ABSTRACT
ABSTRACT
In recent decades, there has been a growing fascination with delivering therapeutic agents through transmucosal (buccal, sublingual, nasal, rectal, and vaginal) drug delivery systems. Drug delivery through oral transmucosal tissue is effective, beneficial, and easily accessible; however, most commercially available formulations are limited to tablets and films, even though there are many formulation approaches and favorable opportunities for the buccal and sublingual routes. When compared to more conventional oral and parenteral dose forms, the buccal mucosa offers several advantages for regulated drug administration over the long term, including improved drug bioavailability and less systemic toxicity. Since the oral mucosa is well-vascularized, medication molecules bypass the liver’s metabolic pathway and reach the systemic circulation straightaway. Additionally, it is an excellent site for the systemic administration of drugs that are not well absorbed and a great alternative for the non-intrusive administration of potent peptide and protein drug molecules. Lots of people are interested in and working on the next generation of mucosal delivery systems for the mouth. This review delves into an array of subjects, including the oral mucosa’s structure, absorption, and permeation pathways; the formulations for oral transbuccal mucosa that improve permeation; and the utilization of existing transbuccal dosage forms to enhance transbuccal drug delivery for the treatment of diverse medical conditions.
INTRODUCTION
In the field of pharmaceutical technology, the phenomenon of mucoadhesion has garnered significant attention since the beginning of the 1980s. New drug delivery technologies have faced competition from improved medication formulations and more sophisticated administration methods throughout the last two decades. Recent decades have seen the successful exploration of transmucosal delivery pathways for a number of medications, with new methods always popping up (Abhanget al., 2014).
The term “transmucosal delivery” describes the method by which a drug is introduced into the bloodstream via the body’s mucous membranes. The process of adhesion may be classified into many terminological subsets based on the specific context in which it takes place. Getting two surfaces “fixed” to each other is the simplest way to describe the process of adhesion. The use of the terms “bioadhesion” and “mucoadhesion” describe adhesion in a biological context and, more specifically, on mucosal membranes, respectively. Bioadhesion is the process by which a polymer, either naturally occurring or artificially produced, binds to a biological surface. One common word for this kind of adhesion when the substrate is a layer of mucus is mucoadhesion (Andrewset al., 2009).
Conventional oral administration of certain medicines may be problematic in light of what is now known about the body’s drug absorption and processing mechanisms. This is because there is no significant correlation between absorption, permeability of the membrane, and bioavailability of these drugs since they are extensively cleared by the liver prior to entering the systemic circulation. Efforts have been made to increase the medications’ bioavailability by the use of absorption enhancers, innovative formulation techniques, and other methods. Recent years have witnessed an uptick in research on Transmucosal Drug Delivery Systems (TMDDSs) as a means to circumvent the limitations of traditional oral dosage forms by transporting medicines across other types of mucosa, such as sublingual, buccal, nasal, ocular, rectal, and vaginal (Heet al., 2016 and Sandriet al., 2020).
Global and regional industry overview, market intelligence, complete analysis, historical data, and forecasts 2023-2030 for the transmucosal medication delivery devices market by type of product (buccal, nasal, sublingual, rectal, urethral, and vaginal routes), application (hospital and residential end users), pain management, addiction treatment, hormonal therapies, and by another region. The worldwide market for transmucosal medication delivery devices was valued at around $41.2 billion in 2022 and is projected to reach $68.1 billion by 2030, expanding at a Compound Annual Growth Rate (CAGR) of about 6.5% from 2023 to 2030 (“Transmucosal Drug Delivery Devices Market Size Report, Industry Share, Analysis, Growth, 2030”).
Several bio-adhesive drug delivery systems, including those for smoking cessation, cough, pain management, and angina pectoris, comprised the worldwide buccal drug delivery industry. The worldwide buccal medication delivery industry was valued at around $3.38B in 2022 and is projected to reach $7.13B by 2030, expanding at a CAGR of about 9.80% from 2023 to 2030 (“Global Buccal Drug Delivery Market-Industry Trends and Forecast to 2030”).
The buccal mucosa is an ideal transmucosal route for the administration of mucoadhesive dosage forms because of its high accessibility and largely immobile smooth mucosa. In comparison with similar non-oral transmucosal drug ingestion approaches, buccal drug delivery has improved bioavailability due to its immediate accessibility to the systemic circulation across the internal jugular vein, which bypasses the liver-specific first-pass metabolism. Drug delivery via the buccal cavity has many advantages, such as a plentiful blood supply, little enzymatic activity, the ability to incorporate absorption enhancers, a painless administration process, and the flexibility to build systems with either multi- or unidirectional release, allowing for local or systemic effects. However, the buccal membrane has a limited permeability and a smaller surface area, thus only a modest dosage may be given by this route. Additionally, there may be restrictions on eating and drinking when using this method (Okuret al., 2021).
Buccal mucosa overview and its suitability
The mucous membrane layer that lines the cheek’s inside is called buccal mucosa. Mucous membranes lining the mouth cavity have a total surface area of 100 cm2. Several separate structures may be made out: the sublingual floor of the mouth, the buccal mucosa, the gingiva, the lining of the lips, and the gums. Oral mucosa consists of stratified squamous epithelium on the outside and basement membrane, lamina propria, and submucosa on the inside (Figure 1). The buccal mucosal lining epithelium is a stratified squamous epithelium that is nonkeratinized and has a surface area of 50.2 cm2 and a thickness of around 500-600 μm. Glycolipids, phospholipids, and cholesterol are abundant in the buccal epithelium’s superficial barrier zone. The essential components of the intercellular lamellae of buccal epithelium include ceramides, cholesterol, and saturated fatty acids. The highly structured gel phase membrane of intercellular lipids inside the epithelium forms a substantial physical barrier for the buccal mucosa (Satheesh Madhavet al., 2012). In the oral mucosa, the main and minor salivary glands release mucus as a component of saliva, however, in most cases, specialized cells such as goblet cells are responsible for mucus synthesizing. The mouth is lined by mucus, a gel-like secretion that consists mostly of glycoproteins that are insoluble in water. Furthermore, it is a visco-elastic hydrogel that mostly includes 1-5% of the water-insoluble glycoproteins listed above, 95-99% water, and a few additional components in minor amounts, including nucleic acids, proteins, enzymes, and electrolytes (Salamat-Milleret al., 2005).
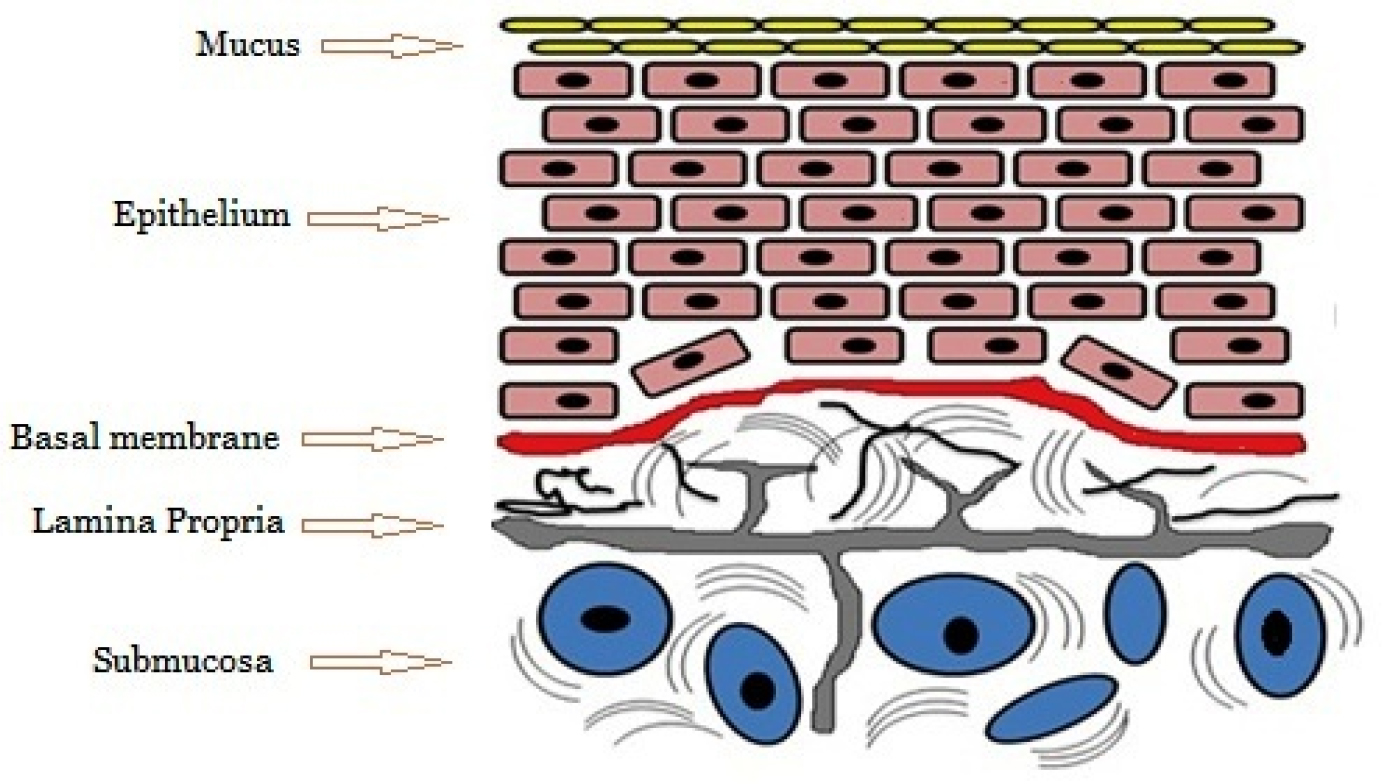
Figure 1:
Buccal mucosal structure.
Transbuccal drug absorption
Several processes, including paracellular or transcellular (simple passive diffusion), active transport, endocytosis, and carrier-mediated diffusion, are mostly responsible for the penetration of different drug substances. The buccal epithelium provides two pathways for passive diffusion: one is intracellular, which entails transportation inside and between cells, and the other route is paracellular, which entails transport via the gaps between cells (Figure 2). The buccal mucosa is mostly permeated by passive diffusion, according to recent findings, with carrier-mediated transport reportedly playing a minor role. Absorption may also occur by endocytosis, in which the drug molecules are engulfed by the cells, but this happens extremely rarely (Krothet al., 2020).
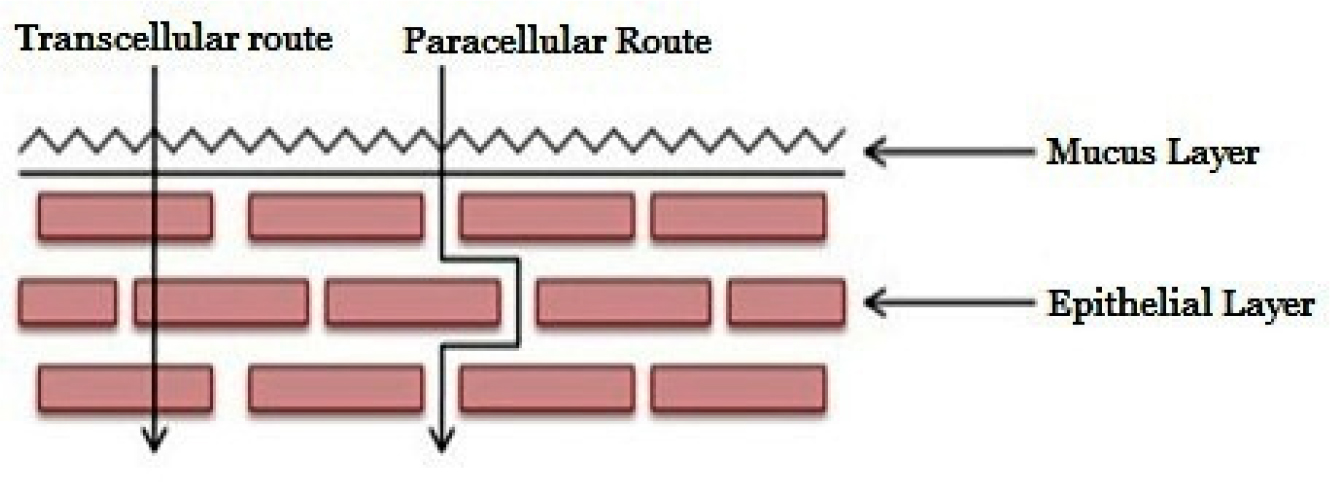
Figure 2:
Tanscellular and paracellular drug absorption.
Factors affecting buccal absorption
Membrane factors
Drug delivery in the buccal cavity is complicated due to the many factors, both independent and dependent, that lower the concentration of absorbable drugs at the mucosal site of absorption. There are a number of factors that affect how quickly and how much of a drug enters the bloodstream, including the thickness of the absorption membrane, the surface area accessible for absorption, the degree of keratinization, the mucus salivary pellicle, the cell renewal rate, and the enzyme content. Incorporating mucoadhesive polymers will allow the dosage form to remain in the buccal region for extended periods of time, even when subjected to tissue movements (Sudhakaret al., 2006).
Salivary glands and saliva
The minor salivary glands are situated in the epithelial region of the buccal mucosa and continuously secrete mucus onto the buccal mucosa’s surface. While mucus aids in the retention of mucoadhesive dosage forms, it can also act as a barrier to the penetration of drugs. A thin layer of saliva, known as pellicle or salivary film, covers the buccal mucosa lining. The composition and movement of the salivary film, which ranges in thickness from 0.07 to 0.10 mm, impact the rate of buccal absorption (McCullochet al., 2018).
Mucoadhesive polymers used in the oral cavity
Mucoadhesive medication delivery techniques rely heavily on bioadhesive polymers. Mucoadhesive dosage form development begins with the identification and evaluation of suitable bio-adhesive polymers for use in the formulation. Another use for polymers is in matrix devices, which regulate the release of medications by embedding them in a polymer matrix. One of the most versatile classes of polymers, bioadhesive polymers have several positive applications in medicine. From naturally occurring polymer compounds to biodegradable grafted copolymers to thiolated polymers, the phrase buccal sticky polymer encompasses a vast and varied class of molecules.
For mucoadhesion to work, mucoadhesive polymers need to have certain structural features such as hydrophobic groups, anionic or cationic charges, molecular weight, chain pliability, and surface energy qualities that promote spreading on mucus tissue. It is common practice to divide mucoadhesive polymers into three broad categories: synthetic, natural, water-soluble, and charged/uncharged. Table 1 provides examples of modern polymers that fall under these categories (Leeet al., 2000 and Zahir-Jouzdaniet al., 2018).
| Criteria | Categories | Examples |
|---|---|---|
| Source | Natural | Agarose, chitosan, guar gum, xanthan gum, carragenan gum, gellan gum and pectin. |
| Semi-natural | Gelatin, sodium alginate and hyaluronic acid. | |
| Synthetic | Cellulose derivatives: Sodium carboxymethyl cellulose, carboxymethyl cellulose, hydroxy ethyl cellulose, hydroxy propyl cellulose, hydroxypropyl methyl cellulose, thiolated carboxymethyl cellulose, methyl hydroxyethylcellulose. | |
| Poly (acrylic acid)-based polymers: Carbopol (carboxy polymethylene), polycarbophil, Polyacrylic acid, poly (methyl vinyl ether-co-methacrylic acid), poly(acrylic acid-co-ethyl hexyl acrylate), poly(alkyl cyanoacrylate), poly(2-hydroxyethyl methacrylate), poly(methacrylate), poly(isobutyl cyanoacrylate) and poly(iso-hexyl cyanoacrylate). | ||
| Others: Poly (N-2-hydroxypropyl methacrylamide) (PHPMAm), Poly (vinyl alcohol), Polyvinylpyrrolidone, polyoxyethylene and thiolated polymers. | ||
| Solubility | Water-soluble | Hydroxypropyl methylcellulose, (cold water), sodium alginate, Sodium carboxymethyl cellulose, carbopol, hydroxy propyl cellulose (waterb38 8C), hydroxyethyl cellulose, polyacrylic acid, polyvinylpyrrolidone, other cellulose derivatives. |
| Water-insoluble | Ethylcellulose, polycarbophil, chitosan (dilute aqueous acids). | |
| Charge | Anionic | Sodium carboxymethyl cellulose, Chitosan-EDTA, Carbopol, carboxymethyl cellulose, Polyacrylic acid, sodium alginate, polycarbophil, xanthan gum and pectin. |
| Cationic | Chitosan, trimethylated chitosan, amino dextran, and Diethylamino Ethyl (DEAE)-dextran. | |
| Non-ionic | Eudragit-NE30D, polyvinylpyrrolidone, polyvinyl alcohol, hydroxyethyl starch, hydroxy propyl cellulose, poly (ethylene oxide), and scleroglucan. | |
| Potential bioadhesive forces | Covalent | Cyanoacrylate |
| Hydrogen bond | Carbopol, polycarbophil, polyvinyl alcohol, and Acrylates poly (methacrylic acid). | |
| Electrostatic interaction | Chitosan, trimethylated chitosan. |
New generation novel mucoadhesive polymers
The new-generation polymers have better chemical interactions because they can create covalent connections with the mucus and the layers of underlying tissues. Apart from thiolated polymers, the latest generation of mucoadhesives is able to stick straight to the surface of cells, bypassing mucus altogether. In contrast to the non-specific processes shown by earlier polymers, these newer ones engage with the surface tissues by covalent bonding or specialized receptors (Waghet al., 2009).
Thiolated mucoadhesive polymers
According to recent research, thiol-group polymers outperform polymers often thought of as mucoadhesive in terms of adhesive characteristics. The development of stronger covalent connections between the polymer and the mucus layer explains the augmentation of mucoadhesion. Thiomers are thiolated polymers that participate in disulfide exchange activities or basic oxidation to engage with cysteine-rich subdomains of mucus glycoproteins. Polymers that have been modified to include a carbodiimide-mediated thiol link have superior mucoadhesive capabilities. Thiolated polymers are a promising new class of mucoadhesive materials due to their enhanced tensile strength, high cohesiveness, quick swelling, and water absorption behavior, among other desirable mucoadhesive qualities.
New mucoadhesive polysaccharide polymers, Thiolated Xanthan Gum (TXG), and S-Protected Thiolated Xanthan gum (STX) were created and studied by Alhakamy et al.; they were then used to make repaglinide mucoadhesive tablets. The enhanced interactions of macromolecules responsible for increasing the mucosal adhesion strength of thiolated gum may explain why STX’s viscosity increases significantly. The STX formulations including repaglinide that were designed to be mucoadhesive demonstrated the greatest strength and residence duration in terms of ex vivo mucoadhesion. The regulated release of repaglinide over 16 hr was achieved through the matrix’s increased cross-linkage and cohesiveness in the thiolated and S-protected thiolated formulations (Alhakamyet al., 2022).
Lectin-mediated, target-specific mucoadhesive polymers
Lectins are proteins that exist naturally and are essential in biological recognition processes that include proteins and cells. Although lectins are also present in bacteria, the vast majority of lectins come from plants. To infect a host organism, some bacteria utilize lectins to bind to its cells. Using suitable cytoadhesives that can attach to mucosal surfaces may improve mucosal delivery. Among these systems, lectins have received the greatest amount of attention from researchers. There is a class of proteins and glycoproteins called lectins that share the ability to bind reversibly to certain carbohydrate residues. Lectins have two options after attaching to mucosal cells: either they stay on the cell’s surface or are taken within by the body via endocytosis in receptor-mediated adhesion. In addition to facilitating targeted particular attachment, lectin-based platforms may provide a means of regulated drug delivery of macromolecular medicines via active cell-mediated drug absorption, making these systems multifunctional. Although lectins provide several benefits over first-generation platforms, it should be noted that these polymers are partially inactivated due to the loss of mucus. The mucus layer serves as a first, completely reversible binding site, and then lectin-mediated drug delivery systems are distributed to the cell layer. Hence, this phenomenon has been seen as beneficial (Clarket al., 2000 and Lehr 2000).
Lectins’ primary role in the natural environment
Several cell monolayers have been shown to exclusively and safely bind N-acetylglucosamine (GluNAc) when lectin from tomato fruit (Lycopersicum esculentum) is used. Tomato lectin has been shown to bind rat intestinal epithelium in a safe manner, without causing any damage to the membrane. The binding values of tomato lectin to rat intestinal rings may be inhibited to 83%, 80%, and 75%, respectively, by competitive sugars like (GlcNAc)4, the monomer of (GlcNAc)4, and N-acetyllactosamine. This proves that N-acetylglucosamine is the specific target of tomato lectin binding (Marothiaet al., 2023).
The principal functions of plant-based lectins in the natural environment include:
Bacterial adhesion
Researchers have lately looked at the sticky qualities of bacterial cells, which are a more complex adhesion mechanism. Fimbriae are unique parts of bacterial cell surfaces that allow them to cling to other cells or inanimate objects. This is how bacteria can stick to their targets. Bacterial polymers that are long and threadlike and found outside of cells significantly contribute to many illnesses. The binding moiety of some receptors binds bacterial fimbriae. The presence of fimbriae on bacterial surfaces is significantly correlated with their virulence. This method is appealing because, like plant lectins, it can enhance the drug’s residence duration on mucus and its receptor-specific interaction (Bernkop-Schnurch and Walker 2001).
Mucoadhesive polymers as enzyme inhibitor and permeation enhancer
Research shows that some mucoadhesive polymers can block enzymes. This discovery is especially significant for delivering medicinal molecules like polypeptide and protein medicines, which are highly susceptible to enzymatic degradation. One possible explanation for why some medications become more soluble in the gut when there are a lot of mucoadhesive polymers around is that these polymers may loosen up tight junctions by soaking up water from the epithelial cells. Dehydration and consequent cell shrinkage are outcomes of water absorption by a dry, swellable polymer. The resulting potential is an enlargement of the intercellular gaps. Oral medication administration has been made possible by using multipurpose matrices, including chitosan, polyacrylates, and cellulose derivatives. These matrices have a high buffer capacity, enzyme-inhibiting capabilities, permeation-enhancing effects, and mucoadhesive qualities. One effective oral mucoadhesive medication administration method is using certain mucoadhesive polymer compounds, such as chitosan, cellulose derivatives, and polyacrylates, which may interfere with enzymes and increase penetration (Gaboret al., 2004 and Baruaet al., 2016).
Biopolymers
Interestingly, the oral transmucosal drug delivery system is the most prevalent among the other delivery methods. In recent years, polymers derived from natural sources in drug delivery systems have been a popular subject of study. The extract of Ocimum basilicum, the seeds of Sesamum indicum, the leaves of Bombax malabaricum, the kernels of Helianthus annus, the seeds of Lallimantia royalena, Cucurbita maxima fruit pulp, and the fruit pulp of Annona squamosa are a few examples of biopolymers.
The transbuccal mucoadhesive drug delivery system
In general, dosage forms created for transbuccal medication distribution should be tiny and flexible enough to be suitable for patients and not irritate them. In addition to these desirable qualities, transbuccal mucoadhesive dosage forms include:
- It should have a high drug-loading capacity.
- Release of the medication under control.
- Release that is typically and ideally unidirectional.
- Features that are bioadhesive in nature.
- The floor is smooth.
- Without any flavor/Tastelessness.
- Convenient application (Hua 2019).
Buccal drug delivery systems
There are many advantages connected with the buccal route of medication administration, which is backed by a substantial amount of research from the scientific community. These advantages include the following:
- Excellent patient accessibility.
- Nonkeratinized mucosa.
- Low enzymatic activity.
- Broad drug absorption area.
- Enhanced control of plasma levels.
- Decreased variation in bioavailability.
- Fewer side effects.
- Minimal fluctuations.
- A simple removal of the dose form (Navamanisubramanianet al., 2017).
Design of buccal dosage form
For the buccal dosage form, two types may be distinguished, which are as follows:
The matrix type: The buccal dosage form developed in a matrix configuration has a mixture of additives, adhesives, and an active pharmaceutical ingredient.
Reserviour type: The buccal dosage form constructed in a reservoir system has a cavity distinct from the adhesive and retains the medicine and additives. An impermeable backing is placed to regulate the medicine is delivery direction, decrease the amount of distortion and disintegration that occurs when the tablet or patch is in the mouth, and avoid the loss of the drug (Repkaet al., 2004).
Sublingual drug delivery systems
In addition to being handy, easily accessible, and usually well accepted, the sublingual mucosa is moderately permeable, which allows for quick absorption and acceptable bioavailabilities of a wide variety of medications during administration. Out of all of these pathways, the sublingual route has received the most attention from researchers. There are two distinct types of sublingual dosage forms: those made up of tablets that dissolve quickly and those made up of soft gelatin capsules filled with liquid medications (Kannaet al., 2023).
The formulation of a sublingual dosage form
Specifically, the following are the activities of the excipients that are used for the sublingual dosage form:
A. Disintegrants: The number and kind of disintegrants utilized during the formulation process are very important factors in producing fast disintegration.
B. Effervescent agents: In contrast, effervescent agents have a significant role in the process that is being described.
C. Saccharides: The incorporation of water-soluble excipients, such as saccharides, enhances the tablet matrix’s wettability, which ultimately results in a quick breakdown.
D. Taste-masking agents: Many formulations contain sweeteners, flavors, and other taste-masking agents to disguise medications’ disagreeable tastes. Sugar excipients have an endothermic heat of dissolution, meaning they dissolve rapidly in saliva. Because of the pleasant sensation they provide in the mouth, they work well with other tastes and sublingual pills (Palemet al., 2015).
Oral transbuccal drug delivery systems
The optimal performance of an oral transbuccal medication delivery method hinges on a number of critical requirements. It is not just a matter of preference, but a necessity for the material to adhere quickly to the mucosal surface and maintain a firm contact to prevent displacement. The system’s efficacy may be influenced by ambient pH, but the bioadhesion performance should remain unaffected. High drug loading, full drug release, and easy administration are other essential features that contribute to the overall effectiveness of an oral transbuccal drug delivery system. The transbuccal mucoadhesive drug delivery system that is currently being developed represents the following kinds of dosage forms: medications such as transbuccal tablets, transbuccal mucoadhesive patches or films, microparticles, and nanoparticles are also available (Montenegro-Nicolini and Morales 2017).
Transbuccal tablets/lozenges
Many various formulations are available, but the three most popular forms of prochlorperazine available are buccal tablets, fentanyl stick lozenge, and nitroglycerin sublingual, which have likely undergone the greatest development. Holding the transmucosal tablets in the mouth allows the medication contents to be released for absorption directly via the oral mucosa. These solid dosage forms are made by compressing powder mixtures; when put on the oral mucosa, they may either dissolve or stick, depending on the excipients used. They can transport medications into the mouth or mucosal surface in several directions. Another option for achieving unidirectional medication delivery is for the dosage form to include an impermeable backing layer. The active ingredient, excipients, and maybe a second impermeable layer to enable unidirectional drug delivery are typically contained in a matrix containing a bioadhesive polymer, such as cellulose derivatives or polyacrylic acids, either alone or in combination (Samanthulaet al., 2022 and Silvaet al., 2015).
Transbuccal mucoadhesive films/patches
Medications can be delivered directly to a mucosal membrane using flexible transmucosal patches or films. In addition to this, they provide several distinctive characteristics, such as a relatively quick beginning of drug delivery, prolonged drug release, and superiority over creams and ointments in terms of the fact that they give a measured dosage of the medication to the site and have less variability either between individuals or within individuals. It is possible to maintain control over the medication concentrations and ensure that the drug is continually supplied for 10 to 12 hr since these systems are closed and the formulations are shielded from saliva. Incorporating an impermeable backing layer into the patches or film dosage form is yet another method that may be used to accomplish the goal of unidirectional medicine administration. Zilactin, used for treating canker sores, cold sores, and lip sores, is one example of a buccal adhesive film already in use in the commercial sector (Dinteet al., 2023 and Patlollaet al., 2021).
Transbuccal microparticles and nanoparticles
The benefits of transbuccal microparticles are identical to tablets, yet due to their physical qualities, they can establish close contact with a greater mucosal surface area. Microparticles are challenging to formulate, which is one of the reasons why their usage for transmucosal administration is uncommon in the current context. Despite this, this medication administration method will be developed to overcome the limits of other dosage forms. Compared to matrix tablets or patches, oral administration methods based on microparticles and nanoparticles often exhibit superior performance. These tiny immobilized carriers have a longer transbuccal mucoadhesive residence time because of their comparatively small size, which allows them to diffuse into the mucous gel layer.
Advances in drug delivery technology have centered on how pharmacokinetic characteristics affect therapeutic effectiveness and how important it is to target drugs to particular action sites. Nanotechnology is leading the charge among these new technologies for buccal medication delivery. Because of their regulated drug release profile, nanocarriers may prolong the time loaded pharmaceuticals spend in the systemic circulation, increasing bioavailability and leading to a steady-state plasma concentration with fewer adverse effects. So, to make medications more bioavailable, polymer matrices, including microparticles and nanocarriers, are often administered buccally (Macedoet al., 2020).
Commercial buccal adhesive drug delivery systems
Drug delivery solutions based on oral transmucosal devices are presently being developed and commercialized by many businesses. The majority of formulations that are sold on the market are in solid dosage forms like lozenges and tablets. Several other hormonal medications, like intranasal sprays of calcitonin and vasopressin, have markedly improved insulin administration via the buccal and pulmonary routes. With Pfizer and Nektar Therapeutics’ Exubera® receiving approval, the insulin delivery sector made great strides. Products that have been authorized for oral transmucosal delivery are included in Table 2. A small number of companies have been successful in this endeavor. Table 3 displays the names of the firms whose technological platforms are being developed for oral transmucosal medication delivery systems (Royet al., 2009).
| Drug | Product Name | Manufacturer | Dosage form | Uses |
|---|---|---|---|---|
| Acyclovir | Lauriad | BioAlliance Pharma | Buccal Tablet | Herpes Labialis |
| Androgen (Oxymetholone) | Anadrol-50 | Thomson Healthcare Products | Oral patch | Hormonal Agent |
| Buprenorphine HCl | Subutex | Reckitt Benckiser | Sublingual Tablet | Low Back Pain (LBP) |
| Buprenorphine HCl and Naloxone HCl | Suboxone | Reckitt Benckiser | Sublingual Tablet and Buccal Film | Opioid Dependence |
| Cannabis-derived (Nabiximols) | Sativex | GW Pharmaceuticals | Oromucosal Spray | Muscle stiffness and spasms (Spasticity) |
| Fentanyl Citrate | Actiq | Cephalon | Lozenges (Stick) | Opioid Analgesic |
| Fentora | Cephalon | Buccal Tablet | Opioid Analgesic | |
| Onsolis | Meda Pharmaceutical Inc | Buccal Film | Opioid Analgesic | |
| Glyceryl Trinitrate | NitroMist | NovaDel | Lingual -Spray | Anti-Angina |
| Suscard | Forest Laboratories | Buccal Tablet | Angina Pectoris | |
| Insulin | Oral-lyn | Generex Biotechnology | Buccal Spray | Diabetes Mellitus |
| Exubera® | Nectar Therapeutics, Inc./Pfizer/ | Pulmonary Spray | Diabetes Mellitus (Human insulin) (rDNA origin) | |
| Miconazole | Loramyc | BioAlliance Pharma | Buccal Tablet | Oropharyngeal Candidiasis |
| Nicotine | Nicorette | GSK Consumer Health | Chewing Gum | Smoking Cessation Agent |
| Nicotinelle | Novartis Consumer | Lozenge | Smoking Cessation Agent | |
| Nicoderm CQ | Pfizer Pharmaceuticals | Oral Film | Smoking Cessation Agent | |
| Nitroglycerine | Nitrostat | Pfizer Pharmaceuticals (pDavis) | Sublingual Tablet | Anti-angina |
| Prochlorperazine | Buccastem | Alliance Pharmaceuticals Limited | Buccal Tablet (Controlled) | Nausea and vomiting caused by migraines |
| Testosterone | Striant SR | Columbia Pharmaceutical | Buccal Tablet (Controlled) | Hypogonadism |
| Zolpidem | Zolpimist | NovaDel | Oral Spray | Insomnia |
| Technology | Company |
|---|---|
| Bio Erodible MucoAdhesive (BEMA) | BioDelivery Sciences International Inc |
| MedRo mucoadhesive spray technology | Med Pharm Ltd. |
| OraDisc (Disc) | Uluru Inc |
| Oral spray (RapidMist) | Generex Biotechnology |
| RapidFilm technology | Labtec Pharma |
| Sativex Buccal Spray | GW Pharmaceuticals |
| Sublingual tablets | Transcept Pharmaceutical Inc |
| Thinsol (Edible film technology) | Bioenvelop |
| VersaFilm (Quick release wafer technology) | IntelGenx |
| XGel (Films), OraDisc (Disc), WaferTab (Film strip) | Meldex |
Future perspective of transbuccal drug delivery research
There has been a lot of recent work on improving the management of systemic drug delivery and local drug targeting by focusing on delivery systems in specific regions of the mouth cavity, including the transbuccal, over long periods. Although different therapies are available, the challenges of drug permeability and regulated medication release by buccal drug administration persist. The buccal mucosa has been the target of many drug-NP loading techniques for both local and systemic delivery. Much research and development effort has gone into developing hydrogels that include nanoparticles and mucoadhesive buccal formulations, especially films. Recent research has focused on the possibility of directly administering vaccination antigens to different mucosal locations to protect mucosal surfaces adequately against the colonization and invasion of pathogenic organisms. Furthermore, mucosal adjuvants are usually necessary because mucosal vaccination induces inadequate immune responses. Therefore, the primary goals of developing successful mucosal vaccines are better mucosal antigen delivery and the discovery of novel and efficient mucosal adjuvants (Formicaet al., 2022; Lochheadet al., 2019).
CONCLUSION
Oral transmucosal drug delivery is an exciting new option that shows promise for overcoming the drawbacks of traditional oral drug delivery and parenteral administration. Transmucosal delivery, particularly buccal and sublingual delivery, has advanced significantly beyond traditional dosage forms on account of the ease of accessibility and avoidance of hepatic metabolism. New methods are constantly being developed to address the ever-present difficulty of medication administration over the oral mucosa. It has been suggested for a long time that the transbuccal route, in addition to other routes (such as pulmonary, nasal, ocular, rectal, and vaginal), might be used as a potential method of drug administration for the systemic distribution of medications that have a low or inconsistent bioavailability. This is an area where plenty of companies have found success, so it’s clear that there’s an opportunity for improvement. Sublingual tablets, buccal films, buccal tablets, and pulmonary spray have greatly enhanced the delivery of several medications, including insulin, administered via the intranasal and buccal routes. Prochlorperazine buccal tablets (Buccastem), fentanyl lozenges (Actiq), nitroglycerin sublingual (Nitrostat), testosterone (Striant SR), and insulin pulmonary spray (Oral-lyn and Exubera) are possibly the most well-known and advanced transbuccal medications currently available. This success story should inspire us all to continue pushing the boundaries of drug delivery.
Cite this article:
Samanthula KS, Bairi AG, Kothapally D. Transbuccal Drug Delivery Systems: A Comprehensive Review of Recent Approaches. J Young Pharm. 2025;17(3):495-503.
ABBREVIATIONS
| TMDDSs | Transmucosal Drug Delivery Systems |
|---|---|
| CAGR | Compound Annual Growth Rate |
| HPC | Hydroxypropyl Cellulose |
| PHPMAm | Poly (N-2-hydroxypropyl methacrylamide) |
| EDTA | Ethylenediaminetetraacetic acid |
| DEAE-dextran | Diethylamino ethyl-dextran |
| TXG | Thiolated Xanthan Gum |
| STX | S-Protected Thiolated Xanthan Gum |
| GlcNAc | N-Acetylglucosamine |
| LBP | Low Back Pain |
| BEMA | BioErodible MucoAdhesive |
| NP | Nanoparticles. |
References
- Abhang P., Momin M., Inamdar M., Kar S.. (2014) Transmucosal drug delivery-an overview. Drug Delivery Letters 4: 26-37 https://doi.org/10.2174/22103031113039990011 | Google Scholar
- Alhakamy N. A., Naveen N. R., Gorityala S., Kurakula M., Hosny K. M., Safhi A. Y., Bukhary D. M., Bukhary H. A., Sabei F. Y., Mushtaq R. Y., Murshid S. S., et al. (2022) Development of novel S-protective thiolated-based mucoadhesive tablets for repaglinide: Pharmacokinetic study. Polymers 14: 3529 https://doi.org/10.3390/polym14173529 | Google Scholar
- Andrews G. P., Laverty T. P., Jones D. S.. (2009) Mucoadhesive polymeric platforms for controlled drug delivery. European Journal of Pharmaceutics and Biopharmaceutics 71: 505-518 https://doi.org/10.1016/j.ejpb.2008.09.028 | Google Scholar
- Bakhrushina E., Anurova M., Demina N., Kashperko A., Rastopchina O., Bardakov A., Krasnyuk I., et al. (2020) Comparative study of the mucoadhesive properties of polymers for pharmaceutical use. Open Access Macedonian Journal of Medical Sciences 8(: 639-645 https://doi.org/10.3889/oamjms.2020.4930 | Google Scholar
- Barua S., Kim H., Jo K., Seo C. W., Park T. J., Lee K. B., Yun G., Oh K., Lee J., et al. (2016) Drug delivery techniques for buccal route: Formulation strategies and recent advances in dosage form design. Journal of Pharmaceutical Investigation 46: 593-613 https://doi.org/10.1007/s40005-016-0281-9 | Google Scholar
- Bernkop-Schnurch A., Walker G.. (Array) Multifunctional matrices for oral peptide delivery. Critical Reviews in Therapeutic Drug Carrier Systems in. Critical Reviews in Therapeutic Drug Carrier Systems 18 https://doi.org/10.1615/CritRevTherDrugCarrierSyst.v18.i5.20 | Google Scholar
- Clark M. A., Hirst B. H., Jepson M. A.. (2000) Lectin-mediated mucosal delivery of drugs and microparticles. Advanced Drug Delivery Reviews 43: 207-223 https://doi.org/10.1016/S0169-409X(00)00070-3 | Google Scholar
- Dinte E., Muntean D. M., Andrei V., Boșca B. A., Dudescu C. M., Barbu-Tudoran L., Borodi G., Andrei S., Gal A. F., Rus V., Gherman L.-M., Cadar O., Barabas R., Niculae M., Ilea A., et al. (2023)
In vitro and in vivo characterisation of a mucoadhesive buccal film loaded with doxycycline hyclate for topical application in periodontitis. Pharmaceutics 15: 580 https://doi.org/10.3390/pharmaceutics15020580 | Google Scholar - Dubashynskaya N. V., Petrova V. A., Skorik Y. A.. (2024) Biopolymer drug delivery systems for oromucosal application: Recent trends in pharmaceutical R&D. International Journal of Molecular Sciences 25: 5359 https://doi.org/10.3390/ijms25105359 | Google Scholar
- Formica M. L., Real D. A., Picchio M. L., Catlin E., Donnelly R. F., Paredes A. J., et al. (2022) On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Applied Materials Today 29: Article 101631 https://doi.org/10.1016/j.apmt.2022.101631 | Google Scholar
- Gabor F., Bogner E., Weissenboeck A., Wirth M.. (2004) The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Advanced Drug Delivery Reviews 56: 459-480 https://doi.org/10.1016/j.addr.2003.10.015 | Google Scholar
- He W.-S., Xiong H.-W., Xi D., Luo T.-T., Lu H., Li M.-H., Liu J.-C., Guo Z.-G., et al. (2016) Buccal Transmucosal Delivery System of Enalapril for Improved Cardiac Drug Delivery: Preparation and Characterization. T. and T. Tropical Journal of Pharmaceutical Research 15: 13-18 https://doi.org/10.4314/tjpr.v15i1.2 | Google Scholar
- Hua S.. (2019) Advances in nanoparticulate drug delivery approaches for sublingual and buccal administration. Frontiers in Pharmacology 10: 1328 https://doi.org/10.3389/fphar.2019.01328 | Google Scholar
- Kanna S., Nadendla R. R., Satyanarayana J., Karthikeya V., Sonu M. V., Bhargavi P. N., et al. (2023) Formulation and evaluation of fast-dissolving oral film of Rivaroxaban. Journal of Young Pharmacists 15: 687-695 https://doi.org/10.5530/jyp.2023.15.94 | Google Scholar
- Kaur G., Grewal J., Jyoti K., Jain U. K., Chandra R., Madan J., et al. (2018) In Drug targeting and stimuli sensitive drug delivery systems : 567-626 https://doi.org/10.1016/B978-0-12-813689-8.00015-X | Google Scholar
- Kroth R., Argenta D. F., Conte J., Amaral B. R., Caon T.. (2020) Transbuccal delivery of isoniazid: permeability and drug-surfactant interaction studies. AAPS PharmSciTech 21: 289 https://doi.org/10.1208/s12249-020-01827-5 | Google Scholar
- Lee J. W., Park J. H., Robinson J. R.. (2000) Bioadhesive‐based dosage forms: The next generation. Journal of Pharmaceutical Sciences 89: 850-866 https://doi.org/10.1002/1520-6017(200007)89:7<850:AID-JPS2>3.0.CO;2-G | Google Scholar
- Lehr C. M.. (2000) Lectin-mediated drug delivery: The second generation of bioadhesives. Journal of Controlled Release 65: 19-29 https://doi.org/10.1016/S0168-3659(99)00228-X | Google Scholar
- Lochhead J. J., Kellohen K. L., Ronaldson P. T., Davis T. P.. (2019) Distribution of insulin in trigeminal nerve and brain after intranasal administration. Scientific Reports 9: 2621 https://doi.org/10.1038/s41598-019-39191-5 | Google Scholar
- Macedo A. S., Castro P. M., Roque L., Thomé N. G., Reis C. P., Pintado M. E., Fonte P., et al. (2020) Novel and revisited approaches in nanoparticle systems for buccal drug delivery. Journal of Controlled Release 320: 125-141 https://doi.org/10.1016/j.jconrel.2020.01.006 | Google Scholar
- Marothia D., Kaur N., Jhamat C., Sharma I., Pati P. K.. (2023) Plant lectins: Classical molecules with emerging roles in stress tolerance. International Journal of Biological Macromolecules 244: Article 125272 https://doi.org/10.1016/j.ijbiomac.2023.125272 | Google Scholar
- McCulloch R., Sattar M., Henderson E. M., Lane M. E., Bluebond-Langner M.. (2018) Use of buccal morphine in the management of pain in children with life-limiting conditions: Results of a laboratory study. Palliative Medicine 32: 554-558 https://doi.org/10.1177/0269216317717192 | Google Scholar
- Mishra A., Behura A., Mawatwal S., Kumar A., Naik L., Mohanty S. S., Manna D., Dokania P., Mishra A., Patra S. K., Dhiman R., et al. (2019) Structure-function and application of plant lectins in disease biology and immunity. Food and Chemical Toxicology 134: Article 110827 https://doi.org/10.1016/j.fct.2019.110827 | Google Scholar
- Montenegro-Nicolini M., Morales J. O.. (2017) Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech 18: 3-14 https://doi.org/10.1208/s12249-016-0525-z | Google Scholar
- Navamanisubramanian R., Nerella R., D C., Seetharaman S.. (2017) Use of okra mucilage and chitosan acetate in verapamil hydrochloride buccal patches development; and characterization. Journal of Young Pharmacists 9: 94-99 https://doi.org/10.5530/jyp.2017.9.18 | Google Scholar
- Okur N. Ü., Bülbül E. Ö., Yağcılar A. P., Siafaka P. I.. (2021) Current status of mucoadhesive gel systems for buccal drug delivery. Current Pharmaceutical Design 27: 2015-2025 https://doi.org/10.2174/1381612824666210316101528 | Google Scholar
- Palem C. R., Dudhipala N., Battu S. K., Goda S., Repka M. A., Yamsani M. R., et al. (2015) Combined dosage form of pioglitazone and felodipine as mucoadhesive pellets via hot melt extrusion for improved buccal delivery with application of quality by design approach. Journal of Drug Delivery Science and Technology 30: 209-219 https://doi.org/10.1016/j.jddst.2015.10.017 | Google Scholar
- Patlolla V. G. R., Popovic N., Peter Holbrook W., Kristmundsdottir T., Gizurarson S.. (2021) Effect of doxycycline microencapsulation on buccal films: Stability, mucoadhesion and drug release. Gels 7: 51 https://doi.org/10.3390/gels7020051 | Google Scholar
- Repka M. A., Prodduturi S., Munjal M., Mididoddi P.. (2004) Matrix- and reservoir-based transmucosal delivery systems: Tailoring delivery solutions. American Journal of Drug Delivery 2: 173-192 https://doi.org/10.2165/00137696-200402030-00003 | Google Scholar
- Roy S., Pal K., Anis A., Pramanik K., Prabhakar B.. (2009) Polymers in mucoadhesive drug-delivery systems: A brief note. Designed Monomers and Polymers 12: 483-495 https://doi.org/10.1163/138577209X12478283327236 | Google Scholar
- Salamat-Miller N., Chittchang M., Johnston T. P.. (2005) The use of mucoadhesive polymers in buccal drug delivery. Advanced Drug Delivery Reviews 57: 1666-1691 https://doi.org/10.1016/j.addr.2005.07.003 | Google Scholar
- Samanthula K. S., Kumar CB M., Bairi A. G., Satla S. R.. (2022) Development, and evaluation of muco-adhesive buccal tablets of hydralazine hydrochloride. Brazilian Journal of Pharmaceutical Sciences 58: Article e18635 https://doi.org/10.1590/s2175-97902020000318635 | Google Scholar
- Sandri G., Ruggeri M., Rossi S., Bonferoni M. C., Vigani B., Ferrari F., et al. (2020) buccal drug delivery. Nanotechnology for Oral Drug Delivery. The Translator : 225-250 https://doi.org/10.1016/B978-0-12-818038-9.00013-2 | Google Scholar
- Satheesh Madhav N. V., Semwal R., Semwal D. K., Semwal R. B.. (2012) Recent trends in oral transmucosal drug delivery systems: An emphasis on the soft palatal route. Expert Opinion on Drug Delivery 9: 629-647 https://doi.org/10.1517/17425247.2012.679260 | Google Scholar
- Silva B. M. A., Borges A. F., Silva C., Coelho J. F. J., Simões S.. (2015) Mucoadhesive oral films: The potential for unmet needs. International Journal of Pharmaceutics 494: 537-551 https://doi.org/10.1016/j.ijpharm.2015.08.038 | Google Scholar
- Sudhakar Y., Kuotsu K., Bandyopadhyay A. K.. (2006) Buccal bioadhesive drug delivery-A promising option for orally less efficient drugs. Journal of Controlled Release 114: 15-40 https://doi.org/10.1016/j.jconrel.2006.04.012 | Google Scholar
- Wagh M. P., Joshi O. U., Patel J. S., Jain V. R.. (2009) Thiomers: A new generation of mucoadhesive polymers. Research Journal of Pharmacy and Technology 2: 250-255. PID=2009-2-2-52 https://doi.org/10.1016/j.jconrel.2006.04.012 | Google Scholar
- Zahir-Jouzdani F., Wolf J. D., Atyabi F., Bernkop-Schnürch A.. (2018)
In situ gelling and mucoadhesive polymers: Why do they need each other?. Expert Opinion on Drug Delivery 15: 1007-1019 https://doi.org/10.1080/17425247.2018.1517741 | Google Scholar
