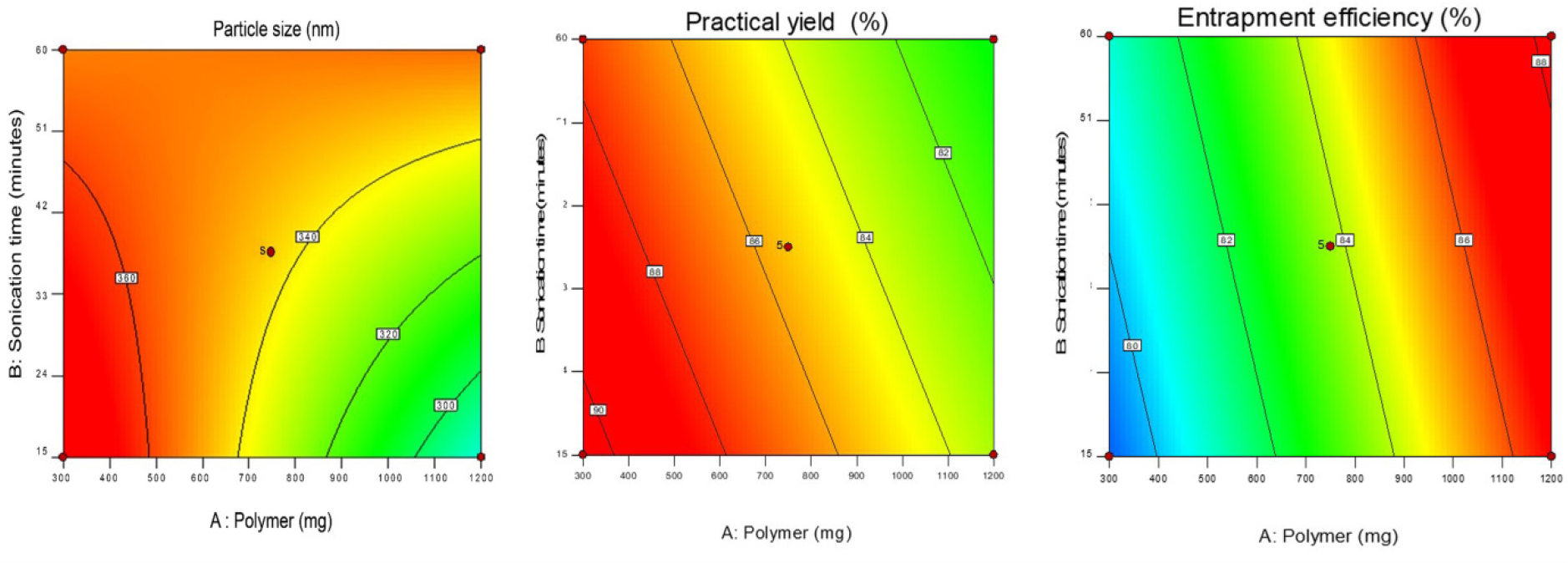
Figure 2:
Factorial contour Response surface plot presenting the interaction between the polymer concentration (A), sonication frequency (B) and sonication time(C) on particle size (Y1), entrapment efficiency (Y2) and practical yield (Y3).
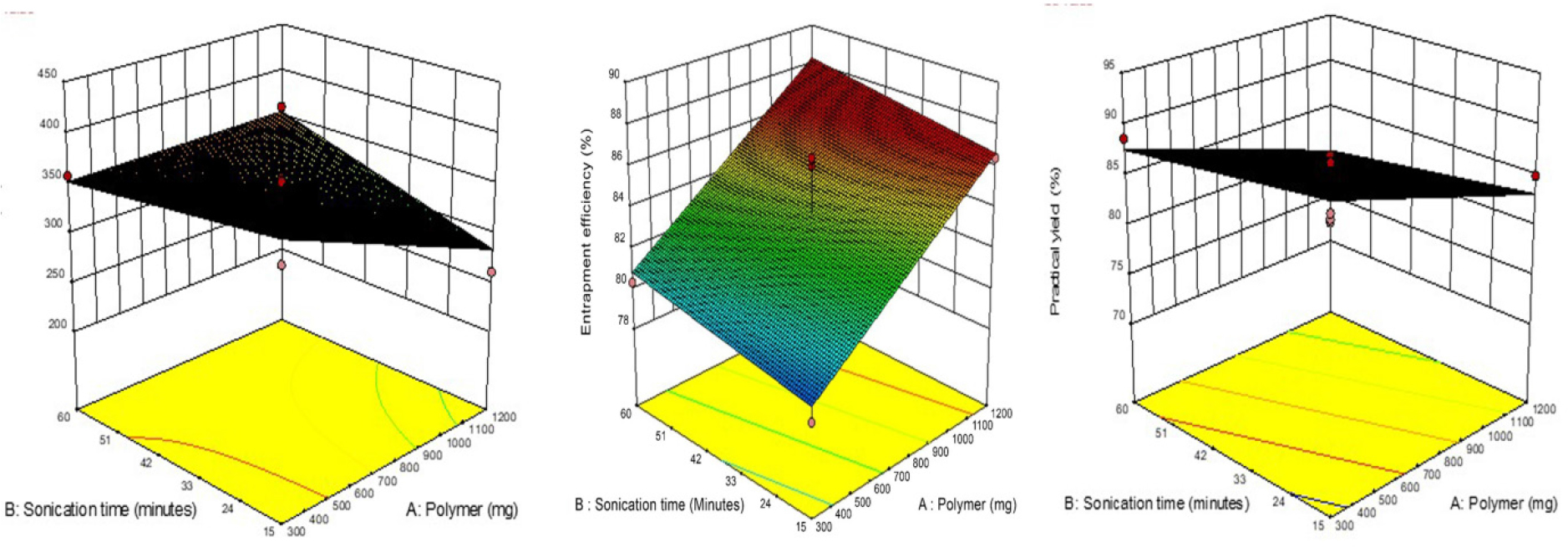
Figure 3:
Response surface plot presenting the interaction between the polymer concentration (A), sonication frequency (B) and sonication time (C) on particle size (Y1), entrapment efficiency (Y2) and practical yield (Y3).
The optimal formulation of FAVI nanoparticle was discovered based on factorial model. Finally, the optimized formula exhibited improved size, EE, and practical yield, specifically measuring 343 nm, 83%, and 85% correspondingly. In order to validate these values, three batches of nanoparticles were synthesized separately, in accordance with the anticipated levels of A, B, and C. The acquired Y1, Y2, and Y3 values exhibited a high level of concordance with the projected values. The findings of this investigation indicate the efficacy of an optimized approach in properly calculating the operational parameters required for manufacturing of FAVI nanoparticle.
Characterization of FAVI nanoparticles
The preparation of FAVI nanoparticles was conducted using the solvent evaporation method, whereby PMMA was employed as the biodegradable polymer. The nanoparticles in the improved formulation exhibited a smooth and spherical morphology, as depicted in Figure 4. The nanoparticle formulation that was optimized was utilized for an invitro drug release using pH 7.4 phosphate buffers. The first medication release was abrupt, then transitioning to a continuous release. The medication exhibited an initial release of 2.8% after 30 min, followed by a steady release of 73% at the 24-hr mark.

Figure 4:
SEM image of Favipiravir nanoparticle.
DISCUSSION
The utilization of Design of Experiments (DOE) has proven to be an effective approach in reducing process variance and achieving a significant product yield with a consistent distribution of particle size. The Box-Behnken design was employed, alongside other methodologies, to optimize and analysis the primary,17 interaction and quadratic effects of process variables on size, EE and practical yield. The aforementioned architectural design is well-suited for the purpose of examining quadratic surfaces response and build models based on 2nd order polynomial equations. The design composed of a series of centrally located points, as well as a cluster of points denoted at the mid-points of every edge of the multidimensional cube. The aforementioned designs exhibit the property of being rotational and require three levels for each factor.
The preparation of FAVI nanoparticles was conducted using the solvent evaporation method, whereby PMMA was employed as the biodegradable polymer. This method is relatively simpler to prepare in comparison to other methods. The preparation of a polymer and drug suspension involved the dissolution of both components in an organic solvent. The organic phase was introduced into an aqueous poloxamer solution. The organic solvent employed in this procedure exhibited a fast-partitioning behaviour into the surrounding aqueous phase, leading to the precipitation of the polymer on surface of the drug particles.18 The subsequent volatilization of the confined solvent led to the formation of polymeric nanoparticles.
The production of these shapes was achieved through the complete saturation of polymer and the low rate of solvent diffusion. This resulted in the creation of smooth, spherical particles that were uniformly dispersed. Furthermore, the particles displayed a uniform surface texture, and all traces of the solvent were completely eliminated from the nanoparticles.19
The prompt and efficient release of the medication was helped by the adhesion of drug particles to the surface, whereas the prolonged release was ascribed to the progressive release from a polymeric matrix.20
CONCLUSION
The FAVI nanoparticles procedure was optimized by 3-level factorial design. From preliminary investigations, polymer concentration, sonication frequency, and time of sonication were the most important variables affecting particle size, EE, and practical yield. These elements’ quantitative effects at various levels were anticipated by polynomial equations. Response surface model was used to anticipate the optimal levels of these elements to produce nanoparticles with uniform in size, high EE, and practical yield. According to these adjusted levels, one nanoparticle formulation was made. The optimized process for FAVI nanoparticle manufacturing was feasible because observed responses matched predicted values. The factorial design allows large-scale FAVI nanoparticle synthesis with homogeneous particle size distribution, excellent entrapment efficiency, and practical yield.
Cite this article
Venugopal V, Meenachisundram SG, Ramadoss K, Jayaprakash N. Quality by Design and Optimization of Favipiravir Nanoparticles Using 3-Level Factorial Design by Box-Behnken Method. J Young Pharm. 2023;15(4):696-703.
ACKNOWLEDGEMENT
This research work was supported by intramural research grant under seed money grant (SBV/ IRC /SEED MONEY/114/2022) of Sri Balaji Vidyapeeth (Deemed to be University) Puducherry, India and the authors are declared that they have no conflict of interest.
ABBREVIATIONS
| FAVI | Favipiravir |
|---|---|
| PMMA | Poly-Methyl-Metha-Acrylate |
| EE | Entrapment efficiency |
| SEM | Scanning electron microscope |
| BCS | Biopharmaceutical Classification System |
| HPLC | High Performance Liquid Chromatography |
References
- Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond). 2016;11(6):673-92. [PubMed] | [CrossRef] | [Google Scholar]
- Gao J, Gu H, Xu B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc Chem Res. 2009;42(8):1097-107. [PubMed] | [CrossRef] | [Google Scholar]
- Hadjipanayis CG, Bonder MJ, Balakrishnan S, Wang X, Mao H, Hadjipanayis GC, et al. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small. 2008;4(11):1925-9. [PubMed] | [CrossRef] | [Google Scholar]
- Kim K, Fisher JP. Nanoparticle technology in bone tissue engineering. J Drug Target. 2007;15(4):241-52. [PubMed] | [CrossRef] | [Google Scholar]
- Camerin M, Moreno M, Marín MJ, Schofield CL, Chambrier I, Cook MJ, et al. Delivery of a hydrophobic phthalocyanine photosensitizer using pegylated gold nanoparticle conjugates for the in vivo photodynamic therapy of amelanotic melanoma. Photochem Photobiol Sci. 2016;15(5):618-25. [PubMed] | [CrossRef] | [Google Scholar]
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329-47. [PubMed] | [CrossRef] | [Google Scholar]
- Salata OV. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):3 [PubMed] | [CrossRef] | [Google Scholar]
- Saini R, Saini S, Sharma S. Nanotechnology: the future medicine. J Cutan Aesthet Surg. 2010;3(1):32-3. [PubMed] | [CrossRef] | [Google Scholar]
- Mishra D, Hubenak JR, Mathur AB. Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy. J Biomed Mater Res A. 2013;101(12):3646-60. [PubMed] | [CrossRef] | [Google Scholar]
- Vora A, Tiwaskar M. Favipiravir. J Assoc Physicians India. 2020;68(8):91-2. [PubMed] | [Google Scholar]
- Naydenova K, Muir KW, Wu LF, Zhang Z, Coscia F, Peet MJ, et al. Structure of the SARS-CoV-2 RNA-dependent RNA polymerase in the presence of favipiravir-RTP. Proc Natl Acad Sci U S A. 2021;118(7):e2021946118 [PubMed] | [CrossRef] | [Google Scholar]
- Venugopal V, Kumar KJ, Muralidharan S, Parasuraman S, Raj PV, Kumar KV, et al. Optimization and in vivo evaluation of isradipine nanoparticles using Box-Behnken design surface response methodology. OpenNano. 2016;1:1-15. [CrossRef] | [Google Scholar]
- Gidwani B, Vyas A. Preparation, characterization, and optimization of altretamine-loaded solid lipid nanoparticles using Box-Behnken design and response surface methodology. Artif Cells Nanomed Biotechnol. 2016;44(2):571-80. [PubMed] | [CrossRef] | [Google Scholar]
- Vaculikova E, Placha D, Pisarcik M, Peikertova P, Dedkova K, Devinsky F, et al. Preparation of risedronate nanoparticles by solvent evaporation technique. Molecules. 2014;19(11):17848-61. [PubMed] | [CrossRef] | [Google Scholar]
- Duse PV, Baheti KG. Bioanalytical method development and validation for the determination of favipiravir in spiked human plasma by using RP-HPLC. J Pharm Res Int. 2021;33:275-81. [PubMed] | [CrossRef] | [Google Scholar]
- Braga GK, Oliveira WP. Manufacturing Drug Loaded chitosan microspheres by spray drying: development, characterization, and potential use in dentistry. Dry Technol. 2007;25(2):303-10. [CrossRef] | [Google Scholar]
- Hao J, Fang X, Zhou Y, Wang J, Guo F, Li F, et al. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int J Nanomedicine. 2011;6:683-92. [PubMed] | [CrossRef] | [Google Scholar]
- Takeuchi I, Hida Y, Makino K. Minoxidil-encapsulated poly (L-lactide-co-glycolide) nanoparticles with hair follicle delivery properties prepared using W/O/W solvent evaporation and sonication. Bio Med Mater Eng. 2018;29(2):217-28. [PubMed] | [CrossRef] | [Google Scholar]
- Kietzke T, Neher D, Landfester K, Montenegro R, Güntner R, Scherf U, et al. Novel approaches to polymer blends based on polymer nanoparticles. Nat Mater. 2003;2(6):408-12. [PubMed] | [CrossRef] | [Google Scholar]
- Oh Y, Kim SH. Hydrogel-shelled biodegradable microspheres for sustained release of encapsulants. J Polym Sci. 2022;60(11):1700-9. [CrossRef] | [Google Scholar]
