ABSTRACT
Background
Analytical Quality by Design (AQbD) enhances method robustness more effectively than traditional methods. Risk assessment and factor screening studies enabled to detection of analytical method parameters. The present study describes an AQbD enabled stability indicating RP-HPLC method for simultaneous determination of the phytochemical markers, Chlorogenic acid and Berberine in standard and clear stone drops, a Homeopathic marketed formulation. Currently no published methods reported based on stability indicating RP-HPLC technique for determination of these markers in any herbal formulations.
Materials and Methods
Two factors at three levels were considered using Central Composite Design for the method optimization. Statistical and graphical analyses were employed to assess the individual and combined interaction effects of critical method parameters on critical method responses.
Results
The optimized mobile phase was Methanol: 0.1% Formic acid (41.67:58.33% v/v) with 1.1 mL/min rate of flow, at 343 nm. Chlorogenic acid was eluted at 3.534 min and Berberine was eluted at 5.140 min respectively with 8 min run time. The method was validated as per ICH Q2 R1 guidelines and performed stress degradation studies; all validation parameters were within acceptable limits.
Conclusion
The established HPLC technique was useful as quality control tool for assessment of phytochemical markers in various traditional medicines and successfully applied to determine Chlorogenic acid and Berberine in Marketed Homeopathic formulation.
INTRODUCTION
Herbal products are widely used in traditional medical systems (Parasuramanet al., 2014) and are considered as complementary and alternative medicines around globally. Despite the availability of allopathic medicines, herbal formulations gained popularity due to their lower toxicity and minimal side effects (Siddique and Sarwat, 2022). Clear stone drops, is a polyherbal formulation widely used for Urolithiasis treatment in Homeopathy. Herbal formulations are extensively marketed worldwide, but the lack of stringent regulations and standardization often compromises patient safety and product efficacy. Hence, World Health Organization established policies and protocols for assessing the quality and safety of herbal medicines (Wanget al., 2023).
Marker-based quality control testing is one of the quality assessment methods for herbal medicines (Kushwahaet al., 2010) Chlorogenic acid and Berberine are the major active phytochemical markers present in Berberis vulgaris, one of the key component of Clear stone drops and also Chlorogenic acid is present in most of the herbs in Clear stone drops. Chlorogenic acid (Figure 1A) is a poly phenolic compound (Santana-Galvez et al., 2017), rich in various herbs, known for its antioxidant, anti-inflammatory, antimicrobial and reno protective properties (Miaoand Xiang, 2020; Fauziet al., 2024; Nguyenet al., 2024). Berberine (Figure 1B) is a quaternary isoquinoline alkaloid employed in several traditional systems for its antidiabetic, antiarrhythmic, antihypertensive, antiobesity, anticancer, anti-hyperlipidemia, anti-inflammatory and anti-microbial and anti-lithogenic properties (Neaget al., 2018; Ochet al., 2020).
Conventional HPLC method development is often trial-and-error-based, while the Analytical Quality by Design (AQbD) offers holistic approach (Tomeet al., 2019) uses statistical tools and Design of Experiments (DOE) for more efficient, cost-effective and robust method optimization (Daset al., 2017). AQbD ensures regulatory compliance, enhances method reliability and reduces development costs (Parket al., 2022). There are no reported methods for determining the stability of these phytochemical markers in a combined formulation in standards as well as in herbal formulations. A few HPLC techniques for the analysis of Chlorogenic acid and Berberine individually or in association with other phytochemicals have been reported. However, the current phytochemicals in the reported methods had a higher amount of organic phase (Atlabachewet al., 2021; Soudagaret al., 2023), more retention time (Chaudhary and Patel 2020; Chaowuttikulet al., 2020; Atlabachewet al., 2021; Soudagaret al., 2023) and were less sensitive ((Chaudhary and Patel 2020; Chaowuttikulet al., 2020; Atlabachewet al., 2021; Wanget al., 2013; Sahani and Jain, 2019; Soudagaret al., 2023). Stability-enabled AQbD based HPLC approach ensures that the methods are consistent, reliable and revalidation is not required. Hence, the present research aims to establish a stability evaluating, simple, accurate, precise, robust and cost-effective RP-HPLC technique for simultaneous quantification in standards and also extended for the estimation of Chlorogenic acid and Berberine in Clear stone drops-a Homeopathic poly herbal formulation.
MATERIALS AND METHODS
Chemicals
Chlorogenic acid and Berberine standards were purchased from Yucca Phytochemicals Pvt. Ltd., Mumbai, India. Homeopathic polyherbal formulation (Clear stone drops) was bought from the local market in Tirupati.
Instrument
HPLC (Shimadzu Prominence, Japan) with a binary high-pressure gradient solvent delivery pump (LC-20AD) system with a UV detector (SPD-20A) and the software operated was Lab Solutions. Design expert software (Stat-Ease) trial version 23.1.1.0 was employed as Central Composite Design (CCD) for method optimization.
Preparation of working standard and sample solution
Stock solutions of Chlorogenic acid (1000 μg/mL) and Berberine (1000 μg/mL) were prepared separately by dissolving 10 mg of each standard in water and Methanol, respectively. Stock solution (100 μg/mL) was made by diluting 1 mL of each stock solution in a 10 mL volumetric flask with mobile phase. The working solution (1μg/mL) was prepared by transferring 0.1 mL of stock solution-II into a 10 mL flask with mobile phase.
10 mL of the sample (Clear stone drops) was extracted with petroleum ether and Methanol, followed by sonication, evaporation and reconstitution in Methanol. The working sample solution was prepared similarly to the standard solution. The extracted sample was then analyzed by HPLC to identify Chlorogenic acid and Berberine markers in clear stone drops.
Selection of Analytical wavelength
Prepared 1 μg/mL solutions of Chlorogenic acid and Berberine, scanned in a UV visible spectrophotometer by Overlaying the UV spectra, 343 nm was selected (Figure 2).
Chromatographic conditions for initial and final optimized method
The initial development was performed on Shiseido Spolar C18 (250 mmx4.6 mm, 5 μm) column employing a mobile phase, Methanol: 0.1% Formic acid (40:60%v/v), at 1 mL/min flow and detected using UV detector at 343 nm. The variables were carefully selected and their interactions were thoroughly explored using Design expert software and chromatographic conditions were optimized. The final method optimization was done using Shiseido spolar C18 (250mmx4.6 mm, 5 μm) column with mobile phase of Methanol: 0.1% Formic acid (41.67: 58.33%v/v) with 1.1 mL/min flow and eluted at 343 nm.
Method development using Design expert software
DOE software is typically used for enhancing method optimization by varying parameters to maximize knowledge, decreases trials, save time and money. The key step in AQbD method development is defining the Analytical Target Profile (ATP). For the present work, the ATP is to establish a more robust RP-HPLC technique that lacks the need for revalidation to quantify Chlorogenic acid and Berberine with ideal system suitability and less analysis time. Percentage of organic solvent (%Methanol v/v) in the mobile phase (A, % v/v) and flow rate (B) were considered as factors or Critical Method parameters (CMPs, Table 1). The Critical Method Responses (CMRs), selected were Plate count of Chlorogenic acid (PC), Tailing Factor (TF) of Chlorogenic acid and Retention Time (RT) of Berberine. Thirteen sets of experimental trials were constructed using two factors, three-level CCD (Table 2). After conducting the 13 experimental trials, the collected data was studied utilizing statistical regression to establish the relation between variables and to determine the Method Operable Design Region (MODR). Analysis of Variance (ANOVA) was employed to evaluate the significant impact of the selected CMPs on the selected CMRs. Contour plots, Perturbation plots, normal plots of residuals and 3D surface plots depict the interaction impact of CMPs and CMRs. The MODR was then utilized to predict optimal chromatographic parameters based on the goals, with the variables being optimized using the response surface method.
| Factor | Name | Minimum | Maximum | Coded Low | Coded High | Mean | Std. Dev. |
|---|---|---|---|---|---|---|---|
| A | Methanol ratio | 32.93 | 47.07 | -1 ↔ 35.00 | +1 ↔ 45.00 | 40.00 | 4.08 |
| B | Flow rate | 0.8586 | 1.14 | -1 ↔ 0.90 | +1 ↔ 1.10 | 1.0000 | 0.0816 |
| [CMP1] | [CMP2] | [CMR1] | [CMR2] | [CMR3] | |||
|---|---|---|---|---|---|---|---|
| Std | Run | Space Type | A:Methanol ratio | B: Flow rate | PC of Chlorogenic acid | TF of Chlorogenic acid | RT of Berberine |
| 1 | 13 | Factorial | 35 | 0.9 | 2392 | 1.439 | 11.901 |
| 2 | 5 | Factorial | 45 | 0.9 | 3190 | 1.248 | 4.718 |
| 3 | 1 | Factorial | 35 | 1.1 | 2097 | 1.435 | 10.555 |
| 4 | 6 | Factorial | 45 | 1.1 | 3110 | 1.212 | 3.716 |
| 5 | 12 | Axial | 32.93 | 1 | 2008 | 1.441 | 13.493 |
| 6 | 10 | Axial | 47.07 | 1 | 3265 | 1.141 | 3.418 |
| 7 | 9 | Axial | 40 | 0.86 | 2898 | 1.409 | 8.404 |
| 8 | 3 | Axial | 40 | 1.14 | 2615 | 1.37 | 6.294 |
| 9 | 7 | Center | 40 | 1 | 2887 | 1.401 | 6.569 |
| 10 | 4 | Center | 40 | 1 | 2891 | 1.401 | 6.479 |
| 11 | 8 | Center | 40 | 1 | 2881 | 1.404 | 6.375 |
| 12 | 2 | Center | 40 | 1 | 2886 | 1.407 | 6.242 |
| 13 | 11 | Center | 40 | 1 | 2891 | 1.401 | 6.147 |
Forced degradation studies
Degradation studies were conducted under acid, alkaline, oxidative, neutral, thermal and photolytic conditions to assess method stability. Solutions of Chlorogenic acid and Berberine were exposed to 0.1N HCl, 0.1N NaOH, 3% H2O2, HPLC water refluxed at 60°C for 30 min and placed in oven and UV light, injected into the HPLC column and calculate the % degradation.
Validation
The validation of the optimized method was conducted as per international conference on Harmonization (ICH) Q2R1 Guidelines and stability studies as per ICH Q1 R2 guidelines.
RESULTS
Using DOE software, 13 random experimental trials were conducted and the relationship between the interaction of CMPs and CMRs was ascertained using a response surface type of DOE employing a CCD design to show the method’s robustness. ANOVA results showed that the models for CMR1, CMR2 and CMR3 were significant (Table 3).
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| CMR 1 : PC of Chlorogenic acid | ||||||
| Model | 1.822 | 5 | 3.645 | 8848.29 | <0.0001 | significant |
| A-Methanol ratio | 1.610 | 1 | 1.610 | 39081.72 | <0.0001 | |
| B-Flow rate | 75121.23 | 1 | 75121.23 | 1823.73 | <0.0001 | |
| Lack of Fit | 219.54 | 3 | 73.18 | 4.25 | 0.0978 | not significant |
| CMR 2 – TF of Chlorogenic acid | ||||||
| Model | 0.1123 | 5 | 0.0225 | 971.68 | <0.0001 | significant |
| A-Methanol ratio | 0.0878 | 1 | 0.0878 | 3800.05 | <0.0001 | |
| B-Flow rate | 0.0011 | 1 | 0.0011 | 48.96 | 0.0002 | |
| Lack of Fit | 0.0001 | 3 | 0.0000 | 6.16 | 0.0558 | not significant |
| CMR 3 -RT of Berberine | ||||||
| Model | 111.18 | 5 | 22.24 | 651.50 | <0.0001 | significant |
| A-Methanol ratio | 99.90 | 1 | 99.90 | 2927.09 | <0.0001 | |
| B-Flow rate | 3.55 | 1 | 3.55 | 104.13 | <0.0001 | |
| Lack of Fit | 0.1216 | 3 | 0.0405 | 1.38 | 0.3696 | not significant |
The predicted R-square values of all the 3 responses, R2 (0.9991), R2 (0.9912) and R2 (0.9906), were in acceptable deal with the adjusted R-squared values of 0.9997, 0.9975 and 0.9963, respectively. The differences between adjusted and predicted R2 values were below 0.2 for each response. Graphical analysis using Normal residual plots, contour plots, perturbation plots and 3D surface plots demonstrates the interaction between factors and responses (Figures 3A, B, C, D).
After thorough analysis of design space specifications, selected the most ideal one to achieve our target with desirability (Figure 4). Derringer’s desirability bar graph showed the individual and combined desirability levels for optimized experimental conditions. Chlorogenic acid and Berberine were eluted at 3.534±0.2 and 5.140±0.2 min with a run time of 8 min, suggesting that the optimized chromatographic conditions (Table 4, Figure 5) were appropriate for the simultaneous determination of Chlorogenic acid, Berberine and remains unaffected by alterations in the experimental conditions, proving the robustness of the method. The chromatograms of stress-tested solutions were recorded and the % degradation of Chlorogenic acid was identified as 1.55 to 4.99; Berberine was 0.75 to 4.08 (Table 5, Figure 6), respectively.
| % Organic phase | Flow rate | Plate count of Chlorogenic acid | Tailing factor of Chlorogenic acid | Retention time of Berberine | Desirability |
|---|---|---|---|---|---|
| 41.67 | 1.100 | 2879.151 | 1.338 | 5.103 | 0.568 |
| Optimized values by Instrument | 2873 | 1.325 | 5.140 | – | |
| Difference | 6.151 | 0.013 | 0.037 | – | |
| Degradation Study | Chlorogenic acid | Berberine | ||
|---|---|---|---|---|
| %Assay | %Degradation | %Assay | %Degradation | |
| Acid | 98.22 | 1.78 | 98.52 | 1.48 |
| Alkali | 95.01 | 4.99 | 97.58 | 2.42 |
| Oxidative | 95.24 | 4.76 | 95.92 | 4.08 |
| Neutral | 98.45 | 1.55 | 99.25 | 0.75 |
| Thermal | 97.11 | 2.89 | 97.1 | 2.9 |
| Photolytic | 96.58 | 3.42 | 98.02 | 1.98 |

Figure 1:
(A) Structure of Chlorogenic acid and (B) Berberine.

Figure 2:
UV spectrum.
The established method was further verified according to ICH guidelines. The standard, blank and sample chromatograms from the specificity studies demonstrate that no peaks interfered with the analyte peaks, suggesting that the current method was specific. The system’s appropriateness was evaluated by Plate count (NTP), %RSD of Peak area, tailing factor and Resolution. All the parameters were within the specified limits (Table 6). The current method showed a linearity for Chlorogenic acid and berberine (0.25-1.5 μg/mL), with correlation values of 0.9996 and 0.9993 respectively (Table 6, Figure 7). The low values of LOD and LOQ (Table 6) indicated that this method was highly sensitive. The % recovery of phytochemicals was within permitted limits (Table 6), revealing that the current method was accurate and precision with % RSD of the peak areas was below 2 (Table 6). Robustness and stability studies confirmed that the method was reliable and the phytochemicals remained stable. All results were within the specified limits (Table 6).
| Parameter | Chlorogenic acid | Berberine | ICH limits |
|---|---|---|---|
| System suitability% RSD of Peak areaNTPTFResolution | |||
| 1.368 | 0.465 | %RSD NMT 2 | |
| 2980 | 3885 | NTP-More than 2000 | |
| 1.312 | 1.299 | TF-NMT 2 | |
| – | 5.569 | Resolution-NLT 2 | |
| Linearity Range (μg/mL) | 0.25-1.5 | 0.25-1.5 | – |
| Regression Equation | y=37561x-184.96 | y=46115x+29.036 | – |
| Correlation coefficient | R2-0.9996 | R2-0.9993 | R2-NLT 0.999 |
| LOD, LOQ (μg/mL) | 0.040, 0.122 | 0.053, 0.160 | – |
| % Recovery | 99.333-99.907 | 99.707-100.167 | 98-102% |
| Intra and Inter day Precision %RSD of peak area | 0.008-0.029 | 0.007-0.035 | %RSD NMT 2 |
| 0.009-0.061 | 0.004-0.055 | %RSD NMT 2 | |
| Robustness% RSD of Peak area for flow rate and% RSD of Peak area for Mobile phase | 0.016-0.036 | 0.008-0.037 | %RSD NMT 2 |
| 0.016-0.085 | 0.008-0.390 | %RSD NMT 2 | |
| Stability% variation | 0.08 | 0.75 | NMT 2 |
| Amount of markers in sample | 0.872 | 1.099 | – |

Figure 3:
Graphical representation of interaction effects of responses. A-Normality plot; B –Contour Plot; C-Perturbation plot; D-3D response surface plot.

Figure 4:
Derringer’s desirability A) Ramps, B) Bar graph.
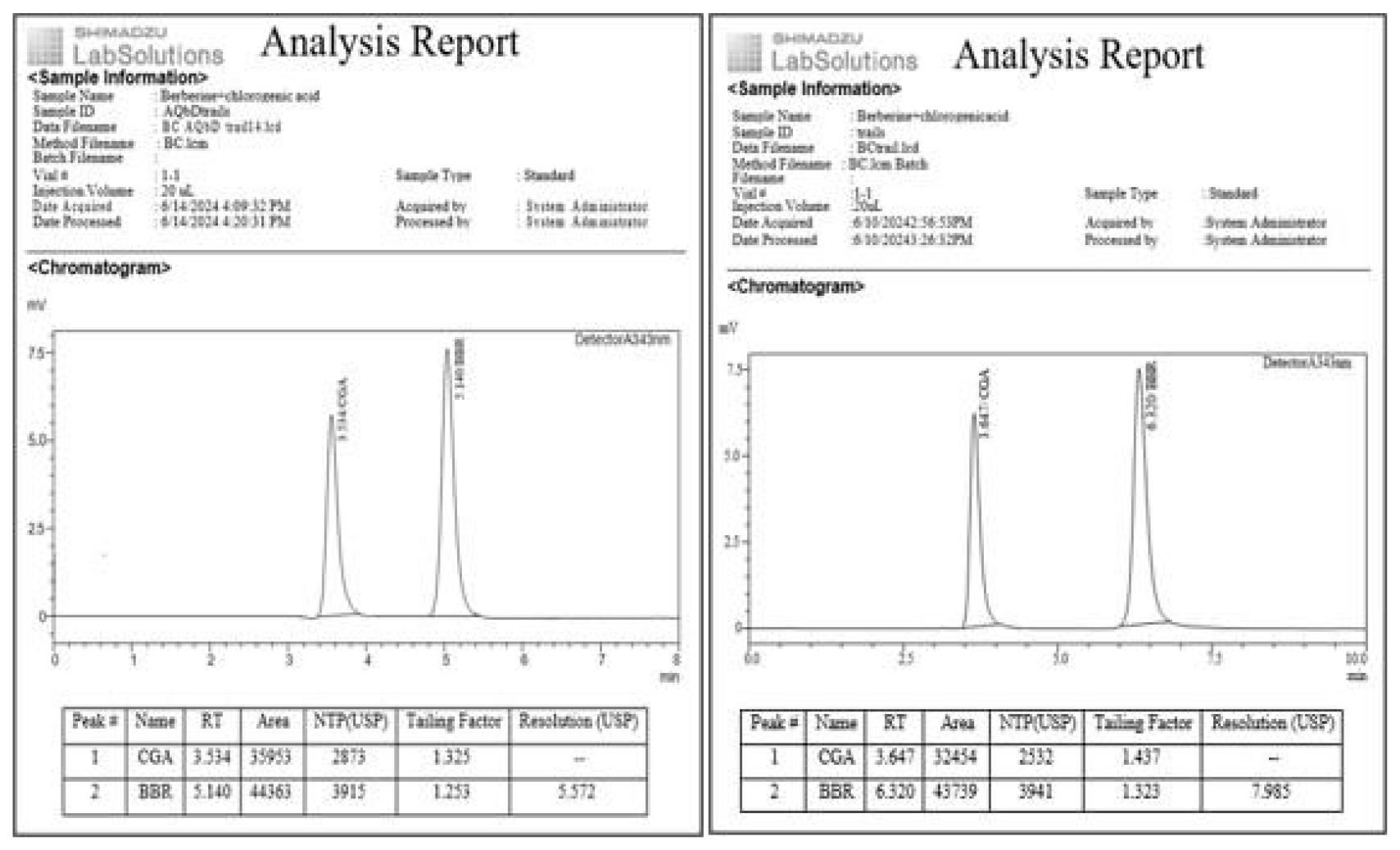
Figure 5:
Initial and final optimized Chromatograms.
Quantification of Phytochemicals in herbal formulation
Injected 6 replicates of 20 μL test sample solution into the column and the amount of Chlorogenic acid and Berberine in the herbal formulation was evaluated using a regression equation (Table 6).
DISCUSSION
The Stability-enabled AQbD based HPLC approach ensures consistency, reliability, accurately assessing the quality of phytochemicals without the need for revalidation. Previous studies reported that several methods existed for analysis of Chlorogenic acid and Berberine individually no methods reported for stability indicating simultaneous quantification of Chlorogenic acid and Berberine by AQbD-based HPLC method. Hence, the present research aims to establish a stability evaluating, simple, accurate, precise, robust and cost-effective RP-HPLC technique using AQbD principles with defining ATP and DOE software. When the model was subjected to a lack of fit test, the findings revealed a non-significant lack of fit value, resulting in a greater p-value than the model’s F-value. Statistical analysis (ANOVA, Regression coefficient) CCD seemed to be more suitable for fixing the quadratic model as per method optimization. Perturbation plots showed how a response changes when each factor changes location from a reference point while the other factors remained constant. All these graphical models enable the exploration of the design space. The target was to maximize the plate count (>2532), minimize the tailing factor (<1.437) and minimize retention time (<6.320). Analysis of Variance (ANOVA) plays an important role in validating the robustness of a method by systematically evaluating the effects of multiple factors on the method’s performance. The quadratic equations for all model responses were:

Figure 6:
Degradation Chromatograms of Chlorogenic acid and Berberine.
Equation 1 indicates increasing Methanol ratio leads to an increase in plate Count, but increase in Methanol and flow rate at high values decreases the plate count. Equation 2 indicates that higher Methanol ratio and flow rate, reduces tailing factor. Equation 3 indicates Increasing Methanol ratio or flow rate reduce retention time. From desirability function the results infers that plate count increases with an increase in % of Methanol ratio and flow rate; while tailing factor, decreases with lower Methanol ratio and flow rate. Retention time is minimized with moderate values. The combined desirability of 0.568 indicates overall moderate performance. Through the experiments, a mobile phase comprising 41.67% (v/v) Methanol and 0.1% formic acid buffer (58.33% v/v), using Shiseido Spolar C18 (250 mm x4.6 mm, 5 μm) at a column temperature of 25+2°C at a flow rate of 1.1 mL/min, detection at 343nm, consistently produced the best results. The developed method was further validated as per ICH Q2 R1 and stability guidelines. The developed method shows that % degradation was <10 in all stress-exposed conditions and elution of the degrading peak were not seen at the elution time of the analyte peaks. This indicates that the phytochemicals Chlorogenic acid and Berberine were stable and the developed method was specific. The results of validation parameters indicate values within acceptance limits.
Generally, herbal formulations contain multiple constituents with variations in their concentration. Developing this stability-indicating AQbD based HPLC method ensures the quality, safety and maximum efficacy of phytochemicals with therapeutic benefits.

Figure 7:
Linearity plot of Chlorogenic acid and Berberine.
CONCLUSION
Implementing the AQbD strategy has aided in the development of a more robust HPLC method, leading to a reduction in cost and analysis time. The method involved a multivariate study of the interaction effects of CMPs on responses to identify the best-performing system and the design space. This approach offers a real-world understanding that facilitates the chromatographic optimization and can be employed in the future. This method can be applied to various traditional medicines containing complex poly herbal formulations to assess the quality of formulations by integrating with advanced detection techniques.
Cite this article:
Cite this article: Kasetti K, Bandhakavi S. Stability Indicating Simultaneous Quantification of Chlorogenic Acid and Berberine in Homeopathic Polyherbal Formulation by AQbD Based HPLC. J Young Pharm. 2025;17(2):319-28.
ACKNOWLEDGEMENT
The authors wish to thank Sri Vasavi Institute of Pharmaceutical Sciences, Tadepalligudem for providing facilities to do work.
ABBREVIATIONS
| AQbD | Analytical Quality by Design |
|---|---|
| ANOVA | Analysis Of Variance |
| ATP | Analytical target Profile |
| CCD | Central Composite Design |
| CMPs | Critical Method Parameters |
| CMRS | Critical Method Responses |
| DoE | Design of Experiments |
| HCL | Hydrochloric acid |
| H2O2 | Hydrogen peroxide |
| ICH | International Conference on Harmonization |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MODR | Method Operable Design Region |
| NaOH | Sodium Hydroxide |
| NLT | Not Less Than |
| NMT | Not More Than |
| NTP | Number of Theoretical Plates |
| PC | Plate Count |
| RP-HPLC | Reverse Phase High performance Liquid Chromatography |
| RSD | Relative standard Deviation |
| RT | Retention Time |
| TF | Tailing factor |
| UV | Ultra violet Visible spectrophotometer |
References
- Atlabachew M, Abebe A, Alemneh Wubieneh T, Tefera Habtemariam Y. Rapid and simultaneous determination of trigonelline, caffeine and chlorogenic acid in green coffee bean extract. Food Science and Nutrition. 2021;9(9):5028-5035. [CrossRef] | [Google Scholar]
- Chaowuttikul C, Palanuvej C, Ruangrungsi N. Quantification of chlorogenic acid, rosmarinic acid and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Brazilian Journal of Pharmaceutical Sciences. 2020;56 Article e17547 [CrossRef] | [Google Scholar]
- Chaudhary P, Patel HU. RP-HPLC and spectrophotometric determination of rutin trihydrate, berberine chloride and trigonelline hydrochloride in antidiabetic Polyherbal Formulations. Research Journal of Pharmacy and Technology. 2020;13(7):3293-3299. [CrossRef] | [Google Scholar]
- Das V, Bhairav B, Saudagar RB. Quality by design approaches to analytical method development. Research Journal of Pharmacy and Technology. 2017;10(9):3188-3194. [CrossRef] | [Google Scholar]
- Fauzi A, Titisari N, Noor MHM, Azlan A, Hamzah H. Therapeutic effect of Morus alba leaf extract and chlorogenic acid on inhibiting the progression of kidney disease. Cogent Food and Agriculture. 2024;10(1) Article 2301841 [CrossRef] | [Google Scholar]
- Kushwaha SK, Kushwaha N, Maurya N, Rai AK. Role of markers in the standardization of herbal drugs: A review. Archives of Applied Science Research. 2010;2(1):225-229. [CrossRef] | [Google Scholar]
- Miao M, Xiang L. Pharmacological action and potential targets of chlorogenic acid. Advances in Pharmacology. 2020;87:71-88. [CrossRef] | [Google Scholar]
- Neag MA, Mocan A, Echeverría J, Pop RM, Bocsan CI, Crişan G, Buzoianu AD, et al. Berberine: Botanical occurrence, traditional uses, extraction methods and relevance in cardiovascular, metabolic, hepatic and renal disorders. Frontiers in Pharmacology. 2018;9:557 [CrossRef] | [Google Scholar]
- Nguyen V, Taine EG, Meng D, Cui T, Tan W. Chlorogenic acid: A systematic review on the biological functions, mechanistic actions and therapeutic potentials. Nutrients. 2024;16(7):924 [CrossRef] | [Google Scholar]
- Och A, Podgórski R, Nowak R. Biological activity of berberine-A summary update. Toxins. 2020;12(11):713 [CrossRef] | [Google Scholar]
- Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of Ayurveda. Pharmacognosy Reviews. 2014;8(16):73-80. [CrossRef] | [Google Scholar]
- Park G, Kim MK, Go SH, Choi M, Jang YP. Analytical quality by design (AQbD) approach to the development of analytical procedures for medicinal plants. Plants. 2022;11(21):2960 [CrossRef] | [Google Scholar]
- Sahani S, Jain V. A novel RP-HPLC method for simultaneous estimation of berberine, quercetin and piperine in an ayurvedic formulation. International Journal of Applied Pharmaceutics. 2019;11(1):94-99. [CrossRef] | [Google Scholar]
- Santana-Gálvez J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017;22(3):358 [CrossRef] | [Google Scholar]
- Siddique H, Sarwat M. Herbal medicines: A boon for healthy human life. 2022 [CrossRef] | [Google Scholar]
- Soudagar M, Kurangi B, Chethan Kumar HB, Chimagave S, Patil U, Kolambkar S, et al. Development and validation of a simple stability-indicating HPLC method for the quantitation of berberine in pharmaceuticals, ayurvedic, homeopathic products and novel nanoformulation. Analytical Chemistry Letters. 2023;13(3):244-256. [CrossRef] | [Google Scholar]
- Tome T, Žigart N, Časar Z, Obreza A. Development and optimization of liquid chromatography analytical methods by using AQbD principles: Overview and recent advances. Organic Process Research and Development. 2019;23(9):1784-1802. [CrossRef] | [Google Scholar]
- Wang H, Chen Y, Wang L, Liu Q, Yang S, Wang C, et al. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Frontiers in Pharmacology. 2023;14 Article 1265178 [CrossRef] | [Google Scholar]
- Wang J-C, Zhang Q, Cai D-F. Stability‐indicating validated HPLC method for analysis of berberine hydrochloride and trimethoprim in pharmaceutical dosage form. Journal of Chemistry. 2013;2013(1) Article 360812 [CrossRef] | [Google Scholar]
